Journal of Gynecological Research and Obstetrics
The effect of adsorbent-antioxidant vaginal gel on high-risk HPV clearance
Songül Alemdaroğlu1*, Gonca Çoban Şerbetçioğlu2, Gülşen Doğan Durdağ1, Şafak Yılmaz Baran1, Şirin Aydın1 and Hüsnü Çelik1
2Department of Obstetrics and Gynecology, Baskent University School of Medicine Izmir Hospital, Izmir, Turkey
Cite this as
Alemdaroğlu S, Şerbetçioğlu GÇ, Durdağ GD, Baran ŞY, Aydın Ş, et al. (2022) The effect of adsorbent-antioxidant vaginal gel on high-risk HPV clearance. J Gynecol Res Obstet 8(3): 048-053. DOI: 10.17352/jgro.000117Copyright License
© 2022 Alemdaroğlu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The purpose of this study is to investigate the effect of an adsorbent-antioxidant vaginal gel, which contains micronized silicon dioxide and antioxidant deflamin, on high-risk human papillomavirus (hr-HPV) clearance within the follow-up periods specified in the guidelines.
Methods: In the study, the data of 52 patients infected with hr-HPV using vaginal gel for 3 months and 115 patients who were not using vaginal gel were analyzed retrospectively. Demographic characteristics, Liquid-Based Cytology (LBC) at the time of presentation, colposcopy findings, and cervical biopsy results of both groups were investigated. After the LBC and hr-HPV results were evaluated at the end of the control period, both groups were compared in terms of hr-HPV clearance. The level of statistical significance was taken as 0.05 in all tests.
Results: The two groups had similar demographic data, cytological findings, colposcopy findings, and cervical biopsy results (p > 0.05). During similar follow-up periods (13.6 ± 3.2 vs. 14.1 ± 3.4 months; p > 0.05), there was no significant difference in terms of hr-HPV clearance (46.2% vs. 51.3%; p > 0.05). Furthermore, the initial and follow-up cytology results of the patients were similar (p > 0.05).
Conclusions: A significant effect of the absorbent antioxidant vaginal gel on hr-HPV clearance at approximately 1-year follow-up could not be demonstrated.
Introduction
Cervical Cancer (CC) is the 4th most common cause of cancer-related deaths with an incidence of 6.5% in women, and its incidence and mortality are expected to increase in the next 20 years [1]. Persistent infection with high-risk human papillomavirus (hr-HPV) (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) is the most significant predictive factor for CC development following almost all cervical intraepithelial neoplasms (CIN) [2]. Although the lifetime risk of HPV exposure is 80%, studies have shown that approximately 90% of infections regress within 1-2 years [3]. The understanding of these features of HPV enabled it to be included in CC screening programs together with cytology and used as a screening test alone to determine follow-up intervals [4,5].
After understanding the role of persistent infection with hr-HPV in CIN and cancer development in cervical carcinogenesis, attention has focused on HPV prevention and CIN therapies [6]. Difficulty in accessing vaccines, a decrease in protection at delayed ages, or the inability to completely prevent HPV transitions with the use of condoms stand out as an important problem (source). Surgical treatments applied in CIN were shown to be associated with complications such as preterm childbirth and cervical stenosis [7]. The usage of medical treatments, which have been tested to date, has not continued due to serious side effects (Imiquimod, Interferon) or insufficient response (green tea, metronidazole-containing gel, 5-fluorouracil (5-FU) vaginal cream) [8-10]. For these reasons, methods that could be an alternative treatment option in CINs and could increase HPV clearance are important research topics.
As a solution to this important problem, the adsorbent-antioxidant vaginal gel was proposed as an alternative in recent years. This vaginal gel adsorbs pathogens (bacteria, viruses, fungi, cell debris, irritant particles) from the cervical surface with a high concentration of micronized silicon dioxide (SiO2) [11]. The antioxidant deflamin (a combination of biologically activated selenium-containing sodium selenite and citric acid) in its content neutralizes the absorbed and bound pathogens with its antioxidant properties [11]. It is reported that Vaginal Gel promotes remission in cases with abnormal cervical smears and CIN1-2, and there is a decrease in the prevalence of hr-HPV three months after its use [12,13].
In this study, our purpose was to investigate the effect of the vaginal gel on hr-HPV clearance by comparing the hr-HPV test results of patients with and without absorbent-antioxidant vaginal gel usage following the detection of positive hr-HPV DNA.
Materials-methods
This study was carried out by the retrospective analysis of patients who underwent HPV-DNA screening in a tertiary center between January 2018 and September 2020. Patients who were hr-HPV DNA positive during this interval and used adsorbent-antioxidant vaginal gel for 3 months were included in the vaginal gel group. Among 1404 patients who had HPV-DNA screening in the same period, patients in whom HPV genotype tests were performed twice and who were infected with at least one hr-HPV and did not use vaginal gel were included in the control group.
In both groups, patients with additional malignancies, patients who had less than 6 months between the two HPV acquisition periods, those who had an HPV test once, and those who were infected with only low-risk HPV genotypes (6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, CP6108) at the time of presentation, those who had immunosuppressive diseases or a history of using immunosuppressive drugs, those whose results were carcinoma in situ/adenocarcinoma in situ and those who had a suspicion of malignancy as a result of smear and cervical biopsy, those who were pregnant during the follow-up period, and those who underwent hysterectomy were excluded from the study.
Gravidity, parity, additional systemic diseases, HPV vaccination status, smoking habits, and the number of cigarettes used in both groups were evaluated. Additional systemic diseases were grouped as HT ± DM and others. The vaccination statuses of the patients were examined. In the HPV test (Diege HC2 DNA collection device®) used in our center, 37 different genotypes, namely 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 from the high-risk group and 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39, and CP6108 from the low-risk group are screened. HPV genotypes of both groups at the initial presentation were determined, and only patients with high-risk genotypes were included in the study. The results were collected in 2 groups as 16 ± 18 and others according to the American Society of Colposcopy and Cervical Pathology (ASCCP) guidelines [14]. The results of cervical smear tests performed with liquid-based cytology (LBC) at the initial and follow-up presentations of the patients were recorded as normal, infectious, insufficient, Atypical Squamous Cells Of Undetermined Significance (ASCUS), Low-Grade Intraepithelial Lesion (LG-SIL), High-Grade Intraepithelial Lesion (HG-SIL), and atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H) according to the Bethesda system. After the first presentation, the results of the patients who underwent colposcopy according to the ASCCP guidelines were evaluated [14]. Colposcopy findings were grouped as normal, inadequate (if transformation zone could not be evaluated due to cervical inflammation, scar, postmenopause, bleeding, etc.), presence of acetowhite epithelium, and atypical vascularity. Cervical biopsy was performed in patients where deemed necessary according to their colposcopy findings. Patients with insufficient colposcopy, patients with cervical biopsy results as CIN 2-3, and patients who underwent Loop Electrosurgical Excision Procedures (LEEP) in the presence of colposcopy findings incompatible with cytological abnormality in LBC were determined.
During the follow-up presentation, if the HPV screening result was negative, or the HPV genotype present in the initial presentation was not detected, the case was accepted as ‘cleared’. Provided that the same genotypes were detected in both presentations, the existing genotype was maintained, or an additional genotype was detected, it was accepted as ‘persisted’, and thus, HPV clearance was categorized.
Statistical analysis
The statistical analyses were performed by using the SPSS version 26.0 (SPSS, Inc. Chicago, IL) statistical software package. The data are expressed as median and range for the continuous variables. The binary variables are reported as frequencies and percentages. Chi-squared tests were used to evaluate the differences in the categorical variables between groups. Independent-samples t-test was used to compare the continuous variables that were normally distributed, and the Mann-Whitney U test was used to compare those that were non-normally distributed. The level of statistical significance was set at p < 0.05.
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. This study was approved by Başkent University Institutional Review Board (No. KA KA21/102).
Results
A total of n: 52 (31.1%) patients were included in the vaginal gel group, and n: 115 (68.9%) patients were included in the control group in the study. The mean age of the vaginal gel group was 40.9 ± 9.6 years, while the mean age of the control group was 43.8 ± 9.6 years (p > 0.05). The gravidity and parity values of the two groups were similar (p > 0.05). The patients in the two groups had similar additional systemic diseases in their histories. The smoking statuses of the patients and the numbers of cigarettes consumed per day were also similar in the two groups (p > 0.05) (Table 1). There were no patients who had HPV vaccines in either group.
In the groups, 16 ± 18 and other hr-HPV genotype distributions in the control vs. vaginal gel groups during the initial presentation were found to be 51 (44.3%) vs. 33 (63.5%) and 64 (55.7%) vs. 19 (36.5%), respectively (p < 0.05). There was no statistically significant difference between colposcopy findings after LBC in the initial presentation or cervical biopsy results. The number of LEEP procedures applied to both groups was similar (p > 0.05). The mean interval between hr-HPV genotype evaluation at the initial and control presentations was 14.1 ± 3.4 months in the control group and 13.6 ± 3.2 months in the vaginal gel group (p > 0.05). The control vs. vaginal gel group distribution of hr-HPV genotypes at the follow-up presentation was 26 (22.6%) vs. 17 (32.7%) in the 16 ± 18 group and 48 (41.7%) vs. 21 (40.4%) in the other group. It was observed that 41 (35.7%) vs. 14 (26.9%) patients had negative HPV-DNA test results at the follow-up presentation (p > 0.05). Follow-up LBC results were similar in the two groups (p > 0.05) (Table 2).
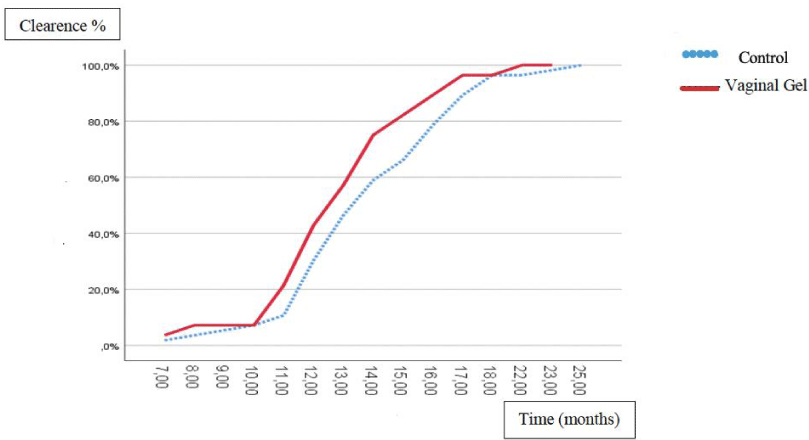
In the comparisons of the results of the HPV-DNA tests performed at the follow-up presentation and those at the initial presentation, clearance was detected in 51.2% of the patients in the control group, while it was detected in 46.2% of the patients in the vaginal gel group (p > 0.05) (Table 3). In the time plot of the clearance rates, it was observed that the vaginal gel group and the control group were similar (Graph 1).
Discussion
The role and importance of primary hr-HPV DNA screening in CC have become prominent. In this study, hr-HPV DNA positive patients were screened retrospectively, and patients who used adsorbent-antioxidant vaginal gel and those who did not use it were compared in terms of HPV-DNA clearance. A similar rate of clearance was detected in the follow-up HPV-DNA tests of the patients with similar demographic data, LBC, colposcopy findings, and cervical biopsy results.
Women experience intense anxiety after learning that they are infected with HPV, during the therapy-free watchful waiting period after abnormal cervical cytological findings, or with the possibility of recurrence after the surgical treatment of CIN [15]. The search for non-invasive medical treatments to support the remission of cervical lesions and clearance of HPV infection continues.
Although the histological regression rates of 179 CIN 2-3 and 62 CIN-1 patients after 8 weeks of ablation treatment with 85% trichloroacetic acid were reported as 87.7% and 80.3%, and HPV 16-18 clearance rates were reported as 73.5% and 75.0%, respectively, the usage of ablative therapy is limited due to the possibility of invasive cancer [16]. In a study comparing different doses of hexaminolevulinate (HAL), photodynamic therapy (PDT), and placebo, it was shown that HAL 5% provided 83% vs. 33% clearance in HPV16-18 at the end of 6 months [17]. In a study that compared 59 patients who used the plant product Gene-Eden-VIR/Novirin for 2-12 months and external follow-up patients in studies reporting HPV cluster times in the literature, the median time in HPV clearance in the former group was found to be 3.5 months, while in the external follow-up patients, it was reported to vary between 6.9 to 20 months [18]. In another study evaluating hr-HPV clearance after the intravaginal application of a zinc-citrate compound for 3 months, clearance was found to be 64.47% in the treatment group, while it was 15.25% (p: 0.000) in the control group [19]. The common result of the aforementioned studies is that HPV clearance was evaluated in 3-6 months after treatment.
In a randomized controlled study evaluating the usage of absorbent-antioxidant vaginal gel for three months, the histological/cytological regression rates in the gel vs. control groups after the 3rd month were found to be 72.2% vs. 25%, and the hr-HPV DNA regression rates were 54.3% vs. 10.6%, respectively (p < 0.001)[13]. In a retrospective study evaluating the effect of the vaginal gel on cytological changes, 77% vs. 6% (PAP III) and 71% vs. 11% (PAP III D) regression rates were reported [12]. Both studies demonstrated the positive effect of the vaginal gel on early cytological results. Most HPV infections (~90%) are known to disappear within 6-18 months of acquisition without any clinical signs or symptoms (transient infections) [20]. In our study, no difference was observed in the groups in terms of cytological changes and hr-HPV clearance in the follow-up periods recommended by ASCCP. However, looking at the HPV clearance curves of the control and vaginal gel groups in our study and the outcomes of 2 studies regarding the usage of vaginal gel in the literature, it may be considered that vaginal gel can accelerate early hr-HPV clearance.
Studies have clearly shown that there are significant differences in the microbial structure, diversity, and composition of species between HPV-negative and HPV-positive women and between healthy women and women with HPV-related diseases [21]. The vaginal microbiota is an important component of local cervical immunity, and this plays an important role in the pathogenicity of HPV infection and the progression of cervical lesions [22]. The fact that micronized silicon dioxide in the contents of vaginal gel is not a selective absorbent against pathogens on the cervical surface suggests that it has no effect on the vaginal microbiota.
The three available HPV vaccines, which are Cervarix® (HPV 16, 18), Gardasil4® (HPV 16, 18, 6, 11), and Gardasil9® (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58) provide almost 100% protection against infection with different HPV genotypes [23-25]. In the study by Hildesheim et al., in which 1,901 (954 vaccine arm; 947 control arm) hr-HPV patients were evaluated, it was shown that the bivalent vaccine did not alter viral clearance and progression to preneoplastic lesions [26]. The effect of vaccines on HPV clearance has not been demonstrated, while in a randomized controlled study by Zarchi et al. regarding the therapeutic effect of vaccines, a 58.7% reduction in CIN 1-3 recurrence was reported in women who received two or more doses of the quadrivalent evaluation of the data of international double-blind randomized controlled trials (FUTURE 1-2) of the quadrivalent HPV vaccine, the recurrence rate in patients who underwent surgery for CIN 2-3 was 6.6% vs. 12.2% in the vaccine vs. control group, respectively, while in the bivalent vaccine study (PATRICIA), these ratios were reported as 0.5% vs. 3.4%, respectively [27,28]. In our study, the vaginal gel did not affect HPV clearance, and furthermore, its effect on histological and cytological results regarding therapeutic effects could not be demonstrated.
In this study, all records and demographic characteristics of the patients in both groups were obtained from medical records, and strict inclusion criteria were applied. The limitation of total number of patients in both groups and the retrospective design are the main limitations of this study. Furthermore, the side effect profile, anxiety scores, and patient compliance during the waiting period of the patients in both groups are not presented. However, to the best of our knowledge, our study is the first study in the literature to present HPV clearance performed in a single center with the longest follow-up intervals.
HPV will continue to be one of the most threatening infectious causes of women’s health issues today and in the near future. Therefore, the search for treatments that will reduce HPV pathogenicity without systemic side effects continues. Although the absorbent-antioxidant vaginal gel seems to be a treatment that can be used in the waiting and follow-up periods of HPV infection, our study could not differentiate its effect on HPV clearance between patients who used the gel and those who did not use the gel in a follow-up interval of approximately 1 year. Studies that investigate the longer-term effects of absorbent-antioxidant vaginal gel in preventing the development of CC and cervical preinvasive lesions are needed.
Statistical analyses were provided by Prof. Meriç Yavuz Çolak, Başkent University, Department of Biostatistics.
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. This study was approved by Başkent University Institutional Review Board (No. KA KA21/102).
Informed consent
Informed consent was obtained from all participants included in the study.
Contributions
SA: writing; ŞYB-GÇŞ -GDD: editing/data collection; ŞA: data collection; HÇ: supervision.
All authors declare that they have participated in the design, execution, and analysis of the paper, and they have read and approved the final version.
- International Agency for Research On Cancer. Available from: https://gco.iarc.fr/today/online-analysis-pie. 2020.
- Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016 Dec 1;2:16086. doi: 10.1038/nrdp.2016.86. PMID: 27905473.
- Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Désy M, Rohan TE. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999 Nov;180(5):1415-23. doi: 10.1086/315086. PMID: 10515798.
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Aug 21;320(7):674-686. doi: 10.1001/jama.2018.10897. PMID: 30140884.
- Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, Ceballos K, Quinlan D, Lee M, Martin RE, Gentile L, Peacock S, Stuart GCE, Franco EL, Coldman AJ. Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial. JAMA. 2018 Jul 3;320(1):43-52. doi: 10.1001/jama.2018.7464. Erratum in: JAMA. 2018 Dec 4;320(21):2273. PMID: 29971397; PMCID: PMC6583046.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep 8;370(9590):890-907. doi: 10.1016/S0140-6736(07)61416-0. PMID: 17826171.
- Jin G, LanLan Z, Li C, Dan Z. Pregnancy outcome following loop electrosurgical excision procedure (LEEP) a systematic review and meta-analysis. Arch Gynecol Obstet. 2014 Jan;289(1):85-99. doi: 10.1007/s00404-013-2955-0. Epub 2013 Jul 11. PMID: 23843155.
- Holmes MM, Weaver SH 2nd, Vermillion ST. A randomized, double-blind, placebo-controlled trial of 5-fluorouracil for the treatment of cervicovaginal human papillomavirus. Infect Dis Obstet Gynecol. 1999;7(4):186-9. doi: 10.1002/(SICI)1098-0997(1999)7:4<186::AID-IDOG4>3.0.CO;2-Z. PMID: 10449266; PMCID: PMC1784744.
- Frost L, Skajaa K, Hvidman LE, Fay SJ, Larsen PM. No effect of intralesional injection of interferon on moderate cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1990 Jul;97(7):626-30. doi: 10.1111/j.1471-0528.1990.tb02552.x. PMID: 2167727.
- Connor JP, Elam G, Goldberg JM. Empiric vaginal metronidazole in the management of the ASCUS Papanicolaou smear: a randomized controlled trial. Obstet Gynecol. 2002 Feb;99(2):183-7. doi: 10.1016/s0029-7844(01)01725-2. PMID: 11814493.
- Deflamed Internetional SRO. Available from: http://www.deflamed.ch/site/?url=products. 2021.
- Huber J, Pötsch B, Gantschacher M, Templ M. Routine Treatment of Cervical Cytological Cell Changes: Diagnostic Standard, Prevention and Routine Treatment of Cervical Cytological Cell Changes - An Assessment of Primary and Secondary Prevention and Routine Treatment Data in the Context of an Anonymous Data Collection from Practicing Gynaecologists; an Academic, Non-Interventional Study. Geburtshilfe Frauenheilkd. 2016 Oct;76(10):1086-1091. doi: 10.1055/s-0042-105286. PMID: 27761030; PMCID: PMC5065416.
- Major AL, Dvořák V, Schwarzová J, Skřivánek A, Malík T, Pluta M, Mayboroda I, Grandjean EM. Efficacy and safety of an adsorbent and anti-oxidative vaginal gel on CIN1 and 2, on high-risk HPV, and on p16/Ki-67: a randomized controlled trial. Arch Gynecol Obstet. 2021 Feb;303(2):501-511. doi: 10.1007/s00404-020-05816-8. Epub 2020 Nov 20. Erratum in: Arch Gynecol Obstet. 2021 Jan 7;: PMID: 33219482; PMCID: PMC7858556.
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012 Apr;137(4):516-42. doi: 10.1309/AJCPTGD94EVRSJCG. PMID: 22431528.
- Sharp L, Cotton S, Carsin AE, Gray N, Thornton A, Cruickshank M, Little J; TOMBOLA Group. Factors associated with psychological distress following colposcopy among women with low-grade abnormal cervical cytology: a prospective study within the Trial Of Management of Borderline and Other Low-grade Abnormal smears (TOMBOLA). Psychooncology. 2013 Feb;22(2):368-80. doi: 10.1002/pon.2097. Epub 2011 Dec 12. PMID: 22162138.
- Geisler S, Speiser S, Speiser L, Heinze G, Rosenthal A, Speiser P. Short-Term Efficacy of Trichloroacetic Acid in the Treatment of Cervical Intraepithelial Neoplasia. Obstet Gynecol. 2016 Feb;127(2):353-9. doi: 10.1097/AOG.0000000000001244. PMID: 26942365.
- Hillemanns P, Garcia F, Petry KU, Dvorak V, Sadovsky O, Iversen OE, Einstein MH. A randomized study of hexaminolevulinate photodynamic therapy in patients with cervical intraepithelial neoplasia 1/2. Am J Obstet Gynecol. 2015 Apr;212(4):465.e1-7. doi: 10.1016/j.ajog.2014.10.1107. Epub 2014 Oct 31. PMID: 25467012.
- Polansky H, Itzkovitz E, Javaherian A. Human papillomavirus (HPV): systemic treatment with Gene-Eden-VIR/Novirin safely and effectively clears virus. Drug Des Devel Ther. 2017 Mar 3;11:575-583. doi: 10.2147/DDDT.S123340. PMID: 28424535; PMCID: PMC5344427.
- Kim JH, Bae SN, Lee CW, Song MJ, Lee SJ, Yoon JH, Lee KH, Hur SY, Park TC, Park JS. A pilot study to investigate the treatment of cervical human papillomavirus infection with zinc-citrate compound (CIZAR®). Gynecol Oncol. 2011 Aug;122(2):303-6. doi: 10.1016/j.ygyno.2011.04.026. Epub 2011 May 24. PMID: 21605892.
- Laudadio J. Human papillomavirus detection: testing methodologies and their clinical utility in cervical cancer screening. Adv Anat Pathol. 2013 May;20(3):158-67. doi: 10.1097/PAP.0b013e31828d1893. PMID: 23574772.
- Gao W, Weng J, Gao Y, Chen X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect Dis. 2013 Jun 10;13:271. doi: 10.1186/1471-2334-13-271. PMID: 23758857; PMCID: PMC3684509.
- Zheng JJ, Song JH, Yu CX, Wang F, Wang PC, Meng JW. Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health. 2019 Aug 12;19(1):109. doi: 10.1186/s12905-019-0806-2. PMID: 31405377; PMCID: PMC6689872.
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, Mayrand MH, Ruiz-Sternberg AM, Stapleton JT, Wiley DJ, Ferenczy A, Kurman R, Ronnett BM, Stoler MH, Cuzick J, Garland SM, Kjaer SK, Bautista OM, Haupt R, Moeller E, Ritter M, Roberts CC, Shields C, Luxembourg A. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017 Nov 11;390(10108):2143-2159. doi: 10.1016/S0140-6736(17)31821-4. Epub 2017 Sep 5. PMID: 28886907.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007 May 10;356(19):1915-27. doi: 10.1056/NEJMoa061741. PMID: 17494925.
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009 Jul 25;374(9686):301-14. doi: 10.1016/S0140-6736(09)61248-4. Epub 2009 Jul 6. Erratum in: Lancet. 2010 Sep 25;376(9746):1054. PMID: 19586656.
- Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M, Sidawy M, Schiller JT, Lowy DR, Herrero R; Costa Rica HPV Vaccine Trial (CVT) Group. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016 Aug;215(2):212.e1-212.e15. doi: 10.1016/j.ajog.2016.02.021. Epub 2016 Feb 16. PMID: 26892991; PMCID: PMC4967374.
- Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, Huh WK, Sings HL, James MK, Haupt RM; FUTURE I and II Study Group. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401. PMID: 22454089; PMCID: PMC3314184.
- Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmerón J, Chow SN, Apter D, Castellsagué X, Teixeira JC, Skinner SR, Hedrick J, Limson G, Schwarz TF, Poppe WA, Bosch FX, de Carvalho NS, Germar MJ, Peters K, Del Rosario-Raymundo MR, Catteau G, Descamps D, Struyf F, Lehtinen M, Dubin G; HPV PATRICIA Study Group. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016 Dec 15;139(12):2812-2826. doi: 10.1002/ijc.30391. Epub 2016 Sep 9. PMID: 27541373; PMCID: PMC5412942.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
