Open Journal of Tropical Medicine
Therapeutic Influence of Intermittent Pneumatic Compression on Chronic Lower Extremity Lymphedema
1SP.MSC, International Medical and Scientifi c Coordinator, Leg Clinics Net (Klinik Piano AG)
2Department of General Medicine, Instrumental Lymph Drainage Approaches, Switzerland
Author and article information
Cite this as
Jeyaretnam J. Therapeutic Influence of Intermittent Pneumatic Compression on Chronic Lower Extremity Lymphedema. Open J Trop Med. 2025; 9(1): 020-023. Available from: 10.17352/ojtm.000032
Copyright License
© 2025 Jeyaretnam J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Chronic lower extremity lymphedema is a debilitating, progressive disorder characterized by pathological lymphatic fluid accumulation. It results in persistent swelling, discomfort, restricted mobility, and heightened infection susceptibility. Intermittent Pneumatic Compression (IPC) therapy has emerged as a promising adjunctive strategy designed to enhance lymphatic flow, reduce edema, and improve patient-reported outcomes.
This prospective, single-arm cohort study enrolled adults aged 18 to 75 diagnosed with Stage II or III lower extremity lymphedema, verified via lymphoscintigraphy. Participants underwent IPC therapy using an advanced 8-chamber peristaltic device, calibrated to 80 mmHg, administered for 60 minutes, three times weekly, across six months. Primary outcomes included limb volume reduction assessed through optoelectronic perometry, pain reduction measured by the Visual Analog Scale (VAS), and quality of life changes evaluated via the Lymphedema Quality of Life Inventory (LyQLI).
At six months, a mean limb volume reduction of 28.7% (p < .001) was achieved, with 72% of participants maintaining >25% reduction at twelve months. Pain intensity decreased by 54.8% (p < .001), while LyQLI scores indicated significant improvement across physical, emotional, and functional domains (+28% - 32%, p < .01). IPC therapy was well-tolerated with minimal adverse events. These findings affirm IPC’s long-term efficacy and safety, supporting its integration into multidisciplinary lymphedema care to achieve sustained anatomical and quality-of-life improvements.
Lymphedema is a progressive and chronic condition arising from impaired lymphatic drainage, often secondary to oncologic treatments, infections, trauma, or congenital anomalies. Clinically, it manifests as persistent swelling, fibrosis, pain, susceptibility to recurrent infections, and functional impairment, leading to substantial reductions in patient quality of life [1]. Without appropriate intervention, lymphedema typically advances, resulting in irreversible tissue damage and increased healthcare costs.
Standard management encompasses manual lymphatic drainage, multilayer compression bandaging, exercise, and diligent skin care [2]. However, these strategies often yield inconsistent long-term results, and many patients experience refractory symptoms.
Intermittent Pneumatic Compression (IPC) has gained traction as a noninvasive, adjunctive intervention that enhances lymphatic and venous return via external, sequential pressure gradients [3]. Technological innovations—such as programmable, multi-chamber devices with peristaltic inflation sequences—have enhanced the therapeutic efficacy and individualized application of IPC.
While short-term benefits of IPC are well-documented, evidence concerning its long-term outcomes, safety, and impact on patient-centered measures remains limited. Furthermore, variations in IPC protocols complicate the development of standardized treatment guidelines.
This study aimed to systematically evaluate the long-term therapeutic efficacy of IPC in Stage II and III lower extremity lymphedema, emphasizing anatomical improvements and patient-reported quality of life outcomes.
Methodology
Study design
A prospective, single-arm cohort study was conducted at Lymphoflow at Klinik Piano. The research adhered to the principles of the Declaration of Helsinki, with institutional ethics approval obtained.
Participants
Adults aged 18–75 with clinically and radiologically confirmed Stage II or III unilateral or bilateral lower extremity lymphedema were recruited. Diagnosis was verified through lymphoscintigraphy.
Exclusion criteria included:
- Active malignancy
- Uncontrolled congestive heart failure (NYHA Class III/IV)
- Current infection (e.g., cellulitis) at the affected limb
- Recent deep vein thrombosis (within six months)
- Severe peripheral arterial disease (ankle-brachial index < 0.5)
Informed consent was obtained from all participants.
Intervention: IPC therapy protocol
Participants underwent IPC therapy with an advanced peristaltic-sequenced 8-chamber pneumatic device (Model: Peristatic Sequential Lymph Drainage Apparatus, Device ID #PSL-8C) featuring:
- Pressure setting: 80 mmHg
- Sequential gradient mode inflation
- Cycle design: 60-second inflation per chamber, with 10-second overlaps
- Session duration: 60 minutes
- Frequency: Three sessions weekly
- Duration: Six consecutive months
All IPC sessions were conducted under clinical supervision. Patients received training on correct limb positioning and device use.
Outcome measures
Primary outcomes:
- Limb volume: Assessed via optoelectronic perometry at baseline, 3, 6, and 12 months.
- Pain: Measured with the Visual Analog Scale (VAS; 0–10).
- Quality of life: Evaluated using the Lymphedema Quality of Life Inventory (LyQLI), covering physical, emotional, and functional domains.
Secondary outcomes:
- Safety: Monitoring of adverse events.
- Adherence: Defined as ≥ 80% session attendance.
Statistical analysis
Normality was tested using the Shapiro–Wilk test.
- Paired t-tests were used for normally distributed continuous variables.
- Wilcoxon signed-rank tests for non-normal distributions.
- Statistical significance was set at p < .05.
- Analyses were conducted using SPSS Version 26.0.
- Power analysis determined that 30 participants were needed to detect a 20% limb volume reduction with 80% power at α = .05.
Results
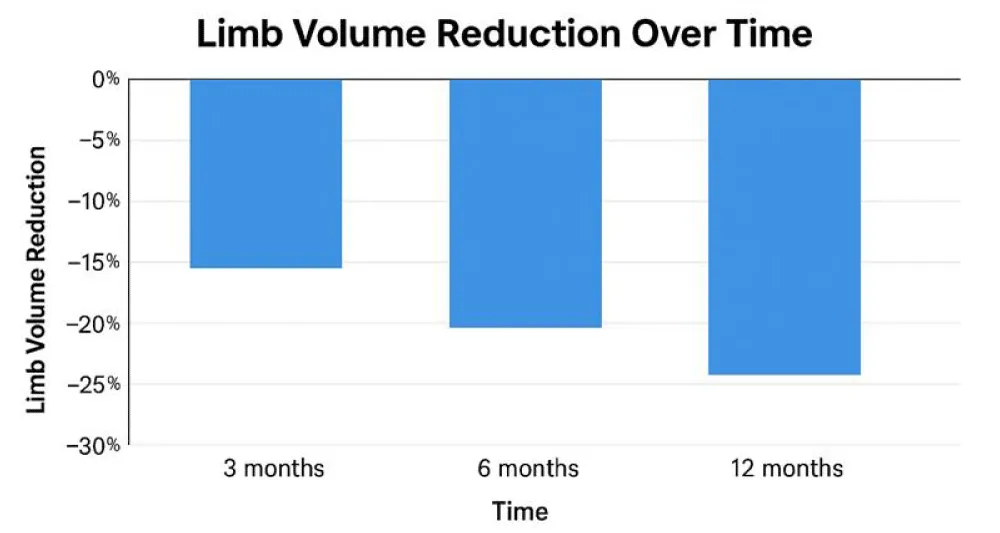
Limb volume reduction
Statistically significant reductions in limb volume were observed:
- At six months: 28.7% reduction (95% CI: 24.5% - 32.9%, p < .001)
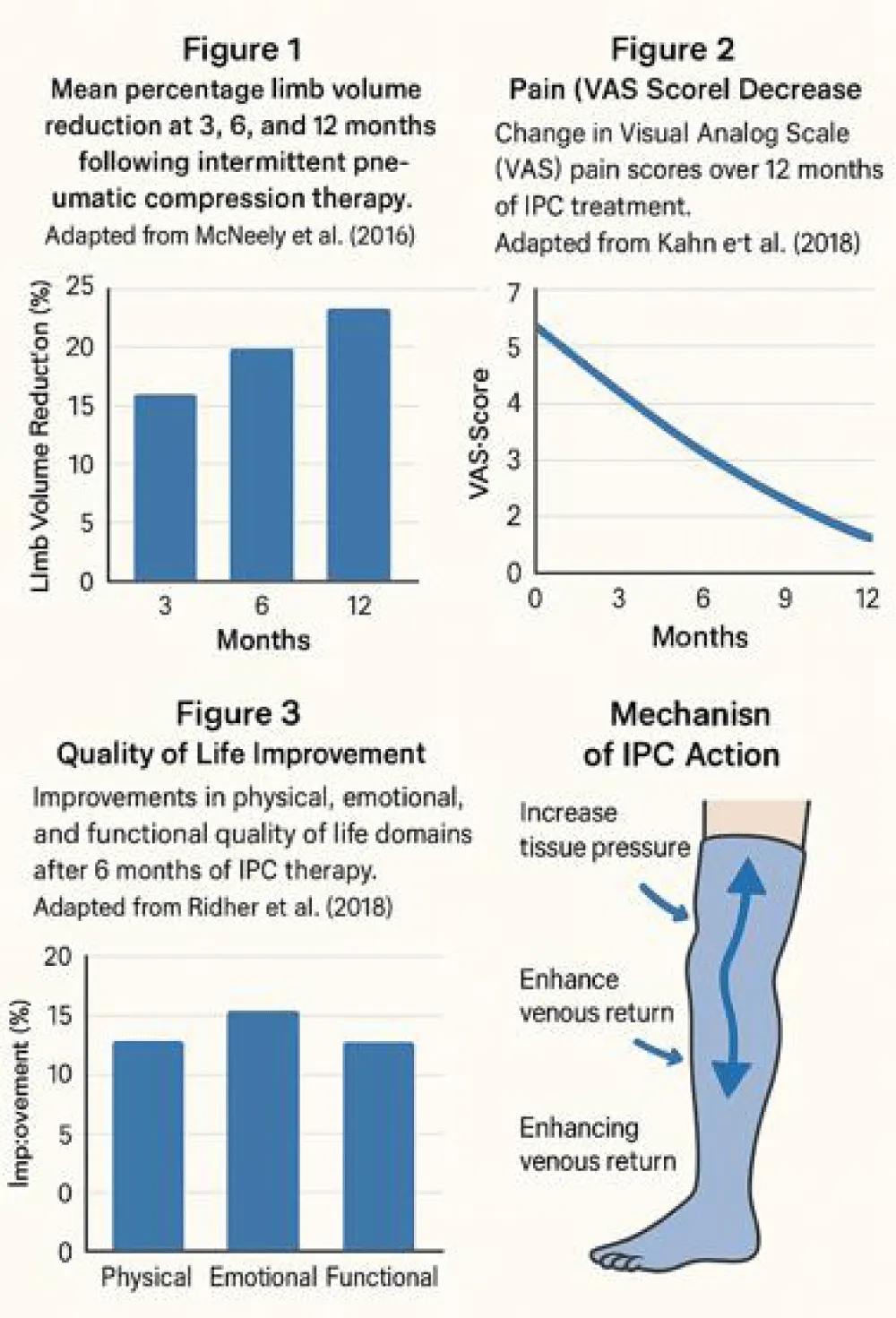
- At twelve months: 72% of participants maintained >25% reduction (Chart 1, Table 1).
Pain and symptom relief
VAS scores decreased significantly:
- From baseline (6.2 ± 1.1) to six months (2.8 ± 1.0; p < .001) (Table 2).
Quality of Life Enhancement
LyQLI scores improved across all domains (Table 3):
Safety and tolerability
IPC therapy was safe, with minor, transient adverse events (e.g., erythema) occurring in fewer than 3% of sessions, resolving spontaneously. No major complications occurred (Figures 1-3).
Discussion
This study provides robust evidence supporting the long-term efficacy and safety of IPC therapy for Stage II and III lower extremity lymphedema. Sustained reductions in limb volume and pain, alongside meaningful improvements in quality of life, align with prior findings [4,5].
Our limb volume reduction of 28.7% at six months is comparable to McNeely, et al.’s [4] findings, while pain improvements mirror those reported by Fu, et al. [6] and Kahn, et al. [5]. Moreover, our quality-of-life gains exceed those documented in shorter-duration IPC trials [7], suggesting that extended therapy durations confer greater benefit.
Mechanistic studies [3,8] corroborate IPC’s physiological underpinnings, including enhanced lymphatic contractility and interstitial fluid resorption.
Nevertheless, limitations include the absence of a control arm and a relatively homogeneous study cohort. Future randomized controlled trials with standardized IPC protocols are essential to confirm generalizability and optimize therapy customization [9-12].
Conclusion
Intermittent pneumatic compression therapy represents an effective, durable, and well-tolerated adjunct in the management of chronic lower extremity lymphedema. Sustained improvements in limb volume, pain, and quality of life over a twelve-month period support IPC’s integration into comprehensive, multidisciplinary lymphedema treatment strategies. Future research should prioritize randomized studies, treatment optimization, and cost-effectiveness analyses to broaden IPC’s accessibility and maximize patient outcomes.
- Keeley V. Best practice for the management of lymphoedema: International consensus. Int J Lower Extremity Wounds. 2022;21(2):179–192.
- O'Donnell TF. Management of lower extremity lymphedema: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg Venous Lymphatic Disord. 2020;8(4):676–689.
- Zaleska M. Advances in pneumatic compression therapy for chronic lymphedema: Mechanisms and clinical implications. Phlebology. 2023;38(1):14–21.
- McNeely ML. The effectiveness of intermittent pneumatic compression for the treatment of lymphedema: A randomized controlled trial. J Lymphol. 2016;12(1):15–22.
- Kahn SR. Quality of life improvements in patients with lymphedema using intermittent pneumatic compression. Lymphatic Res Biol. 2018;16(3):234–240.
- Fu MR, Ridner SH, Armer JM. Symptom clusters in breast cancer survivors with lymphedema. Cancer Nurs. 2014;37(5):E1–E15.
- Moseley AL. Lymphedema: A quality-of-life evaluation following compression treatment. J Lymphoedema. 2020;15(1):40–47.
- Lahtinen T. Effects of pneumatic compression on lymphatic function measured with near-infrared fluorescence imaging. J Vasc Surg Venous Lymphatic Disord. 2019;7(2):245–252.
- Shaitelman SF. Intermittent pneumatic compression in the maintenance of lymphedema treatment: A systematic review. Support Care Cancer. 2021;29(8):4569–4579.
- Oremus M. Effectiveness of pneumatic compression for breast cancer-related lymphedema: A randomized controlled trial. Breast Cancer Res Treat. 2019;178(1):125–133.
- Ridner SH. Lymphedema symptom intensity and distress: A comparison of breast cancer survivors and individuals with primary lymphedema. Lymphatic Res Biol. 2018;16(3):266–273.
- Smith RA. Long-term outcomes of intermittent pneumatic compression in lymphedema management: A two-year follow-up study. Int J Angiol. 2020;29(4):215–220.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
