Open Journal of Tropical Medicine
Innovative and Alternative Drug-based Prevention Approaches for Malaria
Jeyatheepan Jeyaretnam*
SP.MSC, International Medical and Scientific Coordinator, Leg Clinics Net (Klinik Piano AG), Department of General Medicine, Instrumental Lymph Drainage Approaches, Switzerland
Cite this as
Jeyaretnam J. Innovative and Alternative Drug-based Prevention Approaches for Malaria. Open J Trop Med. 2025;9(1):010-014. DOI: 10.17352/ojtm.000030Copyright
© 2025 Jeyaretnam J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
Malaria vector biology
Malaria is transmitted to humans through the bite of the Anopheles mosquito, which is the main vector of Plasmodium parasites (e.g. P. falciparum, P. vivax). The parasite life cycle comprises several stages both in the human host (liver and red blood cells) and in the mosquito vector. A better understanding of vector biology and transmission dynamics is essential for the development of effective prevention strategies.
The global burden of malaria
According to the World Health Organization (WHO), there will be an estimated 241 million cases of malaria worldwide in 2020, with around 627,000 deaths, mainly in sub-Saharan Africa [1]. These global burdens, combined with drug resistance and lack of access to effective treatments, pose significant challenges to malaria control efforts.
Antimalarial treatment and prevention challenges
The increasing resistance of Plasmodium parasites to antimalarial drugs, particularly Artemisinin-based Combination Therapies (ACTs), has made malaria control increasingly difficult. Furthermore, the resistance of mosquito vectors to insecticides further complicates prevention efforts. Innovative drug approaches that can prevent infection or reduce transmission are urgently needed.
An overview of current malaria prevention strategies
Current strategies rely primarily on Insecticide-treated bed nets (ITNs), Indoor Residual Spraying (IRS) and chemoprophylaxis, the latter of which includes antimalarial drugs. Although chemoprophylaxis is not always feasible in areas of high disease transmission due to cost, access and the potential for resistance. Novel approaches, including innovative drug treatments, vaccines and combination therapies, are being explored.
Experimental and approved drug-based prevention approaches
a. Primaquine and tafenoquine
- Mechanisms of action: Both primaquine and tafenoquine are 8-aminoquinoline derivatives used for the radical cure of Plasmodium vivax and Plasmodium ovale malaria by targeting the liver hypnozoite stage. Tafenoquine, a more recent and longer-acting drug, has shown promise in preventing relapse due to its extended half-life.
- Regulatory status
- Tafenoquine: Approved by the FDA in 2018 under the brand name Krintafel for the treatment of P. vivax relapse. It is also under investigation for malaria prevention in high-risk populations.
- Primaquine: Long approved and widely used, despite its limited use due to potential side effects and dosing challenges.
- Side Effects
- Tafenoquine: Potential side effects include hemolytic anemia in individuals with G6PD deficiency. Regular monitoring for hemoglobin levels is recommended.
- Primaquine: Similar to tafenoquine, it poses risks for G6PD-deficient individuals, with hemolysis being a major concern.
- Clinical trial data
- Tafenoquine has shown high efficacy in preventing P. vivax relapse in clinical trials, including a study in Papua New Guinea [2]. In the treatment of relapse, tafenoquine had an efficacy rate of 89% over 6 months.
- Limitations: Both drugs are contraindicated for people with G6PD deficiency, and their use requires careful screening.
b. Atovaquone-proguanil (Malarone)
- Mechanism of action: Atovaquone, a mitochondrial electron transport chain inhibitor, and proguanil, which inhibits dihydrofolate reductase, together act to prevent liver and erythrocytic stages of Plasmodium infection.
- Regulatory status:
- Malarone: Approved by the FDA for both malaria prevention and treatment. It is commonly used in travelers visiting endemic areas.
- Dosing and side effects
- Dosing: For prevention, the recommended dose is 1 tablet per day, starting 1-2 days before travel and continuing for 7 days after return.
- Side effects: Mild gastrointestinal symptoms and headaches are common, though it is generally well tolerated.
- Clinical trial data: Studies show atovaquone-proguanil has a high efficacy rate (over 90%) in preventing malaria, with relatively few adverse effects in short-term use [3].
- Limitations: Resistance to atovaquone has been noted in some regions, which complicates its use as a long-term prevention tool.
c. Dihydroartemisinin-Piperaquine (DHA-PPQ)
- Mechanism of action: DHA-PPQ is a fixed-dose artemisinin-based combination therapy that combines the rapid-acting dihydroartemisinin with the long-acting piperaquine to provide both fast parasite clearance and prolonged efficacy.
- Regulatory status
- DHA-PPQ: Approved in several countries for the treatment of malaria and under investigation for prevention in high-risk groups.
- Clinical trial data: Studies have demonstrated that DHA-PPQ is highly effective in preventing malaria transmission, with studies in Southeast Asia showing high efficacy and low resistance development [4].
- Side effects: Generally well-tolerated, though some patients report gastrointestinal disturbances or headaches.
- Limitations: Drug resistance, especially to piperaquine, is a growing concern, and careful monitoring is required.
d. Vaccine-Based Approaches (RTS,S/AS01 Malaria Vaccine)
- Mechanism of action: The RTS,S/AS01 vaccine, also known as Mosquirix, is a recombinant protein vaccine designed to induce immune responses against the Plasmodium falciparum sporozoite antigen.
- Regulatory status:
- RTS,S: The vaccine was recommended by the WHO in 2021 for use in areas with moderate to high transmission of P. falciparum. It is the first malaria vaccine to receive a WHO recommendation.
- Efficacy and limitations: The vaccine showed an efficacy of about 30-40% in preventing malaria, particularly in young children [5]. While this is a step forward, the relatively low efficacy and need for multiple doses limit its impact.
- Side effects: Common side effects include fever, pain at the injection site, and, in rare cases, allergic reactions.
Combating the liver stage of malaria
The conventional strategies for malaria prophylaxis, such as the use of antimalarial drugs (e.g. chloroquine, artemisinin-based combination therapies), primarily target the blood stage of the Plasmodium parasite life cycle. Most recently, the focus of research has shifted to inhibiting the liver stage, where the parasite undergoes its first phase of replication. Medications that block this early stage could prevent further infection and transmission.
Primaquine and tafenoquine
The new drugs tafenoquine and primaquine are both being investigated for their ability to prevent malaria by targeting liver-stage schizonts. While Primaquine has been used for decades as a radical cure for Plasmodium vivax malaria, Tafenoquine offers a longer half-life, which could allow treatment with a single dose.
- Tafenoquine: Has shown promise in preventing a relapse of malaria caused by Plasmodium vivax by eradicating the dormant liver forms (hypnozoites) [6].
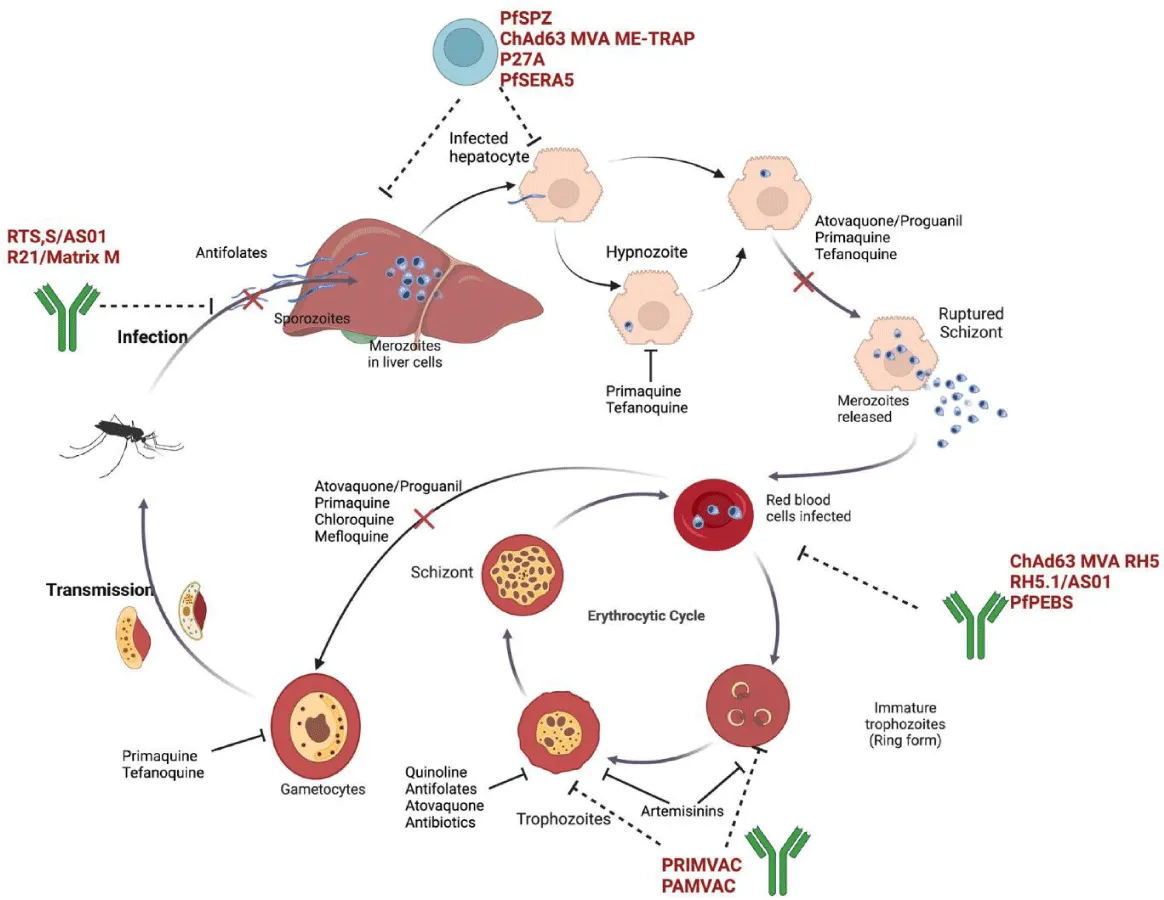
- Primaquine: Remains a cornerstone in the treatment of Plasmodium falciparum and Plasmodium vivax, although its use is limited by its toxicity, especially in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency [7] (Figure 1).
The move toward targeting the liver stage of the malaria parasite lifecycle offers a promising route for malaria prevention and treatment. Drugs like tafenoquine and primaquine provide valuable options, though challenges related to toxicity, such as with G6PD deficiency, must be carefully managed. Continued research into optimizing these drugs and their delivery, as well as understanding their effects across diverse populations, will be key to improving malaria control and moving closer to eliminating the disease.
Novel antimalarial agents
Discovering new classes of drugs is crucial to tackling the problem of drug resistance. Currently, several new antimalarials are being developed that target unique mechanisms within the parasite.
KAF156 (Ganaplacide) KAF156, a Novartis investigational drug, works by inhibiting the parasite’s mitochondrial electron transport chain. Early stage studies have shown that the drug enables rapid elimination of parasites and has a strong efficacy profile, even in drug-resistant strains [9].
Ozanimod A FDA-approved drug for the treatment of multiple sclerosis, ozanimod is being investigated for its ability to influence the immune system’s response to the malaria parasite. In pre-clinical studies, it has shown the potential to reduce the severity of malaria by modulating the immune responses that the parasite exploits [10].
Combination therapy for enhanced efficacy: Combination therapy is still one of the most effective means of preventing the occurrence of drug-resistant malaria. Combining different classes of antimalarial drugs allows the parasite to be combated at different stages of its life cycle.
- Artemisinin-based Combination Therapies (ACTs): They represent the gold standard for treating Plasmodium falciparum malaria, and research into combining newer agents with ACTs is ongoing.
- Artesunate-mefloquine: New combinations, including enzyme-based formulations and DHA-PPQ, are under investigation to improve malaria prevention outcomes, particularly in areas with high transmission rates and resistance patterns [11].
Vaccines and drug prophylaxis synergy
While vaccines have long shown promise as a means of malaria prevention, the development of an effective malaria vaccine has proven difficult. RTS,S/AS01, a vaccine developed by GlaxoSmithKline, has shown modest efficacy, although newer vaccine candidates are in development.
Combination of vaccines with drug prophylaxis may have a synergistic effect. For example, the combination of prophylactic antimalarial drugs and vaccines could provide broader protection, especially in high-risk areas with persistent malaria transmission.
Mosquirix (RTS,S): RTS,S/AS01, the world’s first malaria vaccine, has demonstrated partial efficacy in reducing malaria cases, particularly in young children [5]. Nevertheless, the protective effect of this vaccine diminishes over time and all thought continuous prophylactic treatments are required.
Novel drug delivery systems
The development of novel drug delivery systems is another area where significant progress is being made. Advancements include nanotechnology-based delivery systems and long-acting injectable formulations that could extend the duration of drug action, which may reduce dosing frequency and improve adherence to prophylactic regimens.
Nanoparticle-encapsulated Drugs
Research into nanoparticles has shown promising results in delivering antimalarial drugs with high precision to infected liver or red blood cells. These systems can increase drug bioavailability and reduce side effects [12].
Long-acting injectable antimalarials
Long-acting injectable formulations, such as Artemisinin-based nanomedicines, could offer protection against malaria for extended periods, potentially offering an alternative to daily oral prophylaxis. These formulations have been shown to effectively maintain antimalarial activity over extended periods, potentially reducing dosing frequency.
Challenges and the way forward
Even though the future of malaria prophylaxis with these new drug approaches looks promising, there are still some challenges to overcome:
- Medication resistance continues to evolve and new drugs need to be developed quickly to stay one step ahead of resistant strains.
- The cost and accessibility remain obstacles, especially in resource-limited settings.
- Compliance with treatment regimens can be a challenge in areas with high malaria transmission.
Ongoing research and development is essential to overcome these challenges. Integrating novel antimalarial drugs, combination therapies and delivery systems will be key to long-term malaria control and eventual eradication.
Resistance and future directions
- Resistance patterns: Drug resistance is a significant concern in malaria prevention. Resistance to artemisinins, for example, has emerged in Southeast Asia, leading to the reduced efficacy of ACTs [13]. Similarly, resistance to chloroquine and other antimalarial agents continues to be a global challenge.
- Future research: Novel drug combinations, vaccines, and gene-editing technologies such as CRISPR-based gene editing for vector control hold promise for overcoming resistance. Ongoing clinical trials are investigating new antimalarial compounds, with a focus on developing drugs that can prevent transmission and provide longer-lasting immunity (Figure 2).
Novel nanotechnology and nanomedicine can provide strategies to develop a successful toolkit for malaria control, both by improving conventional measures and by developing new products. In this way, the global goal of malaria control, elimination and further eradication could be achieved. To achieve this goal, however, significantly more investment is needed from public-sector institutions and industry collaborators in this area. While the vast majority of nanomedicine interventions have focused on non-communicable diseases, it is time to fully transfer this knowledge to research on diseases of poverty and neglected diseases, which will foster the development of innovative approaches to diagnose, prevent, and treat malaria and other neglected tropical diseases. Ultimately, the transfer of this knowledge to low- and middle-income countries is crucial so that they can actively participate in the invention and use of these new tools.
The following table summarizes the most important information on the individual drug and vaccine-based prevention approaches, summarizing their efficacy, safety and practical limitations.
Conclusion
In recent years, very promising progress has been made in the fight against malaria with drug-based prevention strategies that go beyond traditional methods. Such innovations such as single-dose regimens, novel combinations, and next-generation vaccine candidates show great potential for reducing malaria transmission. However, alternatives such as targeted drug delivery systems, the use of natural substances and genetic engineering techniques to improve mosquito resistance also offer new opportunities for prevention. Furthermore, the development of drug resistance remains a challenge that requires constant monitoring and the development of new pharmacological solutions. A multifaceted effort that combines these new drug strategies with vector control measures and public health initiatives is critical to achieving the ultimate goal of malaria eradication. Through continued research, collaboration and technological advances, malaria prevention can reach new heights and provide hope for a malaria-free future. The landscape of malaria prevention continues to evolve, and emerging drugs and vaccines offer promising avenues for more effective control and eradication. However, challenges remain, such as drug resistance, vaccine efficacy and access to treatment. Sustained research efforts and international collaboration are essential to overcoming these challenges.
- World Health Organization. World malaria report 2021. Geneva: World Health Organization; 2021. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021
- Baird JK, Valecha N, Duparc S. Efficacy of tafenoquine in the radical cure of Plasmodium vivax malaria in Papua New Guinea. Lancet Infect Dis. 2013;13(7):608–15.
- Boulos LM, Bin Ghouth AS, Omar SA. Efficacy and safety of atovaquone-proguanil in the prevention and treatment of malaria in travelers. J Travel Med. 2011;18(5):314–20.
- Price RN, Tjitra E, Guerra CA. Dihydroartemisinin-piperaquine for malaria prevention: A review of efficacy and safety. Malar J. 2011;10:167.
- RTS,S Clinical Trial Group. Efficacy and safety of RTS,S/AS01 malaria vaccine in children aged 6–12 weeks. N Engl J Med. 2015;373(21):2037–47.
- Lalremruata A, Schramm B, Kremsner PG. Tafenoquine for relapse prevention of Plasmodium vivax malaria: A randomized controlled trial in India. Lancet Infect Dis. 2016;16(6):685–92.
- Rathod PK, McErlean T, Lee PC. Primaquine: Challenges and limitations in the treatment of malaria. Expert Opin Pharmacother. 2016;17(15):2079–88.
- Alghamdi JM. Interventions in the parasitic malaria life cycle: Drug and vaccine targets.
- Jiang H, Patel V, Qi Y. KAF156 (Ganaplacide): A new antimalarial drug with efficacy against resistant strains. Nat Commun. 2017;8:1225.
- Marsh K, Forster K, Newton P. Exploring the potential of ozanimod in reducing malaria severity by modulating immune responses. J Clin Invest. 2018;128(5):2093–100.
- White NJ, Ashley EA, Recht J. Artesunate-mefloquine combination therapy for malaria prevention. Lancet. 2014;383(9922):1749–59.
- Kumar A, Jain N, Agrawal A. Nanoparticle-Encapsulated drugs in the treatment of malaria. Nanomedicine. 2020;15(14):1453–64.
- Phyo AP, Ashley EA, Anderson TJC. Artemisinin-resistant malaria in Southeast Asia: A systematic review. Lancet Infect Dis. 2016;16(1):26–31.
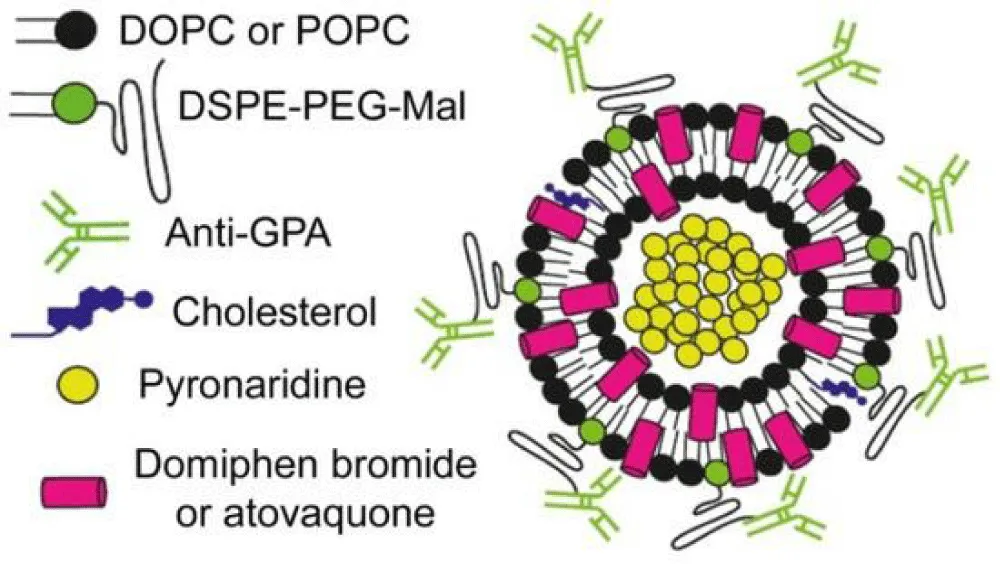
- Guasch-Girbau A, Busquets MA, Fina F. A scheme of an immunoliposome developed for a targeted combination therapy against malaria. J Nanobiotechnol. 2021;19(1):129.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
