Open Journal of Tropical Medicine
Temephos resistance levels in populations of Aedes aegypti (Diptera: Culicidae) from Havana, Cuba
Luis Augusto Piedra1, Yanisley Martínez1, Eric Camacho1, Israel Garcia1, Dayana Rodriguez2, María del Carmen Marquetti1* and Veerle Vanlerberghe3
2Department of Epidemiology, Institute of Tropical Medicine “Pedro Kourí”, La Habana, Cuba
3Emerging Infectious Diseases Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium
Cite this as
Piedra LA, Martínez Y, Camacho E, Garcia I, Rodriguez D, et al. (2023) Temephos resistance levels in populations of Aedes aegypti (Diptera: Culicidae) from Havana, Cuba. Open J Trop Med 7(1): 017-023. DOI: 10.17352/ojtm.000025Copyright
© 2023 Piedra LA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Aedes aegypti chemical control remains an indispensable alternative to prevent dengue, Zika, and Chikungunya outbreaks. Havana City requires constant surveillance due to its bioecological characteristics favor the proliferation of mosquito vectors of these diseases, which constitutes a high risk to the health of its inhabitants. The goal of this study was to determine temephos resistance levels in the populations of Ae. aegypti from five municipalities of Havana. The susceptibility of the larvae was evaluated by bioassays described by the World Health Organization. Aedes aegypti populations evaluated showed high resistance to temephos, with values that oscillated for the FR50 between 26,8 and 82,5 and for the FR90 between 16,6 and 42,5 respectively. The National Control Program of Aedes aegypti in Cuba must promote insecticide rotation policies to avoid or prevent the evolution of temephos resistance in Havana. In addition, an evaluation of the Abate doses applied by the operators in the municipalities studied must be carried out, since this could be influencing resistance development due to operational factors.
Introduction
The approach for the control of Aedes aegypti (L) since the last century is mainly based on environmental sanitation, through community participation and the application of chemical insecticides due to the presence of larval stages in a variety of natural and artificial containers [1,2].
Temephos (organophosphate) is the most widely used pesticide to control Ae. aegypti larvae stages [3-5]. Its intensive use has generated insecticide resistance development in different Ae. aegypti populations associated with metabolic action enzymes in some countries such as such as Brazil [6], the Martinique Islands [7] and India [8].
The first report of temephos resistance in Cuba was in 1997, with the occurrence of a dengue outbreak in Santiago de Cuba [9]. Temephos resistance in Ae. aegypti from Cuba was characterized for the first time through the selection of a temephos-resistant reference strain and it was shown that the mechanism of metabolic action, based on the activity of the enzymes α and β esterases, glutathione s-transferase and monooxygenases were responsible for resistance to this insecticide; although the highest level of correlation of resistance to temephos resulted with esterases, specifically A4 [10]. This esterase has also been identified, as associated with temephos resistance in Ae. aegypti strain from other Latin American countries [11-13].
In a study carried out in Havana, it was shown that only esterase enzymes play an important role in temephos resistance [14] and later it was possible to show that the reversal of temephos resistance was possible due to the mechanism involved in the appearance [15]. Temephos resistance is a phenomenon that has been evolving rapidly, which constitutes a threat to vector control, therefore it is considered to be an issue that needs greater attention in Cuba due to the continued use of this larvicide as part of the interventions carried out by the national control program of Ae. aegypti and Aedes albopictus were established in Cuba in 1981.
The objective of this work was to determine the temephos resistance status in Ae. aegypti populations belonging to five municipalities of Havana, Cuba after more than 40 years of insecticide use.
Methods
Study area
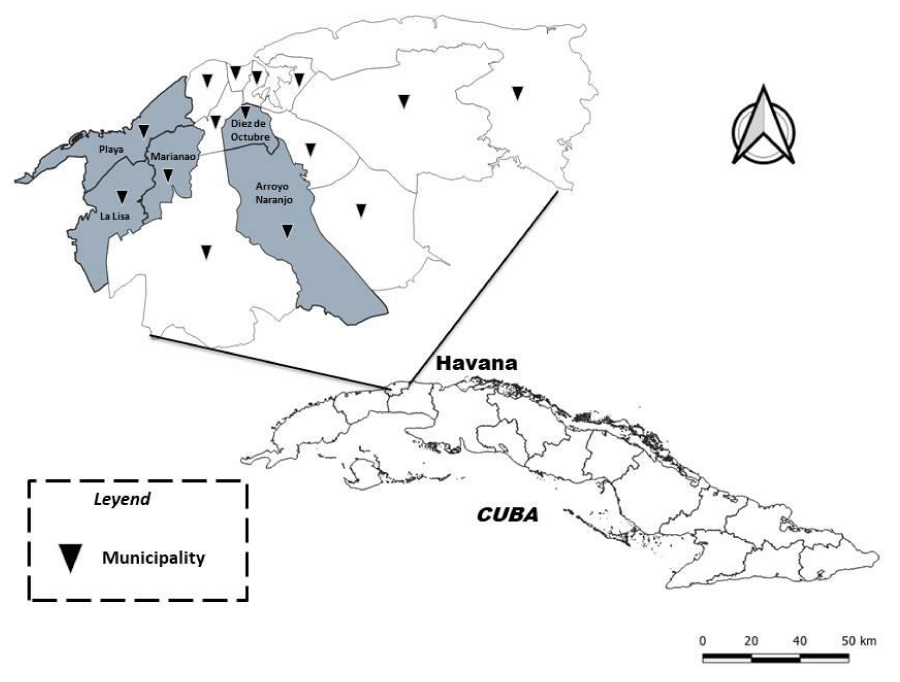
The selected study area to evaluate temephos resistance status in Ae. aegypti population was five municipalities (Playa, Diez de Octubre, Arroyo Naranjo, Marianao, and La Lisa) in Havana province (Figure 1). Playa is located north and west of the capital 23° 05′ 39 ″ N 82° 26′ 56″ W and covers a total area of 35 km2. Diez de Octubre is located west of the capital at 23° 05′ 17″ N 82° 21′ 35″ W and covers a total area of 12.27 km2. Arroyo Naranjo is located at 23° 02′ 37″ N 82° 19′ 58″ W and covers a total area of 83 km2. Marianao is located at 23° 05′ 00″ N 82° 26′ 00″ W and covers a total area of 22 km2. La Lisa is located on the western outskirts of the capital at 23° 01′ 29″ N 82° 27′ 47″ W and covers a total area of 37.5 km2.
Biological material collections were carried out using ovitraps. The collection was carried out in the field from January to June 2022 by personnel from the National Control Program during their routine activities following the sampling protocols established in Cuba for this program [16].
Ae. aegypti strains
Bora-Bora: Aedes aegypti susceptible strain collected in (French Polynesia), free of any detectable resistance mechanism, provided by the Institute Pasteur de Guadeloupe. This strain was used as a control strain in the bioassays performed.
Ae. aegypti colonies maintenance
Aedes aegypti colonies were maintained under the conditions prevalent in the insectarium of the Department of Vector Control in the Institute of Tropical Medicine “Pedro Kourí” (IPK); temperature of 25 ± 2 °C, relative humidity of 75 ± 2% and a photoperiod of 12:12 (Light and Dark) hours were maintained [17]. Larvae for the evaluation were obtained from the F1 generation from eggs collected in each municipality studied. For hatching, the eggs (contained in strips of paper) were placed in 29 x 20 x 4 cm plastic trays with one liter of dechlorinated water to which a minimum amount of larval diet was added, as a stimulus for microbial activity that guaranteed favorable conditions for the hatching of the larvae. Each tray was identified with the name of the strain (place and date of collection). Once the hatching of the first instar larvae was evidenced, and 48 hours after the activation of the eggs, the paper was carefully removed, avoiding the elimination of recently emerged larvae due to their small size attached to the paper. Aedes aegypti colonies maintenance was performed following the established methodology [18]. The morphological characterization of the larvae was carried out in the Vector Control Department of the Pedro Kourí Institute of Tropical Medicine (IPK) using taxonomic keys [18].
Insecticide used
- Temefos 94% purity, supplied by Chemotécnica SA, Buenos Aires, Argentina
Larvae bioassays
The level of susceptibility to the insecticide temephos was evaluated in larval bioassays [19]. Five concentrations of the insecticide were used with four repetitions each and a control by concentration. Twenty-five late third instar and/or early fourth instar larvae of uniform size were placed in plastic cups containing 99 ml of tap water and 1 ml of prepared insecticide solution. Controls contained 99 ml of tap water and 1 ml of acetone. In total (not counting controls), 100 larvae were evaluated for each concentration, and 500 larvae were analyzed for each bioassay. Mortality was determined 24 h after insecticide application.
Data analysis
The results of the larval bioassays were analyzed using the Probit test implemented in the statistical program SPSS Version 21. The Resistance Ratio (RR50) was calculated by comparing the value of lethal concentrations (LC50 and LC90: lethal concentrations that cause 50 and 90% mortality) of field colonies with the Bora Bora strain. Populations were classified as resistant or susceptible using the following criteria: RR50 ≤ 5: Susceptible, 5 < RR50 ≤ 10: Moderate resistance, and RR50 > 10: High resistance [20].
Results
Late third instar and/or early fourth instar larvae from F1 generation of 5 Ae. aegypti populations from Havana province were tested.
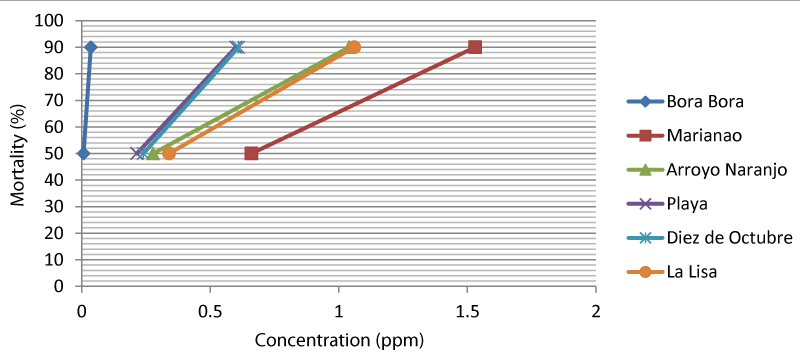
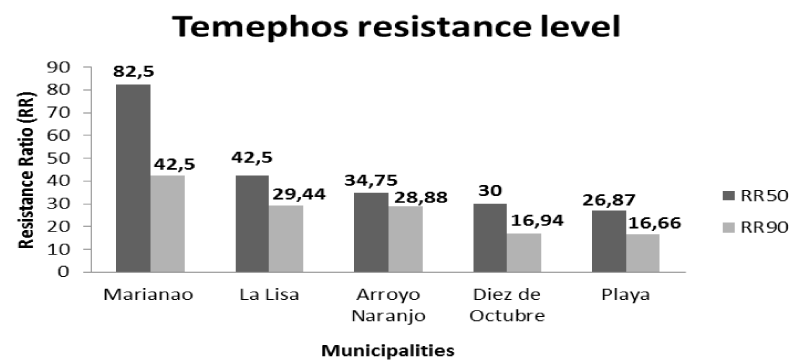
Values of the lethal concentrations (LC50 and LC90), the resistance ratios (RR50 and RR90), and the slope of the probit regression lines for the organophosphate temephos in these Ae. aegypti populations are shown below (Table 1 and Figure 2). The results showed that all populations showed high resistance (RR50 > 10) to temephos, with three municipalities (Arroyo Naranjo, Diez de Octubre, and Playa) showing values 26 < RR50 < 43 (Table 1 and Figure 3). The Marianao population was the notable exception to be considered the highest resistant (RR50 > 80) of all colonies (Table 1 and Figure 3).
Although high resistance was found in the five municipalities of Havana province, it behaved at heterogeneous levels (Figures 2,3). Mosquito populations of La Lisa (RR50 = 42.5, RR90 = 29.44) and Arroyo Naranjo (RR50 = 34.75, RR90 = 28.88) municipalities showed similar resistance ratios (RR), although they were higher than those obtained by Diez de Octubre (RR50 = 30, RR90 = 16.94) and Playa (RR50 = 26.87, RR90 = 16.66) the populations (Table 1 and Figure 3). The resistance ratios in these two Ae. aegypti populations (Diez de Octubre and Playa) were also similar to each other. The Temephos resistance level of the Marianao population (RR50 = 82.5, RR90 = 42.5) was heterogeneous in relation to the rest of the field colonies (Table 1 and Figure 3).
The slope values of the probit regression lines of the field colonies were higher than the Bora Bora strain. Marianao and Diez de Octubre populations showed the highest slope values (slope = 3.5 and 3.1) (Table 1 and Figure 2). This result confirms a homogeneous response to temephos resistance in Ae. aegypti population from both municipalities.
Discussion
The indiscriminate use of chemical insecticides causes resistance in mosquitoes of medical importance and is one of the factors that reduce the chances that vector control programs will be successful [21]. The emergence of resistance to insecticides is a complicated phenomenon involving physiological, genetic, ecological, and behavioral factors combined with insecticide application [22].
Temephos resistance has been reported in some regions of India [5,23], Colombia [24], Martinique [25], and Brazil [26-29]. However, susceptible mosquito populations have been detected in Malaysia [30], Cape Verde [31] and Thailand [32]. Recently, different levels of resistance to temephos were reported in Ae. aegypti populations from Peru [33].
The first report of temephos resistance in an Ae. aegypti population from Cuba occurred in 1997 and coincided with an outbreak of dengue in the municipality of Santiago de Cuba, located in the eastern part of the country [9]. Temephos resistance was also reported in Ae. aegypti populations from Guanabacoa and Playa municipalities during the dengue epidemic in Havana, in 2001-2002 [34,35]. Subsequently, resistance was confirmed in Boyeros municipality in 2006 [36]. A study carried out in 2008 reported high resistance to temephos in Ae. aegypti populations in 15 municipalities of Havana induced by its intensive use [14]. Besides Ae. aegypti has developed resistance worldwide induced by selective pressure due to mismanagement of temephos, causing variations in the susceptibility of mosquito populations, as reported in Colombia [37], Guadalupe Islands, Saint Martin [38], and Brazil [39].
The results of this study showed high resistance (RR50 > 10) to temephos in all populations evaluated with an increase of the resistance ratio in Marianao (RR50 = 82.5 and RR90 = 42.5) and Playa (RR50 = 26.87 and RR90 = 16.66), and a slight reduction in Arroyo Naranjo (RR50 = 34.75 and RR90 = 28.88), Diez de Octubre (RR50 = 30 and RR90 = 16.94) and La Lisa (RR50 = 42 .5 and RR90 = 29.44) compared to the evaluation carried out in 2008 [14]. High levels of temephos resistance have been reported in Acre (Brazil) [40], Tamil Nadu (India) [41], Caldas (Colombia) [42], Pernambuco (Brazil) [43], Martinique [44] and Bahia (Brazil) [45]. Some studies have reported moderate levels of temephos resistance Tocantins (Brazil) [46], Laos (Asia) [47], Paraná (Brazil) [48], Quindío (Colombia) [49], Delhi (India) [50], Sao Paulo and Northeast Region (Brazil) [51]. However, temephos susceptibility was reported in Malaysia [52], Santiago Island (Cape Verde) [53], and Phitsanulok province in Thailand [54]. The slope values obtained by the regression lines (Table 1) reflected that temephos resistance was more homogeneous in the populations of Marianao and Diez de Octubre. The National Vector Control Program applies larvicide monthly in all locations of the country throughout the year, the frequency of which can vary from one month to every 11 days in the case of dengue outbreaks [14]. This explains that the different resistance values are not due to variation in the intensity and frequency of the temephos use, since the control actions carried out by the Campaign were homogeneous for all the municipalities studied. Contrariety these aspects were decisive in the results obtained in Peru [33].
However other factors that could influence the different resistance values of the municipalities are the geographical location of the studied areas and the operational factors related to the incorrect use of the insecticide. Marianao is a highly urbanized municipality that shares land borders with several Havana municipalities where there is a constant flow of people and goods that favor the dispersal of mosquitoes from one place to another with different levels of resistance to insecticides, contrary to La Lisa which it is one of the most peripheral municipalities of the capital. This factor may have influenced the results of this study as reported in Peru [33].
It is known that as the slope is greater, resistance is more homogeneous in the population, that is, they have the same genes and are in the same proportions in individuals [55]. The second factor should be taken into account by the executives of the program in these municipalities, evaluating the quality of the control actions, since an excess or decrease in the doses of temephos applied by the operators could explain this heterogeneity in the resistance values found [22].
On the other hand, the high levels of temephos resistance in Ae. aegypti are of great concern for the control actions carried out by the National Ae. aegypti Control Program, which should be carried out more frequently, since some authors have reported a significant decrease in the residual effect of temephos in highly resistant mosquito populations (RR > 10) being effective for a period of 13 days while with susceptible populations (RR < 10) it has been effective for 18 days [14,56].
The results of this study demonstrate the urgent need to implement new integrated strategies, such as those using alternative insecticides Bacillus thuringiensis var israelensis (Bti) or pyriproxyfene (growth inhibitor) to avoid the continued increase of temephos resistance in these municipalities. A successful strategy carried out in the capital municipality of Boyeros showed how the resistance levels decreased in the Ae. aegypti populations when the temephos application was discontinued and replaced with Bti [2]. Similar results were obtained in Brazil using this biological control and using growth inhibitors [57-59]. It has also been shown that temephos susceptibility can be recovered because its metabolic resistance mechanisms are reversed when its use is discontinued [6,15]. Preferably, these strategies could be carried out by promoting insecticide rotation policies to preserve the effectiveness of insecticides [60] .
The main limitation of this study was that the bioassays of susceptibility to temephos could not be carried out in the populations of the 15 municipalities of Havana because the capacity of our insectary is very limited to work with so many colonies. For this reason, the results could not be compared with studies previously carried out in other municipalities. The following studies will be aimed at determining temephos resistance status and its resistance mechanisms in the rest of the municipalities of Havana.
The recommendations for the National Ae aegypti Control Program would be to apply strategies replacing temephos to the larvicide Bti for a certain period to eliminate selection pressure and reduce resistance levels in Ae. aegypti population from Havana province.
Conclusion
This study shows high levels of temephos resistance in the 5 evaluated Ae. aegypti populations from Havana province. This finding confirms that the implementation of resistance management by the National Ae. aegypti ControlProgram in Cuba is crucial to reversing the evolution of temephos resistance by eliminating selection pressure for a given period that allows mosquito populations to recover susceptibility to this larvicide.
Our sincere gratitude to all workers of the vector control program during the collection of the biological material in the field. We would like to thank Dayana for her collaboration in making the map of the article. This research was supported by the financial support of FA5 Belgian Development Cooperation between the Institute of Tropical Medicine “Pedro Kouri” in Havana, Cuba, and the Institute of Tropical Medicine in Antwerp Belgium.
- Bisset JA, Marquetti MC, Rodríguez MM Contribution of entomological studies about Aedes aegypti and Aedes albopictus. Retrospective analysis and challenges for their control in Cuba, 1981-2016. Rev Cubana Med Trop. 2017; 69(3).
- Rodríguez CM, Bisset CJ, Hernández CH, Ricardo Y, French L, Pérez O, Fuentes I. Caracterización parcial de la actividad de esterasas en una cepa de Aedes aegypti resistente a temefos [Partial characterization of esterase activity in a temephos-resistant Aedes aegypti strain]. Rev Cubana Med Trop. 2012 Jul-Sep;64(3):256-67. Spanish. PMID: 23424802.
- Rawlins SC. Spatial distribution of insecticide resistance in Caribbean populations of Aedes aegypti and its significance. Rev Panam Salud Publica. 1998 Oct;4(4):243-51. doi: 10.1590/s1020-49891998001000004. PMID: 9924507.
- Flores AE, Grajales JS, Salas IF, Garcia GP, Becerra MH, Lozano S, Brogdon WG, Black WC 4th, Beaty B. Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. J Am Mosq Control Assoc. 2006 Dec;22(4):672-7. doi: 10.2987/8756-971X(2006)22[672:MOIRIF]2.0.CO;2. PMID: 17304936.
- Tikar SN, Mendki MJ, Chandel K, Parashar BD, Prakash S. Susceptibility of immature stages of Aedes (Stegomyia) aegypti; vector of dengue and chikungunya to insecticides from India. Parasitol Res. 2008 Apr;102(5):907-13. doi: 10.1007/s00436-007-0848-5. Epub 2008 Jan 4. PMID: 18172687.
- Melo-Santos MA, Varjal-Melo JJ, Araújo AP, Gomes TC, Paiva MH, Regis LN, Furtado AF, Magalhaes T, Macoris ML, Andrighetti MT, Ayres CF. Resistance to the organophosphate temephos: mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010 Feb;113(2):180-9. doi: 10.1016/j.actatropica.2009.10.015. Epub 2009 Oct 30. PMID: 19879849.
- Marcombe S, Carron A, Darriet F, Etienne M, Agnew P, Tolosa M, Yp-Tcha MM, Lagneau C, Yébakima A, Corbel V. Reduced efficacy of pyrethroid space sprays for dengue control in an area of Martinique with pyrethroid resistance. Am J Trop Med Hyg. 2009 May;80(5):745-51. PMID: 19407118.
- Kumawat N, Meena S, Kumari V. Insecticide resistance status of Aedes mosquito vector in India: A review. International Journal of Mosquito Research. 2021; 8(4):20-6.
- Rodríguez MM, Bisset JA, Milá LH, Calvo E, Díaz C, Alain Soca L. Niveles de resistencia a insecticidas y sus mecanismos en una cepa de Aedes aegypti de Santiago de Cuba [Levels of insecticide resistance and its mechanisms in a strain of Aedes aegypti of Santiago de Cuba]. Rev Cubana Med Trop. 1999 May-Aug;51(2):83-8. Spanish. PMID: 10887565.
- Rodríguez MM, Bisset JA, Hernández H, Ricardo Y, French L, Pérez O, Fuentes I. Partial characterization of esterase activity in a temephos-resistant Aedes aegypti strain. Rev Cubana Med Trop. 2012; 64 (3):175–181.
- Rodríguez MM, Bisset JA, Fernández D. Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007 Dec;23(4):420-9. doi: 10.2987/5588.1. PMID: 18240518.
- Lazcano JA, Rodríguez MM, San Martín JL, Romero JE, Montoya R. Evaluación de la resistencia a insecticidas de una cepa de Aedes aegypti de El Salvador [Assessing the insecticide resistance of an Aedes aegypti strain in El Salvador]. Rev Panam Salud Publica. 2009 Sep;26(3):229-34. Spanish. PMID: 20058833.
- Bisset JA, Rodríguez M, Fernández D, Palomino M. Resistencia a insecticidas y mecanismos de resistencia en Aedes aegypti (Diptera: Culicidae) de 2 provincias del Perú [Insecticide resistance mechanisms of Aedes aegypti (Diptera: Culicidae) from two Peruvian provinces]. Rev Cubana Med Trop. 2007 Sep-Dec;59(3):202-8. Spanish. PMID: 23427457.
- Bisset JA, Rodríguez MM, Ricardo Y, Ranson H, Pérez O, Moya M, Vázquez A. Temephos resistance and esterase activity in the mosquito Aedes aegypti in Havana, Cuba increased dramatically between 2006 and 2008. Med Vet Entomol. 2011 Sep;25(3):233-9. doi: 10.1111/j.1365-2915.2011.00959.x. Epub 2011 Apr 18. PMID: 21501201.
- Bisset JA, Rodríguez MM, Piedra LA, Cruz M, Gutierrez G, Ruiz A. Reversal of resistance to the larvicide temephos in an Aedes aegypti (Diptera: Culicidae) laboratory strain from Cuba J Med Entomol. 2019; 1-6.
- Ministry of Public Health from Cuba. Manual of Standards and Technical Procedures. Surveillance and Anti Vector Fight. 2012; 631.
- Pérez O RJ, Bisset JA, Leyva M, Díaz M. Manual of technical indications for insectaries. Editorial Ciencias Médicas. 2004; 53.
- González R. Culicids from Cuba. Editorial Científico Técnica. 2006; 184.
- World Health Organization (1981) Instructions for determining the susceptibility or resistance of mosquito larvaes to insecticides (VBC) 81.0. 6.
- Mazzarri MB, Georghiou GP. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J Am Mosq Control Assoc. 1995 Sep;11(3):315-22. PMID: 8551300.
- Cui F, Raymond M, Qiao CL. Insecticide resistance in vector mosquitoes in China. Pest Manag Sci. 2006 Nov;62(11):1013-22. doi: 10.1002/ps.1288. PMID: 16953491.
- Karunaratne SHP, De Silva P, Weeraratne T, Surendran SN. Insecticide resistance in mosquitoes: Development, mechanisms, and monitoring. Ceylon Journal of Science. 2018; 47 (4):299-309.
- Muthusamy R, Shivakumar MS. Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J Vector Borne Dis. 2015 Jun;52(2):159-65. PMID: 26119549.
- Conde M, Orjuela LI, Castellanos CA, Herrera-Varela M, Licastro S, Quiñones ML. Evaluación de la sensibilidad a insecticidas en poblaciones de Aedes aegypti (Diptera: Culicidae) del departamento de Caldas, Colombia, en 2007 y 2011 [Insecticide susceptibility evaluation in Aedes aegypti populations of Caldas, Colombia, in 2007 and 2011]. Biomedica. 2015 Jan-Mar;35(1):43-52. Spanish. doi: 10.1590/S0120-41572015000100007. PMID: 26148033.
- Marcombe S, Paris M, Paupy C, Bringuier C, Yebakima A, Chandre F, David JP, Corbel V, Despres L. Insecticide-driven patterns of genetic variation in the dengue vector Aedes aegypti in Martinique Island. PLoS One. 2013 Oct 18;8(10):e77857. doi: 10.1371/journal.pone.0077857. PMID: 24204999; PMCID: PMC3799629.
- Macoris Mde L, Andrighetti MT, Otrera VC, Carvalho LR, Caldas Júnior AL, Brogdon WG. Association of insecticide use and alteration on Aedes aegypti susceptibility status. Mem Inst Oswaldo Cruz. 2007 Dec;102(8):895-900. doi: 10.1590/s0074-02762007000800001. PMID: 18209926.
- Araújo AP, Araujo Diniz DF, Helvecio E, de Barros RA, de Oliveira CM, Ayres CF, de Melo-Santos MA, Regis LN, Silva-Filha MH. The susceptibility of Aedes aegypti populations displaying temephos resistance to Bacillus thuringiensis israelensis: a basis for management. Parasit Vectors. 2013 Oct 13;6(1):297. doi: 10.1186/1756-3305-6-297. PMID: 24499507; PMCID: PMC3852962.
- Aguirre-Obando AO, Pietrobon AJ, Bona ACD, Navarro-Silva MA. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in Aedes aegypti populations from Jacarezinho (Brazil) after a Dengue Outbreak. Rev Bras Entomol. 2016; 60 (1):94–100.
- de Sá ELR, Rodovalho CDM, de Sousa NPR, de Sá ILR, Bellinato DF, Dias LDS. Evaluation of insecticide resistance in Aedes aegypti populations connected by roads and rivers: the case of Tocantins state in Brazil. Mem Inst Oswaldo Cruz . 2019; 114:1–10.
- Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors. 2015 Mar 25;8:181. doi: 10.1186/s13071-015-0797-2. PMID: 25888775; PMCID: PMC4377062.
- Rocha HDR, Paiva MHS, Silva NM, de Araújo AP, Camacho DDRDRA, Moura AJFD, Gómez LF, Ayres CFJ, Santos MAVM. Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Trop. 2015 Dec;152:66-73. doi: 10.1016/j.actatropica.2015.08.013. Epub 2015 Aug 22. PMID: 26307496.
- Thongwat D, Bunchu N. Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand. Asian Pac J Trop Med. 2015 Jan;8(1):14-8. doi: 10.1016/S1995-7645(14)60180-2. PMID: 25901918.
- Palomino M, Pinto J, Yañez P, Cornelio A, Dias L, Amorim Q, Martins AJ, Lenhart A, Lima JBP. First national-scale evaluation of temephos resistance in Aedes aegypti in Peru. Parasit Vectors. 2022 Jul 11;15(1):254. doi: 10.1186/s13071-022-05310-x. PMID: 35818063; PMCID: PMC9397858.
- Magdalena Rodríguez M, Bisset JA, Fernández D, Omayda P. Resistencia a insecticidas en larvas y adultos de Aedes aegypti: prevalencia de la esterasa A4 asociada con la resistencia a temefos [Resistance to insecticides in larvae and adults of Aedes aegypti, Havana City: prevalence of A4 esterasa associated with resistance to temephos]. Rev Cubana Med Trop. 2004 Jan-Apr;56(1):54-60. Spanish. PMID: 15849910.
- Bisset JA, Magdalena Rodríguez M, Fernández D, Pérez O. Estado de la resistencia a insecticidas y mecanismos de resistencia en larvas del municipio Playa, colectadas durante la etapa intensiva contra el Aedes aegypti en ciudad de la habana, 2001-2002 [Status of resistance to insecticides and resistance mechanisms in larvae from Playa municipality collected during the intensive campaign against Aedes aegypti in Havana City, 2001-2002]. Rev Cubana Med Trop. 2004 Jan-Apr;56(1):61-6. Spanish. PMID: 15849911.
- Rodríguez MM, Bisset JA, Pérez O, Montada D, Moya M, Ricardo Y, Valdéz V. Situation of the insecticidal resistance and its mechanisms in Aedes aegypti in Boyeros municipality. Rev Cubana Med Trop. 2009; 61(2).
- Grisales N, Poupardin R, Gomez S, Fonseca-Gonzalez I, Ranson H, Lenhart A. Temephos resistance in Aedes aegypti in Colombia compromises dengue vector control. PLoS Negl Trop Dis. 2013 Sep 19;7(9):e2438. doi: 10.1371/journal.pntd.0002438. PMID: 24069492; PMCID: PMC3777894.
- Goindin D, Delannay C, Gelasse A, Ramdini C, Gaude T, Faucon F, David JP, Gustave J, Vega-Rua A, Fouque F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect Dis Poverty. 2017 Feb 10;6(1):38. doi: 10.1186/s40249-017-0254-x. PMID: 28187780; PMCID: PMC5303256.
- Valle D, Bellinato DF, Viana-Medeiros PF, Lima JBP, Martins Junior AJ. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz. 2019;114:e180544. doi: 10.1590/0074-02760180544. Epub 2019 Apr 29. PMID: 31038548; PMCID: PMC6489372.
- Chediak M, G Pimenta F Jr, Coelho GE, Braga IA, Lima JB, Cavalcante KR, Sousa LC, Melo-Santos MA, Macoris Mde L, Araújo AP, Ayres CF, Andrighetti MT, Gomes RG, Campos KB, Guedes RN. Spatial and temporal country-wide survey of temephos resistance in Brazilian populations of Aedes aegypti. Mem Inst Oswaldo Cruz. 2016 May;111(5):311-21. doi: 10.1590/0074-02760150409. Epub 2016 Apr 29. PMID: 27143489; PMCID: PMC4878300.
- Muthusamy R, Shivakumar MS. Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J Vector Borne Dis. 2015 Jun;52(2):159-65. PMID: 26119549.
- Conde M, Orjuela LI, Castellanos CA, Herrera-Varela M, Licastro S, Quiñones ML. Evaluación de la sensibilidad a insecticidas en poblaciones de Aedes aegypti (Diptera: Culicidae) del departamento de Caldas, Colombia, en 2007 y 2011 [Insecticide susceptibility evaluation in Aedes aegypti populations of Caldas, Colombia, in 2007 and 2011]. Biomedica. 2015 Jan-Mar;35(1):43-52. Spanish. doi: 10.1590/S0120-41572015000100007. PMID: 26148033.
- Araújo AP, Araujo Diniz DF, Helvecio E, de Barros RA, de Oliveira CM, Ayres CF, de Melo-Santos MA, Regis LN, Silva-Filha MH. The susceptibility of Aedes aegypti populations displaying temephos resistance to Bacillus thuringiensis israelensis: a basis for management. Parasit Vectors. 2013 Oct 13;6(1):297. doi: 10.1186/1756-3305-6-297. PMID: 24499507; PMCID: PMC3852962.
- Marcombe S, Paris M, Paupy C, Bringuier C, Yebakima A, Chandre F, David JP, Corbel V, Despres L. Insecticide-driven patterns of genetic variation in the dengue vector Aedes aegypti in Martinique Island. PLoS One. 2013 Oct 18;8(10):e77857. doi: 10.1371/journal.pone.0077857. PMID: 24204999; PMCID: PMC3799629.
- Andrighetti MTM, Cerone F, Rigueti M, Galvani KC, Da Graça Macoris ML. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. Dengue Bull. 2008; 32:186–98.
- de Sá ELR, Rodovalho CDM, de Sousa NPR, de Sá ILR, Bellinato DF, Dias LDS. Evaluation of insecticide resistance in Aedes aegypti populations connected by roads and rivers: the case of Tocantins state in Brazil. Mem Inst Oswaldo Cruz. 2019; 114:1–10.
- Marcombe S, Fustec B, Cattel J, Chonephetsarath S, Thammavong P, Phommavanh N, David JP, Corbel V, Sutherland IW, Hertz JC, Brey PT. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl Trop Dis. 2019 Dec 12;13(12):e0007852. doi: 10.1371/journal.pntd.0007852. PMID: 31830027; PMCID: PMC6932826.
- Aguirre-Obando OA, Pietrobon AJ, Bona ACD, Navarro-Silva MA. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in Aedes aegypti populations from Jacarezinho (Brazil) after a Dengue Outbreak. Rev Bras Entomol. 2016; 60(1):94–100.
- Aguirre-Obando OA, Bona ACD, Duque LJE, Navarro-Silva MA. Insecticide resistance and genetic variability in natural populations of Aedes (Stegomyia) aegypti (Diptera: Culicidae) from Colombia. Zoologia. 2015; 32(1):14–22.
- Tikar SN, Mendki MJ, Chandel K, Parashar BD, Prakash S. Susceptibility of immature stages of Aedes (Stegomyia) aegypti; vector of dengue and chikungunya to insecticides from India. Parasitol Res. 2008 Apr;102(5):907-13. doi: 10.1007/s00436-007-0848-5. Epub 2008 Jan 4. PMID: 18172687.
- Macoris Mde L, Andrighetti MT, Otrera VC, Carvalho LR, Caldas Júnior AL, Brogdon WG. Association of insecticide use and alteration on Aedes aegypti susceptibility status. Mem Inst Oswaldo Cruz. 2007 Dec;102(8):895-900. doi: 10.1590/s0074-02762007000800001. PMID: 18209926.
- Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors. 2015 Mar 25;8:181. doi: 10.1186/s13071-015-0797-2. PMID: 25888775; PMCID: PMC4377062.
- Rocha HDR, Paiva MHS, Silva NM, de Araújo AP, Camacho DDRDRA, Moura AJFD, Gómez LF, Ayres CFJ, Santos MAVM. Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Trop. 2015 Dec;152:66-73. doi: 10.1016/j.actatropica.2015.08.013. Epub 2015 Aug 22. PMID: 26307496.
- Thongwat D, Bunchu N. Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand. Asian Pac J Trop Med. 2015 Jan;8(1):14-8. doi: 10.1016/S1995-7645(14)60180-2. PMID: 25901918.
- González G. Resistance to insecticides in the dengue vector mosquito Aedes aegypti (L) in two periods of disease transmission in Mérida, Yucatán. PhD Thesis in Sciences with an Accent in Medical Entomology. Faculty of Biological Sciences, Autonomous University of Nuevo León. 2013; 132.
- Montella IR, Martins AJ, Viana-Medeiros PF, Lima JB, Braga IA, Valle D. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am J Trop Med Hyg. 2007 Sep;77(3):467-77. PMID: 17827362.
- Andrighetti MTM, Cerone F, Riguetti M, Galvani KC, Da Graça Macoris ML. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. Dengue Bull. 2008; 32:186–98.
- Lima EP, Paiva MH, de Araújo AP, da Silva EV, da Silva UM, de Oliveira LN, Santana AE, Barbosa CN, de Paiva Neto CC, Goulart MO, Wilding CS, Ayres CF, de Melo Santos MA. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasit Vectors. 2011 Jan 12;4:5. doi: 10.1186/1756-3305-4-5. PMID: 21226942; PMCID: PMC3035027.
- Rahman RU, Cosme LV, Costa MM, Carrara L, Lima JBP, Martins AJ. Insecticide resistance and genetic structure of Aedes aegypti populations from Rio de Janeiro State, Brazil. PLoS Negl Trop Dis. 2021 Feb 16;15(2):e0008492. doi: 10.1371/journal.pntd.0008492. PMID: 33591988; PMCID: PMC7909666.
- World Health Organization (2019) Guidelines for malaria vector control. Ginebra: World Health Organization. License: CC BY-NC- SA 3.0 IGO.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
