Journal of Gynecological Research and Obstetrics
Gastric-type endocervical adenocarcinoma: Review of clinicopathologic characteristics and recent advances
Michelle Lin1, Kyu-Rae Kim2 and Jae Ro1*
2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
Cite this as
Lin M, Kim KR, Ro J (2020) Gastric-type endocervical adenocarcinoma: Review of clinicopathologic characteristics and recent advances. J Gynecol Res Obstet 6(3): 072-075. DOI: 10.17352/jgro.000091Background: Gastric-type endocervical adenocarcinoma (ECAC) represents a unique variant of endocervical adenocarcinoma that is distinguished by lack of HPV association and an aggressive clinical course with unfavorable prognosis. In this review, we will discuss the clinical and pathologic aspects of gastric-type ECAC, as well as recently described molecular alterations which may be harnessed in future diagnostic and therapeutic strategies.
Results: Clinically, gastric-type ECAC tends to present at an advanced stage with higher rates of extrauterine spread and distant metastasis. Microscopically, gastric-type ECAC is characteristically composed of cells with abundant clear to eosinophilic vacuolated cytoplasm with distinct cell borders, and may demonstrate deceptively benign-appearing morphology. The prognosis of gastric-type ECAC is poor compared to other subtypes of ECAC, as it tends to present with advanced disease and show resistance to conventional chemoradiation therapy. Recent studies have reported a range of molecular alterations in gastric-type ECAC which may have implications in future treatment, including CDKN2A, BRCA2, ERBB2, PIK3CA, ARID1A, and NTRK1/NTRK3 mutations.
Conclusions: Gastric-type ECAC is a relatively recently defined subtype of ECAC with an array of clinical and pathological characteristics that distinguish it from other subtypes of ECAC. As this entity continues to pose difficulties in diagnosis and treatment for pathologists and clinicians alike, continued future investigation is crucial to further our understanding and optimize management of this disease.
Introduction
Gastric-type endocervical adenocarcinoma (ECAC) is a relatively recently characterized subtype of ECAC, defined by its morphologic similarity to gastric pyloric glands and immunophenotypic expression of gastric-type mucin. First recognized and described by Kojima, et al. in 2007, it was added to the WHO classification of tumors in 2014 as a variant of mucinous ECAC, and encompassing Minimal Deviation Adenocarcinoma (MDA) as an extremely well-differentiated form of gastric-type ECAC [1,2]. Recent studies have established gastric-type ECAC as an entity with distinct clinical and pathologic characteristics. Unlike the vast majority of ECACs, gastric-type ECAC is unrelated to high-risk HPV infection [2,3]. Furthermore, in comparison to other ECACs, gastric-type ECAC is associated with more aggressive clinical behavior and a worse prognosis [1-3]. This review will summarize the clinical and histopathologic features of gastric-type ECAC (Table 1), as well as recent advances in the molecular characterization of these tumors that may significantly impact future directions in diagnosis and treatment.
Clinical features
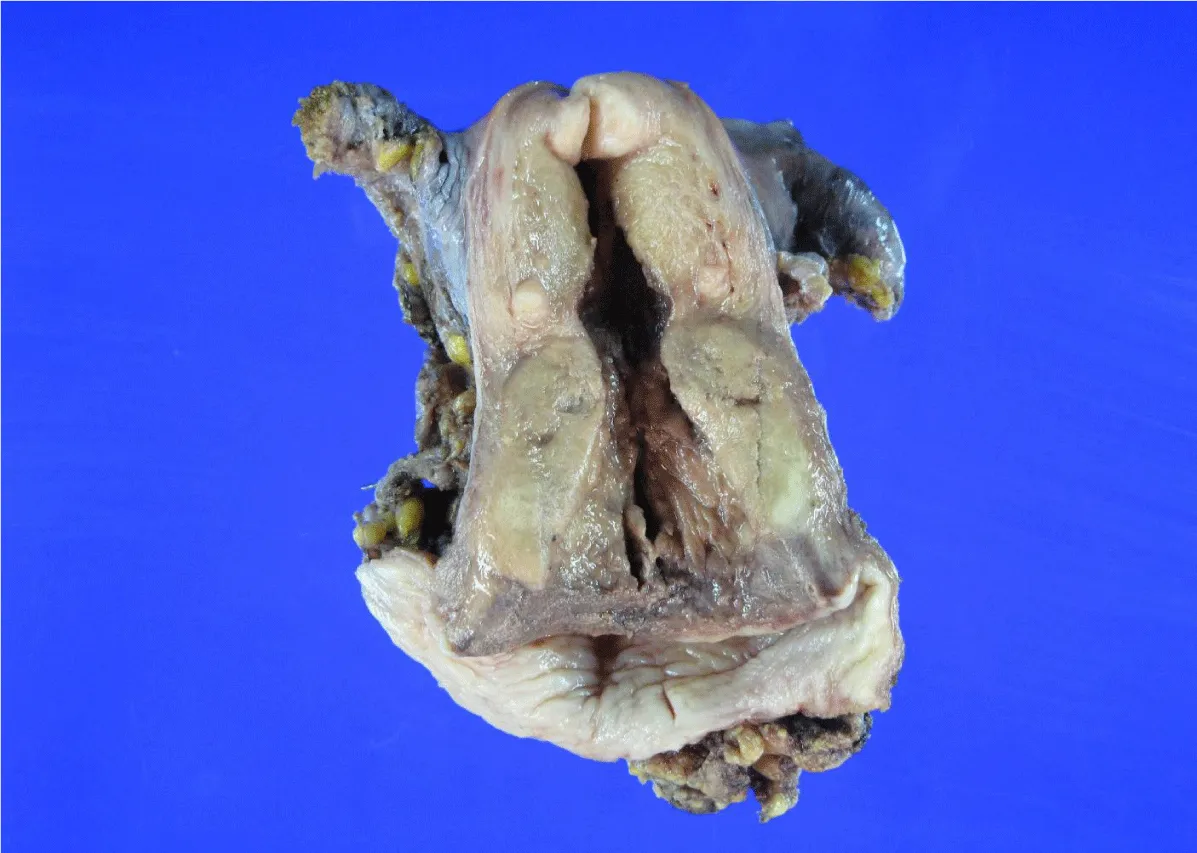
Gastric-type ECAC has been found to represent the second most common subtype of ECAC after usual-type ECAC, comprising approximately 10% of cases worldwide [4]. Although relatively rare in Western countries, it accounts for up to 25% of cases in Japan [2,4]. The mean age of presentation is approximately 50-55 years (approximately one decade older than patients with usual-type ECAC) [2]. Patients may present with abnormal bleeding, watery vaginal discharge, abdominal discomfort, or may be asymptomatic [2,5,6]. Cases may also be detected through cervicovaginal cytologic screening, although the sensitivity, particularly in well-differentiated cases, is not high [5,6]. Gastric-type ECAC has a well-documented association with Peutz-Jeghers syndrome, which is caused by a germline mutation in the STK11 gene on chromosome 19 and also leads to hamartomatous gastrointestinal polyps and increased risk of several types of cancers [2]. Macroscopically, gastric-type ECAC tends to arise from the upper endocervix and form an ill-defined infiltrative mass causing a bulky or “barrel-shaped” cervix (Figure 1), in contrast to usual-type ECAC, which arises from the transformation zone and usually presents as a well-defined mass [7,8]. In addition, gastric-type ECAC often presents at a more advanced stage than other subtypes, with deeper cervical stromal invasion and higher incidence of parametrial and vaginal extension, involvement of other abdominopelvic organs, and metastasis to lymph nodes and distant sites [8,9].
Histopathologic features
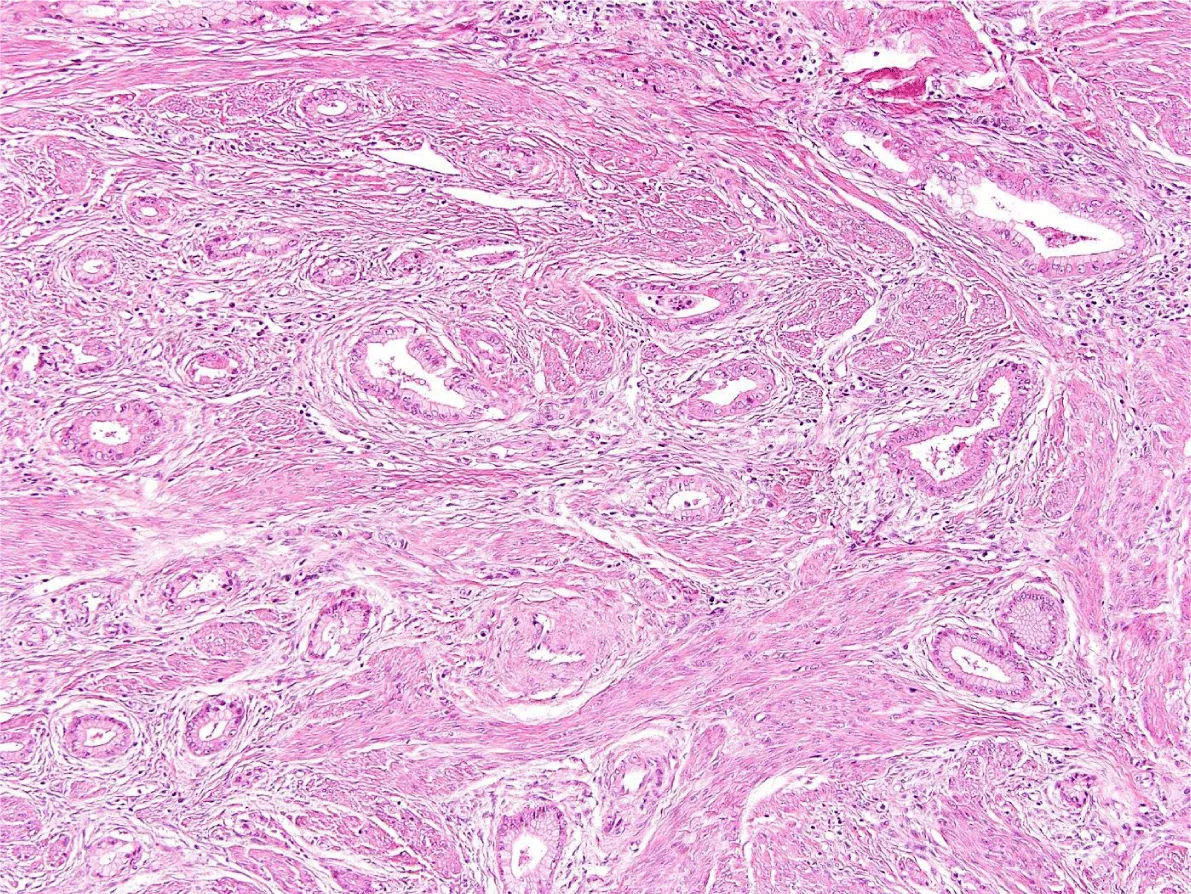
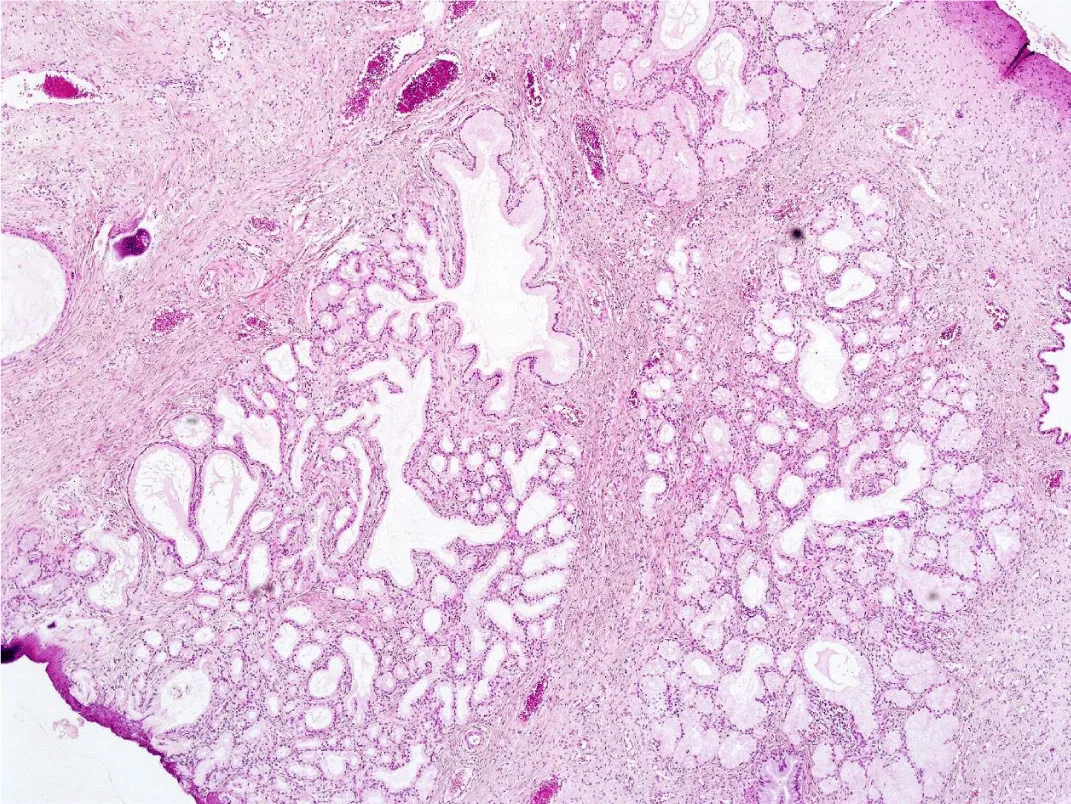
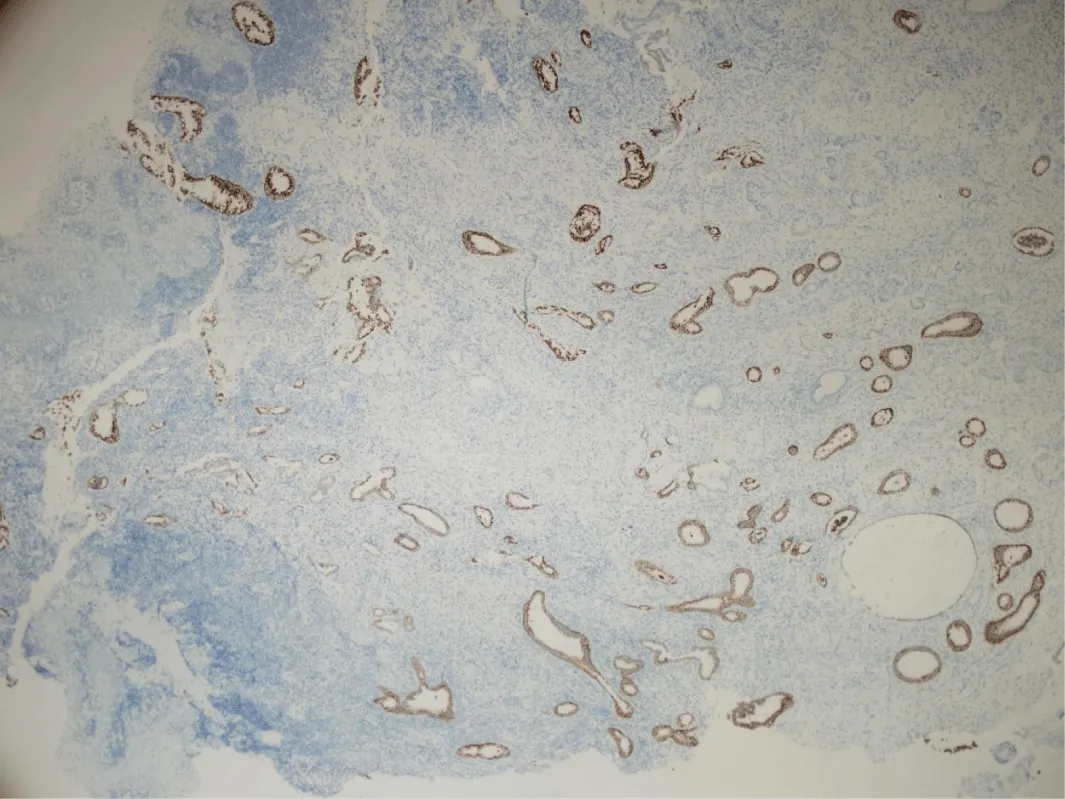
Microscopically, gastric-type ECAC is composed of irregularly shaped, small to cystically dilated glands with varying degrees of architectural complexity including intraluminal papillae and occasionally cribriform and solid growth (Figure 2) [5,6,10]. A desmoplastic stromal reaction may be present around the glands at the deep invasive edge of the tumor [5]. The glands are lined by cells that characteristically contain abundant, clear to pale eosinophilic cytoplasm and have well-defined cellular borders [1,3]. The nuclei are typically basally oriented and show low levels of mitotic activity and apoptosis (in contrast to usual-type ECAC, which generally shows nuclear stratification with readily identifiable apical mitoses and apoptotic bodies) [5,10]. The degree of cytologic atypia in gastric-type ECAC can range from mild to marked; MDA represents an extremely well-differentiated form of gastric-type ECAC composed of glands with cytologically bland nuclei and little to no stromal reaction, causing a deceptive morphologic resemblance to benign endocervical glands [5,8]. In addition, some cases may be seen in association with lobular endocervical gland hyperplasia (LEGH), which appears as a well-demarcated lobular proliferation of small mucinous glands, usually located in the inner half of the endocervical wall (Figure 3) [8,10]. As LEGH often displays gastric-type differentiation and has similar immunophenotypic features and molecular alterations to gastric-type ECAC, it has been hypothesized to represent a precursor lesion to gastric-type ECAC [10-13]. Some cases demonstrate a histologic transition from LEGH to gastric-type ECAC, further supporting this theory [11]. Immunohistochemically, the tumor shows expression of MUC6 (Figure 4) and HIK1083, markers of pyloric-type mucin [2,3,14]. p16 is generally negative or only focally positive, reflecting its HPV-independent pathogenesis [2,14]. Recent studies have shown that up to 50% of cases show a mutant-type pattern of p53 staining (with either diffusely positive or null staining) [2,5,10,14]. PAX8 is positive in 68-88% of cases; although not consistently expressed in gastric-type ECAC, it should be included in the immunohistochemical panel to differentiate these tumors from metastases of gastric adenocarcinomas (which are typically PAX8-negative) [14,15]. In addition, the tumor usually is positive for CK7 and CEA, may be positive for CK20 and CDX2, and is usually negative for ER and PR [13].
Treatment and prognosis
Gastric-type ECAC confers a significantly less favorable prognosis than usual-type ECAC. Patients with gastric-type ECAC tend to present at a higher stage than those with usual-type ECAC; however even when stratifying for stage at presentation, gastric-type ECAC still demonstrates poorer outcomes compared to usual-type ECAC (particularly in early-stage disease) [5,9]. These outcomes include higher rates of lymphovascular invasion, lymph node metastasis, and distant metastasis [5,9]. While gastric-type ECAC shows a propensity to spread to ovaries, abdominal wall/peritoneum, omentum, and urinary tract, other distant sites of metastasis including bowel, liver, lung, bone, and brain have also been documented [8-10]. Patients with gastric-type ECAC also show a higher rate of recurrence with lower progression-free and disease-specific survival (DSS), with Karamurzin, et al. reporting a 42% DSS for gastric-type ECAC compared to 91% for usual-type ECAC [8,9]. Of note, the histologic grade did not affect prognosis, as the survival statistics for cases of MDA did not differ significantly from less differentiated cases of gastric-type ECAC [8]. In addition, patients with gastric-type ECAC display a poorer response rate to chemotherapy and radiotherapy compared to usual-type ECAC [9,16]. In a study of post-neoadjuvant cisplatin and docetaxel-based chemotherapy resection specimens, Kojima, et al. found a 68.8% rate of downstaging upon microscopic examination for usual-type ECAC cases, but a 0% rate for gastric-type ECAC cases [16]. The high rates of chemoresistance and radioresistance in gastric-type ECAC have led investigators to consider alternative molecular-based targeted therapies to potentially achieve better outcomes in these patients.
Recent advances
Recent studies have examined molecular characteristics of gastric-type ECAC and their potential ramifications in diagnosis and treatment. The most frequent recurrent genetic alterations found include mutations in the TP53 and STK11/LKB1 genes, suggesting that these mutations may play important roles in the pathogenesis of gastric-type ECAC [10,15,17]. Of note, germline STK11 mutations have previously been implicated in Peutz-Jeghers syndrome, and somatic SKT11 mutations have been demonstrated in sporadic cases of gastric-type ECAC as well as in the transformation of LEGH to gastric-type ECAC [15,18]. Other alterations found in gastric-type ECAC that may represent actionable mutations for molecular-based targeted therapies include CDKN2A, BRCA2, ERBB2, PIK3CA, ARID1A, and NTRK1/NTRK3 [10,15,17,20]. Garg, et al. also found in their cohort frequent POLE mutations, which have been recently investigated as predictive markers for positive response to immune checkpoint inhibitor therapy [16]. In addition, Jung, et al. and Garg, et al. demonstrated MDM2 amplification in a subset of gastric-type ECAC; as this alteration is currently used to facilitate diagnosis of well-differentiated and dedifferentiated liposarcomas, it may potentially be used in a similar capacity for the identification of gastric-type ECAC cases [10,15].
Conclusions
In conclusion, gastric-type ECAC is a distinctive subtype of ECAC that is not associated with HPV infection and displays an aggressive clinical course with worse outcomes compared to other types of ECACs. Due to its relative rarity in Western countries, HPV and p16 negativity, and potentially bland morphology, this tumor poses diagnostic difficulties and is often under-recognized [6]. In addition, specific treatment options and guidelines for gastric-type ECAC are not well established [9]. However, in the era of widespread HPV immunization, the proportion of non-HPV-associated cervical cancers including gastric-type ECAC may increase in the near future. Therefore, early and efficient detection of these cases by pathologists and optimization of therapeutic modalities by clinicians is critical. To this end, continued clinical, histopathologic and molecular characterization of gastric-type ECAC will further advance our understanding of this entity and future implications in its diagnosis and treatment.
- Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, et al. (2007) Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 31: 664-672. Link: https://bit.ly/3noBA36
- International Agency for Research on Cancer. WHO classification of tumors of the female reproductive organs. IARC, Lyon. 2020.
- Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, et al. (2010) Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol 177: 2169-2175. Link: https://bit.ly/3ixNhAU
- Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, et al. (2018) International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol 42: 214-226. Link: https://bit.ly/30JCJZw
- Pirog EC, Park KJ, Kiyokawa T, Zhang X, Chen W, et al. (2019) Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv Anat Pathol 26: 1-2. Link: https://bit.ly/2GxzUUq
- Turashvili G, Morency EG, Kracun M, DeLair DF, Chiang S, et al. (2019) Morphologic features of gastric-type cervical adenocarcinoma in small surgical and cytology specimens. Int J Gynecol Pathol 38: 263-275. Link: https://bit.ly/36SIZ59
- Park KJ, Kim MH, Kim JK, Cho KS (2018) Gastric-type adenocarcinoma of the uterine cervix: magnetic resonance imaging features, clinical outcomes, and prognostic factors. Int J Gynecol Cancer 28. Link: https://bit.ly/3lyt3sT
- Karamurzin YS, Kiyokawa T, Parkash V, Jotwani AR, Patel P, et al. (2015) Gastric type endocervical adenocarcinoma: an aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol 39: 1449-1457. Link: https://bit.ly/33E2UTb
- Nishio S, Mikami Y, Tokunaga H, Yaegashi N, Satoh T, et al. (2019) Analysis of gastric-type mucinous carcinoma of the uterine cervix—An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol Oncol 153: 13-19. Link: https://bit.ly/33EQkDk
- Jung H, Bae GE, Kim HM, Kim HS (2020) Clinicopathological and Molecular Differences Between Gastric-type Mucinous Carcinoma and Usual-type Endocervical Adenocarcinoma of the Uterine Cervix. Cancer Genomics-Proteomics 17: 627-641. Link: https://bit.ly/34wf9jT
- Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, et al. (2004) Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod Pathol 17: 962-972. Link: https://bit.ly/30G68n7
- Kawauchi S, Kusuda T, Liu XP, Suehiro Y, Kaku T, et al. (2008) Is lobular endocervical glandular hyperplasia a cancerous precursor of minimal deviation adenocarcinoma?: a comparative molecular-genetic and immunohistochemical study. Am J Surg Pathol 32: 1807-1815. Link: https://bit.ly/3loJB6a
- Talia KL, McCluggage WG (2018) The developing spectrum of gastric-type cervical glandular lesions. Pathology 50: 122-133. Link: https://bit.ly/3d6csJD
- Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, et al. (2016) A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol 40: 636-644. Link: https://bit.ly/2SwVSZV
- Garg S, Nagaria TS, Clarke B, Freedman O, Khan Z, et al. (2019) Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing. Mod Pathol 32: 1823-1833. Link: https://bit.ly/2Gp0y1P
- Kojima A, Shimada M, Mikami Y, Nagao S, Takeshima N, et al. (2018) Chemoresistance of gastric-type mucinous carcinoma of the uterine cervix: a study of the Sankai Gynecology Study Group. Int J Gynecol Cancer 28. Link: https://bit.ly/3ldTF22
- Hodgson A, Howitt BE, Park KJ, Lindeman N, Nucci MR, et al. (2019) Genomic Characterization of HPV-related and Gastric-type Endocervical Adenocarcinoma: Correlation With Subtype and Clinical Behavior. Int J Gynecol Pathol. Link: https://bit.ly/3jRhCvX
- Kuragaki C, Enomoto T, Ueno Y, Sun H, Fujita M, et al. (2003) Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest 83: 35-45. Link: https://bit.ly/3nthAMC
- Takatsu A, Miyamoto T, Fuseya C, Suzuki A, Kashima H, et al. (2013) Clonality analysis suggests that STK11 gene mutations are involved in progression of lobular endocervical glandular hyperplasia (LEGH) to minimal deviation adenocarcinoma (MDA). Virchows Archiv 462: 645-651. Link: https://bit.ly/3lipDu3
- Nakamura A, Yamaguchi K, Minamiguchi S, Murakami R, Abiko K, et al. (2019) Mucinous adenocarcinoma, gastric type of the uterine cervix: clinical features and HER2 amplification. Med Mol Morphol 52: 52-59. Link: https://bit.ly/2Sz1Fyd
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
