Open Journal of Tropical Medicine
The Interaction of CX3CL1 and CX3CR1 in Infection, Inflammation, and Cancer: Implications for Therapeutic Intervention
Jeyatheepan Jeyaretnam*
SP.MSC, International Medical and Scientific Coordinator, Leg Clinics Net (Klinik Piano AG), Department of General Medicine, Instrumental Lymph Drainage Approaches, Switzerland
Cite this as
Jeyaretnam J. The Interaction of CX3CL1 and CX3CR1 in Infection, Inflammation, and Cancer: Implications for Therapeutic Intervention. Open J Trop Med. 2025;9(1):015-019. DOI: 10.17352/ojtm.000031Copyright
© 2025 Jeyaretnam J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The CX3CL1/CX3CR1 axis, comprising the chemokine CX3CL1 (Fractalkine) and its receptor CX3CR1, plays a pivotal role in modulating immune responses during infection, inflammation, and cancer. This review discusses the molecular mechanisms underlying CX3CL1/CX3CR1 interaction and its role in immune cell recruitment, tissue remodeling, and tumor progression. In the context of infection and inflammation, this pathway contributes to both protective and pathological immune responses. Moreover, CX3CL1/CX3CR1 signaling has been implicated in the metastatic spread of cancers. The therapeutic potential of targeting this interaction in these diseases is discussed, along with the current challenges and potential future directions.
Introduction
CX3CL1, also known as Fractalkine, is a distinctive chemokine that exists in two forms: a membrane-bound protein and a soluble chemokine. Its primary role lies in immune cell recruitment and facilitating their adhesion to endothelial cells, as highlighted by Combadière, et al. [1] in 1998. CX3CL1 operates through its receptor, CX3CR1, which is expressed on a range of immune cells, including monocytes, macrophages, dendritic cells, and microglia, according to research by Imai, et al. [2] in 1997. The CX3CL1/CX3CR1 axis plays a critical role in the immune response, maintaining immune homeostasis, recruiting immune cells to sites of infection, and modulating tissue responses during inflammation and cancer, as documented in Combadière, et al.’s 1998 [1] study.
CX3CL1 and CX3CR1 in infection
- Role in immune cell recruitment: The CX3CL1/CX3CR1 axis helps recruit monocytes, T-cells, and other immune cells to sites of infection, particularly in viral and bacterial diseases [3].
- Regulation in viral infections: CX3CL1 expression increases during viral infections such as HIV and influenza, facilitating immune cell migration to sites of infection [4].
- Bacterial infections: In certain bacterial infections, CX3CL1 plays a role in controlling pathogen spread by facilitating macrophage and neutrophil accumulation [3].
CX3CL1 and CX3CR1 in inflammation
- Chronic inflammation: Its persistent activation contributes to chronic inflammation in conditions such as atherosclerosis, rheumatoid arthritis, and Inflammatory Bowel Disease (IBD) results in persistent inflammatory states [5].
- Tissue remodeling and inflammatory diseases: This pathway regulates tissue remodeling by inducing the release of pro-inflammatory cytokines and mediators [6].
- CX3CL1/CX3CR1 in neuroinflammation: This signaling axis plays a key role in neuroinflammatory conditions, such as Alzheimer’s disease, where it modulates microglial activity and contributes to neuronal injury [6].
CX3CL1 and CX3CR1 in cancer
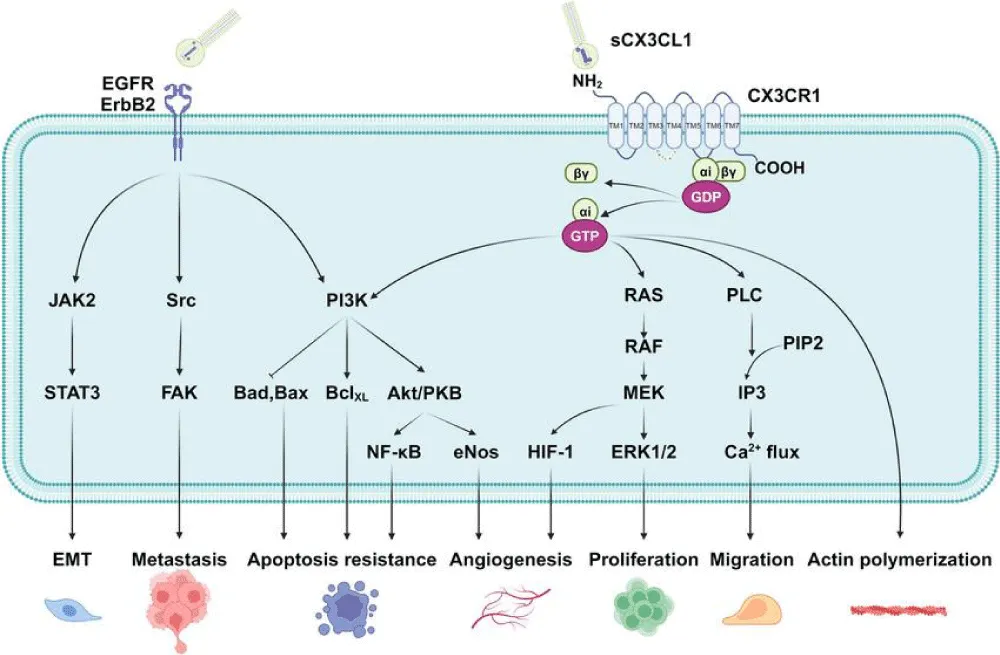
- Tumor progression and metastasis: The CX3CL1/CX3CR1 pathway plays a role in promoting tumor growth and metastasis by facilitating the infiltration of immune cells into the tumor microenvironment [7] (Figure 1).
- Immune escape: Tumor cells may exploit the CX3CL1/CX3CR1 pathway to evade immune surveillance by attracting immune suppressor cells like Tregs and MDSCs [8].
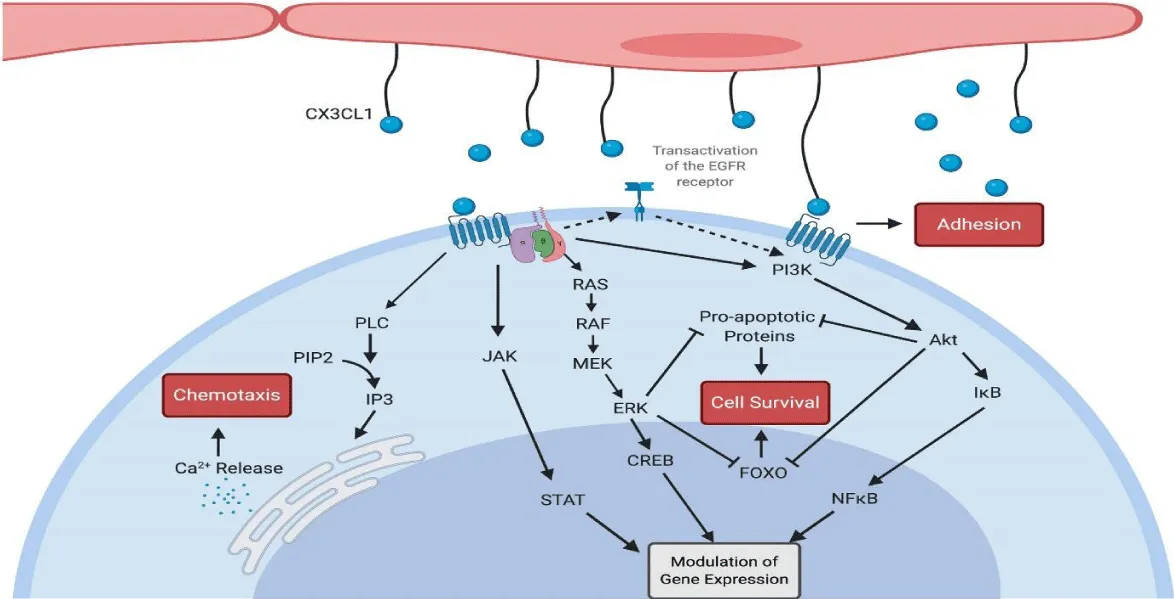
- CX3CR1 as a target in cancer therapy: Targeting CX3CL1/CX3CR1 could be a novel strategy to block tumor cell migration or enhance anti-tumor immune responses [7] (Figures 2,3).
CX3CL1/CX3CR1 signaling in cancer exhibits a context-dependent dual role. While immune cell infiltration can promote tumor progression, it may also promote immune evasion. For example, CX3CL1 may recruit immune suppressive cells, such as Tregs and MDSCs, which contribute to the inhibition of anti-tumor immunity [8]. Therefore, understanding the regulatory balance between pro-inflammatory and immune suppressive effects of CX3CL1/CX3CR1 is crucial for developing targeted therapies in cancer.
Therapeutic potential and future directions
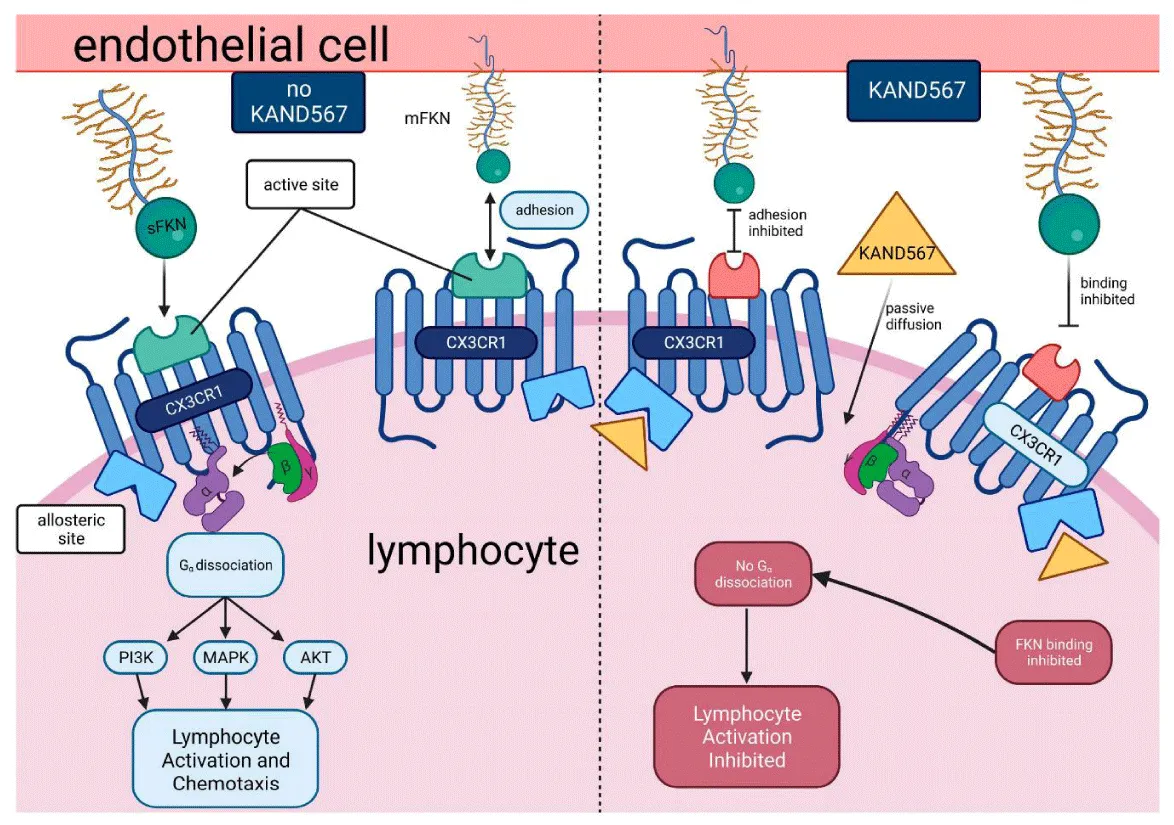
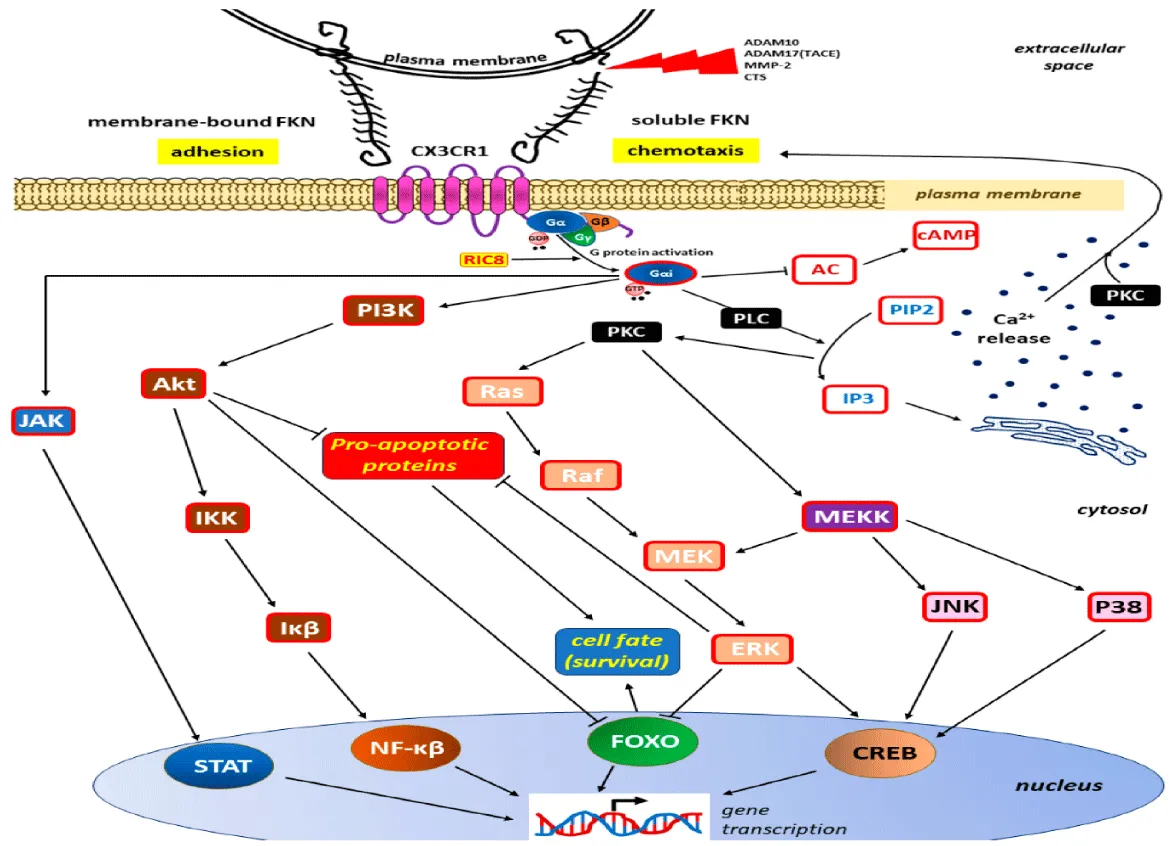
Therapeutic targeting of the CX3CL1/CX3CR1 axis holds significant potential, with strategies like blocking their interaction using monoclonal antibodies or small molecules showing promising results in preclinical studies, as noted by Shimizu, et al. [8] in 2018. However, this approach faces challenges due to the intricate role of CX3CL1/CX3CR1 in simultaneously promoting immune responses and suppressing inflammation. Achieving therapeutic efficacy requires carefully balancing anti-inflammatory effects with immune-boosting actions, as highlighted by Tan, et al. [7] in 2020. Looking ahead, the exploration of CX3CL1/CX3CR1 in personalized medicine and combination therapies may offer novel solutions for addressing cancer, infections, and chronic inflammatory diseases, suggesting an exciting avenue for future research and application, as supported by Shimizu, et al. [8] in 2018 (Figure 4).
Discussion
The CX3CL1/CX3CR1 signaling axis plays a critical and multifaceted role in regulating immune responses in infection, inflammation, and cancer. This chemokine-receptor interaction is essential for immune cell recruitment, tissue homeostasis, and resolution of infection. However, while the axis plays a central role in protective immunity, it may also drive pathological mechanisms, particularly in the context of chronic inflammation and cancer. In chronic inflammatory diseases, prolonged activation of the CX3CL1/CX3CR1 axis can contribute to tissue remodeling, perpetuating a cycle of immune cell activation that exacerbates diseases like atherosclerosis, rheumatoid arthritis, and Inflammatory Bowel Disease (IBD). Notably, the pathway contributes to neuroinflammatory processes in disorders like Alzheimer’s disease, highlights its significant role in the central nervous system, where it influences microglial activation and neuronal damage [6].
In the context of cancer, the CX3CL1/CX3CR1 pathway exhibits dual effects that complicate therapeutic targeting. While it facilitates, CX3CL1 facilitates the recruitment of immune cells into the tumor microenvironment, which could potentially enhance anti-tumor immunity. It may also promote immune evasion by attracting immunosuppressive cells such as Tregs and MDSCs, which inhibit effective anti-tumor responses [7,9]. This dual function underscores the therapeutic complexity associated with targeting this signaling pathway in cancer. Although the CX3CL1/CX3CR1 pathway remains a promising target for immunotherapy, its therapeutic exploitation is complicated by the context-dependent nature of its effects. The impact of CX3CL1/CX3CR1 signaling varies depending on the disease type, stage, and the immune landscape, requiring careful consideration of how to manipulate the pathway to achieve therapeutic benefit without inadvertently promoting immune suppression or inflammation.
The therapeutic potential of targeting the CX3CL1/CX3CR1 axis is increasingly recognized. Monoclonal antibodies and small-molecule inhibitors targeting CX3CR1 are currently under evaluation in preclinical and early clinical trials. These therapies aim to inhibit the recruitment of immune suppressor cells and enhance the activity of tumor-infiltrating lymphocytes, with a focus on cancers such as melanoma and breast cancer [8]. Nevertheless, several therapeutic challenges persist. Modulating this pathway therapeutically must be approached with caution, as it could disrupt essential immune functions in other diseases, such as autoimmune disorders or chronic inflammation. Striking a balance between the pathway’s anti-inflammatory and immunostimulatory roles of CX3CL1/CX3CR1 will be crucial to achieving optimal therapeutic outcomes and minimizing adverse effects.
Looking to the future, research should aim to deepen our understanding of the molecular mechanisms governing CX3CL1/CX3CR1 signaling in various disease contexts. By elucidating the intricate ways in which this pathway influences immune responses within diverse tissue environments and pathological contexts, we can develop more refined, personalized therapeutic approaches. For example, understanding how CX3CL1/CX3CR1 regulates mechanisms of tissue remodeling and fibrogenesis could have significant implications for diseases characterized by excessive scar tissue formation, such as heart disease, liver fibrosis, and pulmonary fibrosis. Additionally, investigation into the crosstalk between this pathway and other immune regulatory mechanisms, such as the PD-1/PD-L1 immune checkpoint, may enable the development of combination therapies that enhance immune responses while overcoming immune evasion mechanisms [7].
Ultimately, the goal is to harness the therapeutic potential of the CX3CL1/CX3CR1 axis in a manner that maximizes its benefits while minimizing any risk of adverse effects. With careful consideration of the context-dependent roles of this pathway and its interactions with other immune mechanisms, we can look forward to more effective treatments for a wide range of diseases, including cancer, chronic inflammation, and infectious diseases. By advancing our understanding of this axis and its therapeutic implications, the path to developing more effective and safer interventions becomes increasingly clear, offering promising therapeutic avenues for patients affected by these complex conditions.
Conclusion
The CX3CL1/CX3CR1 signaling axis is integral to immune responses in infection, inflammation, and cancer, mediating crucial processes, including immune cell recruitment, adhesion, and activation. While this pathway is crucial for protective immunity, it can also contribute to pathological conditions. In chronic inflammatory diseases like atherosclerosis, rheumatoid arthritis, and IBD, prolonged activation of CX3CL1/CX3CR1 exacerbates inflammation and tissue damage. Similarly, in cancer, the pathway both promotes immune cell infiltration, potentially enhancing anti-tumor immune responses, and recruits immunosuppressive cells, such as Tregs and MDSCs, which facilitate tumor immune evasion.
This dual role complicates targeting the CX3CL1/CX3CR1 axis for therapeutic purposes. While preclinical and preliminary clinical evidence suggests that targeting this pathway with monoclonal antibodies or small molecules could offer therapeutic benefits in cancer and chronic inflammation, notable limitations persist. The complex, context-dependent effects of CX3CL1/CX3CR1 signaling require careful modulation to avoid disrupting essential immune functions. Future investigations should aim to elucidate the molecular mechanisms behind its role in various diseases and investigate potential combination therapies involving immunomodulatory agents, such as immune checkpoint inhibitors, to enhance therapeutic outcomes. Ultimately, refining strategies to selectively target CX3CL1/CX3CR1 could offer more effective treatments for cancer, chronic inflammation, and infections.
- Combadière C, Potteaux S, Gao JL. Monocyte chemoattractant protein-1 in atherosclerosis and inflammation. Ann N Y Acad Sci. 1998;839:192-204.
- Imai T, Hieshima K, Nomiyama H. The unique chemokine receptor CX3CR1 and its ligand CX3CL1 in the immune system. Immunol Lett. 1997;56(3):277-82.
- Papadopoulos IM, Moutsopoulos HM, Drosos AA. CX3CL1/CX3CR1 interaction in inflammatory conditions. Autoimmun Rev. 2011;10(9):547-52.
- Sallusto F, Baggiolini M, Lanzavecchia A. CX3CL1/CX3CR1 signaling in immune cell recruitment during infection. Trends Immunol. 2000;21(9):535-42.
- McGill M, Moore S, Weng S. Chronic inflammation and cardiovascular disease: A role for CX3CL1/CX3CR1 in the regulation of leukocyte trafficking. J Immunol Res. 2015;2015:234-42.
- Henkel JS, Beers DR, Appel SH. Microglia in neuroinflammation and Alzheimer's disease. J Neuroimmunol. 2019;337:92-9.
- Tan L, Liu Y, Zhang J. CX3CL1/CX3CR1 axis in cancer and tumor progression. Front Immunol. 2020;11:320-8.
- Shimizu K, Saito H, Kanai Y. The role of CX3CR1 in cancer metastasis and immune evasion. Cancer Immunol Res. 2018;6(7):673-82.
- Tominari T, Tsukamoto H, Yamaoka K. The dual role of CX3CR1 in cancer progression and immune modulation. J Cancer Ther. 2014;5:201-15.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
