Journal of Gynecological Research and Obstetrics
The impact of the body micro-environment on female infertility
Rosita Verteramo, Matteo Pierdomenico, Giulia Gerolin, Pantaleo Greco and Carmelia Milano*
Cite this as
Verteramo R, Pierdomenico M, Gerolin G, Greco P, Milano C (2023) The impact of the body micro-environment on female infertility. J Gynecol Res Obstet 9(1): 029-038. DOI: 10.17352/jgro.000122Copyright License
© 2023 Verteramo R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Infertility influences 15% of reproductive-aged couples in the world and the same percentage has been counted in Italy. Male and female causes of infertility are identified in 20% - 30% and 20% - 35% of couples, respectively; in 10% - 20% of cases, no cause is found. Until the last decade, a lot of studies have analyzed both modifiable factors (tobacco, alcohol, diet factors, overweight, infections) and non-modifiable factors (parental age, low ovarian reserve, etc.). Probably there is a connection between modifiable and non-modifiable causes of infertility, that together increase the failure of reproduction. This review aims to analyze the influence of the microenvironment in the female genital tract, particularly to highlight the current literature, published in Italy, on reproductive tract microbiome in different anatomical locations, vagina, endometrium, Fallopian tubes, the intestinal microbiome, and the possible interaction between such microbial communities and infertility. Finally, based on the data presented in this review, we try to encourage future perspectives and research directions in Italy.
Introduction
Infertility is becoming not only a problem of reproductive health but has also psychological, economic, and medical implications resulting in trauma and stress, especially in societies and cultures that give strong emphasis on childbearing [1]. The primary infertile female is a woman who has never been diagnosed with a clinical pregnancy and meets the criteria of being classified as having infertility. Instead, secondary female infertility applies to a woman unable to establish a clinical pregnancy but who has previously been diagnosed with a clinical pregnancy. The same categorization might be applicable to the male regarding his contribution to the initiation of a pregnancy [2]. Secondary infertility is the most common form of female infertility around the globe, frequently due to reproductive tract infections [3]. In this setting, the human microbiome plays a crucial role in determining the influence of genital tract fertility potential. The original definition of microbiome is “a characteristic microbial community occupying a reasonably well-defined habitat which has distinct physio-chemical properties”, by Whipps JM, et al. and today it is enriched by a dynamic consideration of the microbial activities that result in ecological niches. The variation of the composition of the microbiome can lead to a state of dysbiosis, particularly in the case of stress conditions, where the rapid decrease of microbial diversity promotes the expansion of specific bacteria or pathogens [4]. By some research, it was found that the genital tract accounts for up to 29% of the whole human microbiome, while the urogenital tract contributes up to 9%. Particularly it is the site where lactobacilli dominate the microbial community and contribute to defending women against infectious disease, hence playing a potential pivotal role in reproductive outcomes, such as fertility and gestational length [5]. This article reviews the existing literature in Italy, submitted within recent years, regarding the role of the microbiome in different anatomical locations, vagina, endometrium, Fallopian tubes, intestinal microbiome, and the possible interaction between such microbial communities and infertility, to evaluate the state of art in Italy and to explored and encouraged the future perspectives and research directions in Italy.
Materials and methods
The electronic search databases used were PubMed in January 2023. Papers initially have been consulted on the type and year of publication and only studies published in the last 10 - 5 years were selected. Finally, only articles were included with the Italian affiliation or the Italian author. The keywords used were: “micro-environment “, “ Pregnancy microbiome Italy”, “Vaginal microbiome Italy”, “Endometrial microbiome pregnancy Italy”, “fallopian tubes microbiome”, “infertility and microbiome”, “PID and infertility”. The studies are reported in order of date of publication in Table 1.
Female reproductive tract
The successful birth of healthy offspring, necessary for the continuation of the species, depends on the Female Reproductive Tract (FRT). The Female Reproductive tract arises from the genital ridges, the Mullerian duct system, and sinovaginal bulbs. It is composed of the ovaries, the site of maturation and release of oocytes; the fallopian tubes (FT, also called oviducts) that transport the oocytes to the uterus following ovulation; the uterus where implantation of the embryo occurs and pregnancy takes place; and the cervix which connects the uterus to the vagina and is the entry site for male gametes as well as the birth canal. The regulation of FRT function is complex and is coordinated by hormones of the Hypothalamus-Pituitary-Ovarian (HPO) axis. The differential hormonal responses between the epithelial, stromal, and immune populations provide another layer of regulation locally within the tissues. The endometrium has an essential role in implantation, particularly during the early stages of pregnancy [6]. It is a regenerative, dynamic tissue as it undergoes dramatic changes in response to the ovarian hormones throughout the menstrual cycle. Rising levels of E2 after menstruation mark the start of the proliferative phase during which the functional layer is regenerated and grows considerably. It is thought to regenerate from the basal portion of the glands, which is not shed [7-10]. The basal glands express markers found in other tissue stem cells/progenitors, including SRY-box transcription factor 9 (SOX9), stage-specific embryonic antigen 1 (SSEA1), and CDH2 (11). This might be a stem cell compartment, supported by the observation that CDH2+ cells can form gland-like structures in vitro [11]. After ovulation, during the secretory phase, the increase in progesterone levels triggers the process of decidualization, which results in the specialized differentiation of the glands and stromal cells to prepare for pregnancy, accompanied by characteristic morphological and ultrastructural changes. The glands accumulate glycogen in the subnuclear cytoplasm, and they begin to secrete copious amounts of uterine milk proteins including glycodelin and osteopontin [12]. In the absence of implantation, falling levels of progesterone that result from the involuting corpus luteum trigger menstruation. Decidualization is essential for the establishment of pregnancy and defects in this process might contribute to several disorders of pregnancy (e.g. pre-eclampsia and miscarriage) [13-15].
Composition of reproductive tract microbiome in relation to infertility
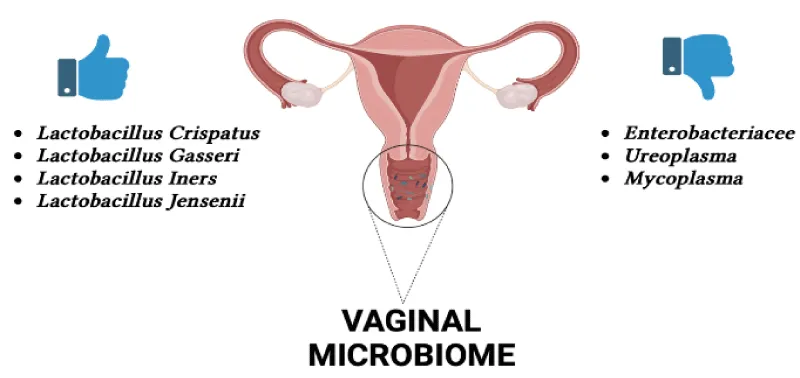
Vaginal microbiome: The vaginal microbiome is a complex and dynamic microecosystem that always undergoes oscillations during the female menstrual cycle and the woman’s whole life. The vaginal mucosa is made up of a stratified squamous nonkeratinized epithelium protected by cervicovaginal secretion [16]. The vaginal mucosa acquires oxygen, glucose, and other nutrients from underlying submucosal tissues through diffusion due to the partial blood supply [17]. This establishes a relatively anaerobic habitat situation. The vagina houses a complex microbial community that subsists in a symbiotic relationship with the host. The vaginal microbiome is made up of the indigenous environment, microorganisms, and their genomes that make up the entire habitat. In women of reproductive age, physiological modifications such as changes in hormone levels cause oscillations in the vaginal microbiome. There are distinct differences between non-pregnant and pregnant women in terms of the vaginal microbiome, with a sharp decline in variety and profusion detected in pregnant women. The prevalence of Lactobacillus spp., Clostridiales Actinomycetales, and Bacteroidales is detected in pregnant women, while in non-pregnant women the prevalence of Lactobacillus spp., Actinobacteria, Streptococcus, Veillonellaceae, Proteobacteria, Prevotella, Bifidobacteriaceae, Bacteroides, and Burkholderiales [18]. The vaginal microbiome modifications over time in a single person and differs significantly between persons due to variables such as sexual activity, douching, chronic stress, regional differences, and race. Now, there are few genotyping studies associated with a healthy vaginal microbiome, but this is an area of research that could benefit from further investigation. Lactobacillus strains thrive in the vaginal anaerobic environment and produce various antimicrobial complexes such as lactic acid, hydrogen peroxide, and bacteriocins, thus contributing to a healthy vaginal microbiome and establishing resistance against infecting pathogens [19]. In the Italian study by Campisciano G, et al [20], the knowledge of female idiopathic infertility and the vaginal microbiome of infertile women suffering from diverse clinical/physiological conditions was related to that of healthy women suffering from bacterial vaginosis. The data of the study are consistent with recent reports documenting that the infertility condition is accompanied by a compositional change in the vaginal microbiota. Resident dysbiosis is frequently determined by the reduction of lactobacilli and the proliferation of a diversity of bacteria, mostly strict anaerobes residing mainly in the gastrointestinal tract (Enterobacteriaceae) and urogenital tract (Ureaplasma) [21]. An irregular distribution of lactobacilli was detected among the cohorts of women considered. A microbiota dominated by lactobacilli is an adequate biomarker of a healthy vaginal ecosystem. Lactobacilli can act as a barrier against the invasion of pathogens as the products of their metabolism, secreted in the cervico-vaginal fluid, play a key role in counteracting both bacterial and viral infections [22]. The most frequently isolated species are Lactobacillus gasseri, Lactobacillus iners, and
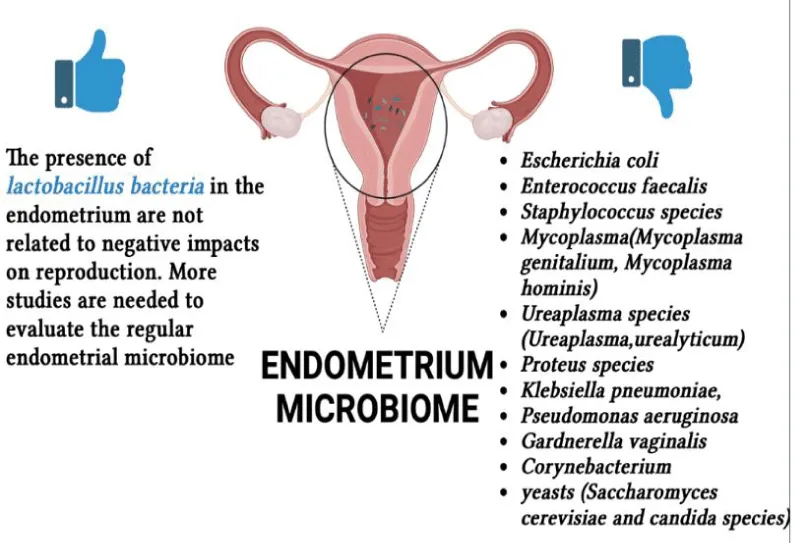
Endometrium microbiome: Knowledge of the regular upper genital tract microbiome is not as several as vaginal. The molecular documentation of bacterial species in the endometrium of asymptomatic patients undertaking hysterectomy for benign indications established that the uterine cavity is not sterile [27]. A statement exciting this dogma proposed the existence of an endometrial microbiota counting diverse microorganisms (Lactobacillus spp., Mycoplasma hominis, Gardnerella vaginalis, and Enterobacter spp.) isolated by traditional microbiological culture techniques of endometrial samples found from hysterectomy. A pathological infection is not always produced by the host-microbiota interactions, a murine model of arising bacterial infection supports the idea that the endometrium might not be as sterile as thought [28]. The presence of no lactobacillus bacteria in the endometrium is correlated with adverse effects on reproductive function and should be measured as an emerging cause of implantation failure and pregnancy loss [29]. Studies suggest that persisting intrauterine bacterial infectious conditions such as chronic endometritis possibly damage the embryo implantation process. The microbial environment in the female reproductive tract, however, remains largely undetermined in infertile patients with a past of Repeated Implantation Failure (RIF) [30,31]. RIF potentially originates in abnormal embryonic factors (such as chromosomal abnormalities, mitochondrial DNA quantity, and oxidative stress) [32], reduced endometrial receptivity (such as hydrosalpinx, endometrial polyps, distorted uterine cavity, and Chronic Endometritis (CE) [33], and systemic factors (such as thrombophilic and immunological factors) [34]. Endometritis is an infectious and inflammatory disorder of the endometrium. Endometritis is histopathologically divided into two categories [35]. Acute endometritis is categorized by micro abscess creation and neutrophil invasion in the endometrial superficial epithelium, gland lumina, and uterine cavity, the other is Chronic Endometritis (CE), the histopathologic features of which are endometrial superficial edematous alteration, high stromal cell density, dissociated development between epithelium and stroma, and infiltration of endometrial stromal plasmacytes [36]. There are currently no universally recognized uniform definitions or conventional diagnostic guidelines for CE, although experts agree that the presence of numerous Endometrial Stromal Plasmacytes (ESPCs) is the most specific and sensitive finding in this pathology [37]. The major cause of CE is microbial infection in the uterine cavity. This is sustained by the fact that some antibiotic treatments actually eliminate ESPCs in the affected patients [38]. The microorganisms detected frequently in endometrium with CE are common bacteria (streptococcus species, Escherichia coli, Enterococcus faecalis, and staphylococcus species), mycoplasma/ureaplasma species (Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma urealyticum), Proteus species, Klebsiella pneumoniae, Pseudomonas aeruginosa, Gardnerella vaginalis, Corynebacterium, and yeasts (Saccharomyces cerevisiae and candida species) [39]. The Italian pilot study performed at the University of Pisa by Cela V, et al. [40] presented that women with RIF who had a main non-lactobacillus microbiota had greater concentrations of inflammatory markers and minimal levels of anti-inflammatory/well-being factors than women with eubiosis, particularly with regard to lactobacillus abundance. The degree of inflammation (i.e. levels of inflammatory factors) was inversely correlated with lactobacilli abundance and in turn, increased in the presence of endometrial pathogens. The concentrations of the cytokines analyzed were related to the embryo implantation process, influencing the number of ETs in patients with RIF. Overall, their data contribute to the documentation of endometrial dysbiosis as a trigger of inflammation-related endometrial changes affecting the embryo implantation course. Also, this study emphasizes the importance of assessing the uterine microbiota in infertile patients, even if they do not have symptomatic/clinical endometritis, and proposes the use of probiotic supplements to recondition endometrial eubiosis. Indeed, although there is a wealth of evidence pointing to the correction of the genital microbiota in relation to elevated inflammation, understanding the risk factors and mechanisms by which it affects genital health, and in particular fertility, is essential in the management of IVF. Another interesting Italian study is that carried out by Riganelli, et al. [41], the aim of their study was to explore structural variations in the vaginal and endometrial microbiota in an attempt to define possible biomarkers related to embryo implantation failure. To this end, they characterized the vaginal (cytobrush) and endometrial (biopsy) microbiota of asymptomatic and infertile women undergoing ART, immediately before egg collection and after hormonal stimulation. In this study, 34 infertile women aged between 22 and 43 years were recruited at the Department of Infertility of the University of Rome La Sapienza between April 2017 and April 2018. They were divided into four groups according to their age and all underwent second-stage assisted reproductive technology with implantation failure. The patients had infertility related to tubal occlusion, endometriosis, ovulatory disorders, or idiopathic infertility. All women underwent a mild/minimal stimulation protocol of recombinant FSH combined with a GnRH antagonist and all reached the embryo transfer stage. Their pilot study showed differences in microbiota composition between the vaginal and uterine habitats, with a greater presence of Lactobacillus in the vagina and a more heterogeneous composition and absence of Lactobacillus in the uterine microbiota. Furthermore, the endometrial microbiota was shown to be different between pregnant and non-pregnant women. These results indicate that when translocation from the vagina to the endometrial area occurs, this can lead to an unsuitable microbiota that may adversely affect IVF (in vitro Fertilization) results. The results suggest that prior assessment of microbiota composition could allow clinicians to restore a favorable environment for IVF outcomes through the use of targeted probiotic and/or antibiotic therapies. The study carried out by Reschini M, et al. [42] reported results from the use of a careful and meticulous method of endometrial sampling. The observation that the taxonomy between the endometrial and vaginal microbiomes differed greatly supports the validity of the sampling method. This difference allowed the researchers to determine that the endometrial samples were not contaminated by vaginal species. Additionally, the study failed to confirm previous findings that a Lactobacillus-dominant endometrial microbiome is beneficial for IVF success and that bacterial vaginosis is detrimental. Instead, the researchers found that higher Shannon and Shannon’s equitability indexes were present in women who became pregnant.
The commonly used transcervical collection method can result in contamination due to the high microbial density in the cervix. The method used in the study aimed to overcome these limitations. The researchers used a double-sheathed catheter for both embryo transfer and endometrial sampling, which may reduce contamination compared to other methods. However, the technique is not perfect and further improvement is difficult to achieve. Trans-myometrial sampling could potentially be more effective, but it requires an invasive and potentially risky abdominal approach and can be exposed to cutaneous microbiome contamination. The use of a catheter for both embryo transfer and endometrial sampling is not a new technique, but the method used in the study included active aspiration, which sets it apart from previous studies. The results from the study showed a lower frequency of Lactobacillus in the endometrial microbiome compared to previous studies using similar methods. The study also found no relationship between Lactobacillus dominance and pregnancy rate with IVF. Instead, the researchers found that higher alpha diversity, as measured by Shannon and equitability indexes, was related to a higher chance of pregnancy. This result provides evidence that a higher biodiversity in the endometrial microbiome, rather than a Lactobacillus-dominant environment, may be beneficial for pregnancy. Endometrium microbiota in relation to infertility is shown in Figure 2.
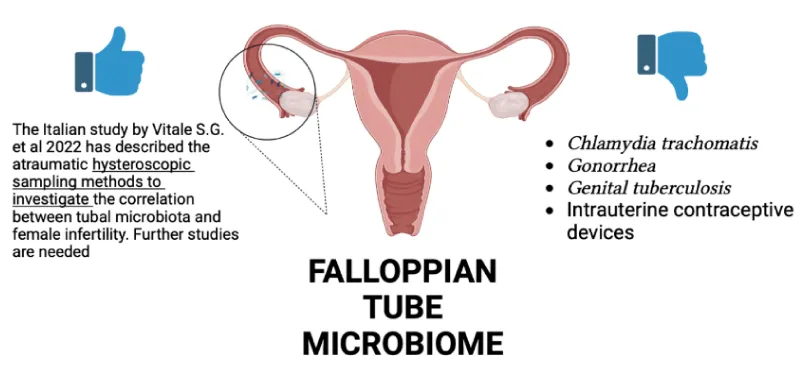
Fallopian Tube microbiome: The Fallopian Tubes are the most important site of the genital tract for woman fertility. They are a part of the female reproductive tract that hosts fertilization and pre-implantation development of the embryo [43]. Current diagnostic approaches, like hysterosalpingography and laparoscopy, cannot correctly identify many subtle causes of tubal dysfunction, yet. Among the causes of tubal infertility, are the anomalies of the tube, infections (Chlamydia trachomatis, Gonorrhea, genital tuberculosis), intrauterine contraceptive devices, endometriosis, complications after abdominal surgery, endometrial polyps, etc. [44]. However, it is known that the most common cause of tubal factor infertility is a pelvic inflammatory disease (PID), creating critical alterations of the tubal epithelium, little attention has been devoted to understanding the tubal modifications caused by the resident microbial population and their interaction with the surrounding tubal epithelium by direct sampling during laparoscopy using a cytobrush or less traumatic ones, the hysteroscopy. The Italian study by Vitale SG, et al. 2022 [44] has described the atraumatic hysteroscopic sampling methods to investigate the correlation between tubal microbiota and female infertility. This study has elucidated that PID affects tubal patency not only with macroscopic structural distortions but also by affecting the tubal epithelium directly, consisting of sloughing and/or destroying of ciliated cells, with subsequent cessation of ciliary activity, disruption of cell junctions, and apoptosis of epithelial cells. So, the consequences of several pathogenic mechanisms are direct cytotoxic effect, immune response, secretion of chemokines, and cytokines. As the study has underlined, the majority data of the studies available in the literature had been obtained by laparoscopic salpingectomy or by direct biopsies of the distal portion; the hysteroscopic approach less invasiveness than the former, but the use of cytobrush has some critical implications worth highlighting (the unavoidable mechanical trauma caused by the cytobrush and the limited flexibility for the microbiological and cytological sampling of the distal tubal lumen). Cause of the lack of consistent research about alternative microbiological methods with the use of hysteroscopy as in Italy as in the rest of the world, the study by Vitale S.G. et al. has encouraged large multicentre well-designed studies using hysteroscopic sampling methods to discover more about the relationship between tubal microbiota and female infertility [44]. Fallopian tube microbiota in relation to infertility is shown in Figure 3.
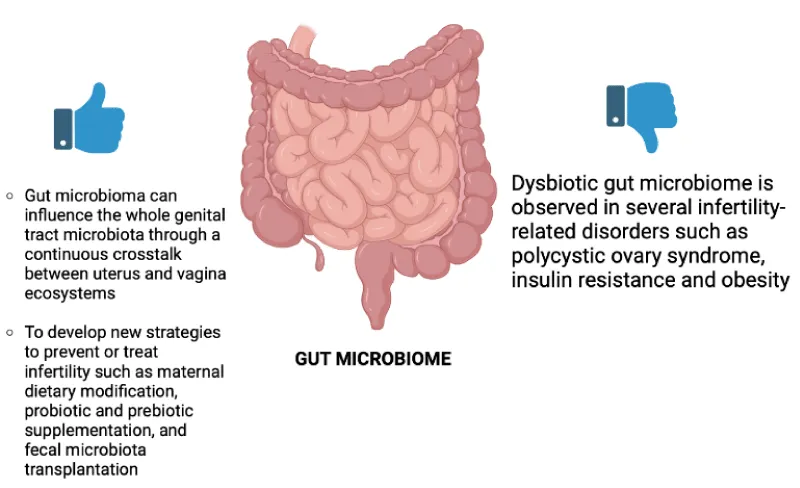
Intestine Microbiome: Always more and more studies have shown that the Gut Microbiome (GM) can influence female fertility [45,46]. The Italian studies, Giampaolino P, et al. 2021 and Fabozzi G, et al. 2022 are the most recent of our research. GM is a complex and dynamic population of microorganisms living in the human gastrointestinal tract that performs biochemical functions otherwise absent in the host. The main GM phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with the former two representing 90% of the whole population [46]. Among the functions of the GM, the studies selected (table) have underlined that there is a direct influence on female fertility to the regulation of the level of sex hormones, by the production of the enzyme β-glucuronidase (GUBS), which is involved in both the xenobiotic and endobiotic metabolism. Indeed, changes to the microbial population encoding the enzyme GUSB, known as the estrobolome, affect the endogenous estrogen metabolism by regulating the enterohepatic circulation of these hormones, with a subsequent impact on the woman’s hormonal balance and, therefore, on her fertility. Instead, indirectly, the GM seems linked to female infertility due to the important relationship that exists between a healthy GM and the immune system [46]. Indeed, dysbiotic GM is observed in several infertility-related disorders such as Polycystic Ovary Syndrome (PCOS), Insulin Resistance (IR), and obesity [45]. the correct balance of the GM plays a key role in female fertility since it has been demonstrated that GM can influence the whole genital tract microbiota through continuous crosstalk between uterus and vagina ecosystems [46]. Noteworthy, oral administration of probiotics influences vaginal microbiota composition and immunity, and different microbial species, such as the Gram-positive Lactobacillus spp. that dominates the vaginal microbiota in physiological conditions, originate from the gut. Moreover, GM dysbiosis can induce bacteria translocation, which impairs the permeability of the intestine and the leakage of bacteria and bacterial products from the gut into the circulation, thus affecting the female genital tract microbiota. Through the studies cited, we pay attention to the possibility of developing new strategies to prevent or treat infertility such as maternal dietary modification, probiotic and prebiotic supplementation, and faecal microbiota transplantation [46]. Intestine microbiota in relation to infertility is shown in Figure 4.
Discussion
State of art in Italy and future research developments
In Italy, more and more studies are evaluating the association between female mycobacterium, fertility, and assisted fertility techniques. More and more patients are making use of techniques in cases of infertility, even in Italy where the average age of first pregnancy is increasing. Knowledge about the microbiome of the regular upper genital tract is not as extensive as that of the vagina. But new studies are assessing how the endometrium has its own microbiota and how this interferes with fertility and assisted fertilisation techniques. Italian studies have evaluated that women with RIF who had a non-lactobacillus major microbiota had higher concentrations of inflammatory markers and lower levels of anti-inflammatory/well-being factors than women with eubiosis, particularly with regard to lactobacillus abundance. The degree of inflammation (i.e. levels of inflammatory factors) was inversely correlated with lactobacilli abundance and in turn, increased in the presence of endometrial pathogens. This brings more attention to the assessment of endometrial well-being not only from a structural but also from a microbiome perspective. A new point of discussion is data on the tubal microbiome; in fact, as seen Italian studies have described hysteroscopic trauma sampling methods to investigate the correlation between tubal microbiota and female infertility, creating new methods on how to investigate the tubes giving new perspectives. Progress has been made on how inflammations such as PID can influence tubal patency by not only structurally altering the tubes but also at the epithelial level. Italian studies are trying to go further in assessing the correlation between microbiota and fertility by also studying how the gut and its microbiota influence fertility and assisted fertilisation techniques related to infertility, such as polycystic ovary syndrome (PCOS), insulin resistance (IR), and obesity. Another point is how the oral intake of probiotics goes to influence the microbiota of the genital tract, which determines the possibility of developing new strategies for studies evaluating the female microbiota, such as supplementation or faecal microbiota transplantation. Infertility and medically-assisted fertilisation techniques are increasingly studied topics, but microbiota still has many unexplored aspects that need stronger evidence, such as the need for more prospective studies to evaluate the female genital tract microbiota, especially for medically-assisted fertilization.
Conclusion
In this review, we explored state-of-the-art research regarding the microbiome and infertility in Italy without taking into consideration other European studies. We researched how each genital trait has been explored in the literature regarding our topic. From this, it emerged that the best-known genital tracts are the vagina and the endometrium, while little is known about the other tracts.
Author contributions
Conceptualization, C.M., R.V. M.P.; writing—original draft preparation, C.M, G.G..; writing—review and editing, C.M., M.P., G.G., R.V., and P.G. All authors have read and agreed to the published version of the manuscript.
The authors are grateful to all members of the research group for their discussions. The figures (Figures 1-3) were created with BioRender.com.
- October. WHO Sexual and Reproductive Health. https://www.who.int/reproductivehealth/topics/infertility/multiple-definitions/en/
- Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018 Dec; 62:2-10. doi: 10.1016/j.clinbiochem.2018.03.012. Epub 2018 Mar 16. PMID: 29555319.
- Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018 Dec; 62:2-10. doi: 10.1016/j.clinbiochem.2018.03.012. Epub 2018 Mar 16. PMID: 29555319.
- Whipps JM. In: Fungi Biol Control Syst. Burge N, editore. Manchester: Manchester University Press. Mycoparasitism and control of plant diseases. 1988; 161-187.
- Vitale SG, Ferrari F, Ciebiera M, Zgliczyńska M, Rapisarda AMC, Vecchio GM, Pino A, Angelico G, Knafel A, Riemma G, De Franciscis P, Cianci S. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int J Mol Sci. 2021 Dec 24;23(1):180. doi: 10.3390/ijms23010180. PMID: 35008605; PMCID: PMC8745627.
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002 Jun;87(6):2954-9. doi: 10.1210/jcem.87.6.8563. PMID: 12050279.
- Ludwig H, Spornitz UM. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann N Y Acad Sci. 1991; 622:28-46. doi: 10.1111/j.1749-6632.1991.tb37848.x. PMID: 2064187.
- Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012 Dec;13(4):235-51. doi: 10.1007/s11154-012-9221-9. PMID: 22847235..
- Ferenczy A. Studies on the cytodynamics of human endometrial regeneration. II. Transmission electron microscopy and histochemistry. Am J Obstet Gynecol. 1976 Mar 15;124(6):582-95. doi: 10.1016/0002-9378(76)90059-4. PMID: 943943.
- Padykula HA. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci. 1991; 622:47-56. doi: 10.1111/j.1749-6632.1991.tb37849.x. PMID: 2064204.
- Nguyen HPT, Xiao L, Deane JA, Tan KS, Cousins FL, Masuda H, Sprung CN, Rosamilia A, Gargett CE. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017 Nov 1;32(11):2254-2268. doi: 10.1093/humrep/dex289. PMID: 29040564.
- Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol. 2004 Jul 20; 2:58. doi: 10.1186/1477-7827-2-58. PMID: 15265238; PMCID: PMC493283.
- Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011 Mar-Apr;17(2):242-53. doi: 10.1093/humupd/dmq037. Epub 2010 Aug 21. PMID: 20729534; PMCID: PMC3039220.
- Garrido-Gomez T, Dominguez F, Quiñonero A, Diaz-Gimeno P, Kapidzic M, Gormley M, Ona K, Padilla-Iserte P, McMaster M, Genbacev O, Perales A, Fisher SJ, Simón C. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci U S A. 2017 Oct 3; 114(40):E8468-E8477. doi: 10.1073/pnas.1706546114. Epub 2017 Sep 18. PMID: 28923940; PMCID: PMC5635883.
- Conrad KP, Rabaglino MB, Post Uiterweer ED. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta. 2017 Dec; 60:119-129. doi: 10.1016/j.placenta.2017.06.005. Epub 2017 Jun 9. PMID: 28693893; PMCID: PMC5718949.
- Pekmezovic M, Mogavero S, Naglik JR, Hube B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019 Dec;27(12):982-996. doi: 10.1016/j.tim.2019.07.006. Epub 2019 Aug 23. PMID: 31451347.
- Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol. 2011 Feb; 204(2):120.e1-5. doi: 10.1016/j.ajog.2010.07.010. Epub 2010 Sep 15. PMID: 20832044.
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466. doi: 10.1371/journal.pone.0036466. Epub 2012 Jun 13. PMID: 22719832; PMCID: PMC3374618.
- Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG. 2017 Mar;124(4):606-611. doi: 10.1111/1471-0528.14390. Epub 2016 Nov 7. PMID: 28224747.
- Campisciano G, Florian F, D'Eustacchio A, Stanković D, Ricci G, De Seta F, Comar M. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J Cell Physiol. 2017 Jul;232(7):1681-1688. doi: 10.1002/jcp.25806. Epub 2017 Feb 16. PMID: 28098358.
- Ghiasi M, Fazaeli H, Kalhor N, Sheykh-Hasan M, Tabatabaei-Qomi R. Assessing the prevalence of bacterial vaginosis among infertile women of Qom city. Iran J Microbiol. 2014 Dec;6(6):404-8. PMID: 25926958; PMCID: PMC4411426.
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011 Apr;118(5):533-49. doi: 10.1111/j.1471-0528.2010.02840.x. Epub 2011 Jan 20. PMID: 21251190; PMCID: PMC3055920.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011 Mar 15;108 Suppl 1(Suppl 1):4680-7. doi: 10.1073/pnas.1002611107. Epub 2010 Jun 3. PMID: 20534435; PMCID: PMC3063603.
- Zanotta N, Campisciano G, Morassut S, Castro-Silva E, Luksa V, Zito G, Luppi S, Martinelli M, Colli C, De Seta F, Ricci G, Suligoi B, Comar M. Emerging role for Ureaplasma parvum serovar 3: Active infection in women with silent high-risk human papillomavi.
- Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F, Tancredi A, Paschero C, Boatti L, Zanotto E, Sidoti F, Bottino P, Costa C, Cavallo R, Benedetto C. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endomet.
- Quaranta G, Sanguinetti M, Masucci L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front Immunol. 2019 Nov 26;10:2653. doi: 10.3389/fimmu.2019.02653. PMID: 31827467; PMCID: PMC6890827.
- Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, Fredricks DN, Eschenbach D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015 May;212(5):611.e1-9. doi: 10.1016/j.ajog.2014.11.043. Epub 2014 Dec 16. PMID: 25524398; PMCID: PMC4754962.
- Racicot K, Cardenas I, Wünsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013 Jul 15;191(2):934-41. doi: 10.4049/jimmunol.1300661. Epub 2013 Jun 10. PMID: 23752614; PMCID: PMC4153356.
- Liu Y, Ko EY, Wong KK, Chen X, Cheung WC, Law TS, Chung JP, Tsui SK, Li TC, Chim SS. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil Steril. 2019 Oct;112(4):707-717.e1. doi: 10.1016/j.fertnstert.2019.05.015. Epub 2019 Jul 18. PMID: 31327470.
- Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R, Jia H. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017 Oct 17;8(1):875. doi: 10.1038/s41467-017-00901-0. PMID: 29042534; PMCID: PMC5645390.
- Franasiak JM, Werner MD, Juneau CR, Tao X, Landis J, Zhan Y, Treff NR, Scott RT. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J Assist Reprod Genet. 2016 Jan;33(1):129-36. doi: 10.1007/s10815-015-0614-z. Epub 2015 Nov 7. PMID: 26547201; PMCID: PMC4717132.
- Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012 May;97(5):1039-43. doi: 10.1016/j.fertnstert.2012.03.010. Epub 2012 Mar 30. PMID: 22464086.
- Xu B, Zhang Q, Zhao J, Wang Y, Xu D, Li Y. Pregnancy outcome of in vitro fertilization after Essure and laparoscopic management of hydrosalpinx: a systematic review and meta-analysis. Fertil Steril. 2017 Jul;108(1):84-95.e5. doi: 10.1016/j.fertnstert.2017.05.005. Epub 2017 Jun 1. PMID: 28579408.
- Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online. 2013 Nov;27(5):530-8. doi: 10.1016/j.rbmo.2013.05.018. Epub 2013 Jun 20. PMID: 23933035.
- Kiviat NB, Wølner-Hanssen P, Eschenbach DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE, Moore DE, Holmes KK. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute sal.
- Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981 Aug;58(2):176-84. PMID: 7254729.
- Kitaya K, Yasuo T. Immunohistochemistrical and clinicopathological characterization of chronic endometritis. Am J Reprod Immunol. 2011 Nov;66(5):410-5. doi: 10.1111/j.1600-0897.2011.01051.x. Epub 2011 Jul 12. PMID: 21749546.
- Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, Marrocchella S, Greco P, Resta L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015 Feb;30(2):323-30. doi: 10.1093/humrep/deu292. Epub 2014 Nov 10. PMID: 25385744.
- Cicinelli E, De Ziegler D, Nicoletti R, Tinelli R, Saliani N, Resta L, Bellavia M, De Vito D. Poor reliability of vaginal and endocervical cultures for evaluating microbiology of endometrial cavity in women with chronic endometritis. Gynecol Obstet Invest. 2009;68(2):108-15. doi: 10.1159/000223819. Epub 2009 Jun 11. PMID: 19521097.
- Cela V, Daniele S, Obino MER, Ruggiero M, Zappelli E, Ceccarelli L, Papini F, Marzi I, Scarfò G, Tosi F, Franzoni F, Martini C, Artini PG. Endometrial Dysbiosis Is Related to Inflammatory Factors in Women with Repeated Implantation Failure: A Pilot Study. J Clin Med. 2022 Apr 28;11(9):2481. doi: 10.3390/jcm11092481. PMID: 35566605; PMCID: PMC9101226.
- Riganelli L, Iebba V, Piccioni M, Illuminati I, Bonfiglio G, Neroni B, Calvo L, Gagliardi A, Levrero M, Merlino L, Mariani M, Capri O, Pietrangeli D, Schippa S, Guerrieri F. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front Cell Infect Microbiol. 2020 Jul 14;10:350. doi: 10.3389/fcimb.2020.00350. PMID: 32760681; PMCID: PMC7372811.
- Reschini M, Benaglia L, Ceriotti F, Borroni R, Ferrari S, Castiglioni M, Guarneri D, Porcaro L, Vigano' P, Somigliana E, Uceda Renteria S. Endometrial microbiome: sampling, assessment, and possible impact on embryo implantation. Sci Rep. 2022 May 19;12(1):8467. doi: 10.1038/s41598-022-12095-7. PMID: 35589752; PMCID: PMC9120179.
- Li S, Winuthayanon W. Oviduct: roles in fertilization and early embryo development. J Endocrinol. 2017 Jan;232(1):R1-R26. doi: 10.1530/JOE-16-0302. PMID: 27875265.
- Vitale SG, Carugno J, D'Alterio MN, Mikuš M, Patrizio P, Angioni S. A New Methodology to Assess Fallopian Tubes Microbiota and Its Impact on Female Fertility. Diagnostics (Basel). 2022 Jun 2;12(6):1375. doi: 10.3390/diagnostics12061375. PMID: 35741185; PMCID: PMC9221911.
- Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, Improda FP, Verrazzo P, Zara G, Buonfantino C, Borgo M, Riemma G, Angelis C, Zizolfi B, Bifulco G, Della Corte L. Microbiome and PCOS: State-of-Art and Future Aspects. Int J Mol Sci. 2021 Feb 19;22(4):2048. doi: 10.3390/ijms22042048. PMID: 33669557; PMCID: PMC7922491.
- Fabozzi G, Rebuzzini P, Cimadomo D, Allori M, Franzago M, Stuppia L, Garagna S, Ubaldi FM, Zuccotti M, Rienzi L. Endocrine-Disrupting Chemicals, Gut Microbiota, and Human (In)Fertility-It Is Time to Consider the Triad. Cells. 2022 Oct 22;11(21):3335. doi: 10.3390/cells11213335. PMID: 36359730; PMCID: PMC9654651.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
