Journal of Gynecological Research and Obstetrics
PlGF, sFlt-1 and sFlt-1/PlGF ratio: Promising markers for predicting pre-eclampsia
Ola H El-Demerdash1, Mona M Zaki1, Mohamed O El Maraghy1, Doaa M A Elzoghby1, Marwa A Abdel-Wahed1* and Ahmed M Mamdouh2
2Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Cite this as
El-Demerdash OH, Zaki MM, El Maraghy MO, Elzoghby DMA, Abdel-Wahed MA, et al. (2018) PlGF, sFlt-1 and sFlt-1/PlGF ratio: Promising markers for predicting pre-eclampsia. J Gynecol Res Obstet 4(2): 018-023. DOI: 10.17352/jgro.000052Objective: To evaluate placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) as early predictors of pre-eclampsia.
Methods: Cohort study at Department of Obstetrics and Gynecology, Ain Shams University Hospitals, enrolling eighty pregnant women between 14 and 19 weeks’ gestation, sera were collected, and stored at -80°C. Thirty women developed pre-eclampsia, fifty continued to be normotensive and their second blood samples were collected. Serum sFlt-1 and PlGF were assayed using chemiluminescence. All enrolled subjects were divided into Q1, Q2, Q3 and Q4 according to serum levels of sFlt-1, PlGF and sFlt-1/PlGF.
Results: The combined sFlt-1/PlGF ratio (cut-off 40) and PlGF (cut-off 328 pg/mL) had high overall diagnostic performance for early prediction of pre-eclampsia during the second trimester. The Odds ratio for the occurrence of pre-eclampsia was 5.4 in Q4 versus Q3 women and 4.05 higher in Q3 versus Q2 women. Women in Q2 and Q1 had similar risk for developing pre-eclampsia.
Conclusion: The combined use of sFlt-1/PlGF ratio with PlGF or sFlt-1, improved the predictive and diagnostic performance of each separate marker.
Introduction
Pre-eclampsia is a hypertensive disorder complicating 5-8% of pregnancies [1]. It is characterized by hypertension and proteinuria and is a leading cause of maternal mortality in 15–20% of pregnant women in developed countries versus 40–80% in developing countries [2,3].
Pre-eclampsia is a state of angiogenic imbalance associated with endothelial dysfunction within specific vascular beds (4). The abnormalities in the development of the placental vasculature could occur early, weeks to months before the development of the clinical manifestations of pre-eclampsia [5].
Placental growth factor (PlGF), a member of the vascular endothelial growth factor (VEGF) family, is one of the main factors that play a key role in the remodeling process of maternal arteries in normal pregnancy. This growth factor is released into the bloodstream by the migrating trophoblasts [6]. The soluble fms-like tyrosine kinase-1 (sFlt-1), also known as soluble VEGF receptor- 1, is a variant of the VEGF receptor, which is secreted from endothelial cells, monocytes, and placenta. The sFlt-1 is capable of binding with both VEGF and PlGF, consequently regulating their action [7]. Alterations in maternal serum concentrations of PlGF and sFlt-1 have been reported to precede the onset of clinically identifiable pre-eclampsia as early as the first trimester [6].
The aim of our study is to assess the clinical utility of PlGF and sFlt-1 as early predictors of preeclampsia.
Materials and Methods
This cohort study was done in the Department of Obstetrics and Gynecology, Ain Shams University Hospitals, Egypt, between October 2014 and January 2016. Preliminary, 432 randomly selected pregnant women in the second trimester (between 14 and 19 weeks’ gestation) were participated and only eighty continued till the end of the study. Gestational age was calculated from the last normal menstrual period and by an early obstetric ultrasonography. Women enrolled in this study were further divided into 4 quarters (Q1, Q2, Q3 and Q4) according to the serum levels of sFlt-1, PlGF and sFlt-1/PlGF ratio during the second trimester (Table 1).
The Ethics Committee of Ain Shams University Hospitals approved this study and a written consent was obtained from each subject enrolled. Thirty women developed pre-eclampsia and fifty continued to be normotensive. Pre-eclampsia was diagnosed in this study according to the diagnostic criteria of American College of Obstetrics and Gynecology, 2013 [8].
Inclusion criteria were singleton normotensive pregnant women during their second trimester (between 14 and 19 weeks’ gestation) and maternal age more than 18 years. All included subjects were Egyptian. Exclusion criteria were renal diseases, systemic lupus erythematosus (or any other autoimmune disease), chronic corticosteroid therapy, women with multiple pregnancies, major fetal congenital anomalies or chromosomal abnormalities.
All pregnant women included in this study were subjected to full history taking and thorough clinical examination with special emphasis on edema and blood pressure measurement, which was taken in a semirecumbent position, with a supported arm and appropriately sized cuff using a manual sphygmomanometer.
First blood samples were collected on enrollment centrifuged at 1000 x g for 15 minutes after complete blood clotting and aliquots were stored at -80°C. Second blood samples were collected on admission during the third trimester. Laboratory investigations done in the second trimester for exclusion of any clinical abnormality included complete blood count (CBC), prothrombin time (PT), international normalized ratio (INR), random serum glucose (RSG), renal function tests (BUN, creatinine), liver function tests including AST and ALT, and urine protein testing by dipstick in a random urine sample. These tests were repeated in the third trimester to assess the clinical severity of pre-eclampsia.
The analysis of CBC was done using Coulter LH 750 Cell Counter, measurement of PT was performed using Stago Compact Autoanalyzer using Neoplastine CI Plus supplied by Diagnostica Stago and serum chemistry was performed using Beckman Coulter AU480 Autoanalyzer. Serum sFlt-1 and PlGF were quantified by chemiluminescence immunoassay technique using reagents provided by EIAAB Science Co., Gaoxin Road, Wuhan, China. The used analyzer reader for chemiluminescence absorbance was Promega E7031 Glomax.
Sample size calculation
Based on the published data of 88% average diagnostic sensitivity and 82% average diagnostic specificity for sFlt-1, PlGF and sFlt-1/PIGF ratio [9]. A minimum total sample size of 188 participants to obtain a minimum of 15 pre-eclamptic patients had achieved a power of 90%. The assumed prevalence of the disease was 0.08 and the target level of significance was 0.05 [10]. The calculation was done using PASS program version 15.
Statistical analysis
Statistical analysis was done using SPSS software version (V. 23.0, IBM Corp., USA, 2015). The values for the biochemical markers were expressed as mean and standard deviation in case of parametric data and as median and interquartile ranges in case of skewed data, while categorical variables were summarized using frequency measures. Student´s t test, Wilcoxon’s rank sum test (Mann-Whitney U) and Chi-square test were used for comparative analysis.
Receiver operator characteristic (ROC) curves were constructed and optimal cut-off values for the biomarkers were established by the best sensitivity and specificity. Multi-ROC curve analysis was used to assess the diagnostic performance of the combined use of more than one test, where the right angle at the upper left corner is the best diagnostic threshold (cut-off) of the parameter being varied.
Odds ratio measures the association between an exposure and an outcome and how many times the risk was present among diseased individuals compared to that among non-diseased ones. In all statistical analyses, P<0.05 was considered significant.
Results
The descriptive and comparative statistics of the demographic data are shown in tables 1-3.
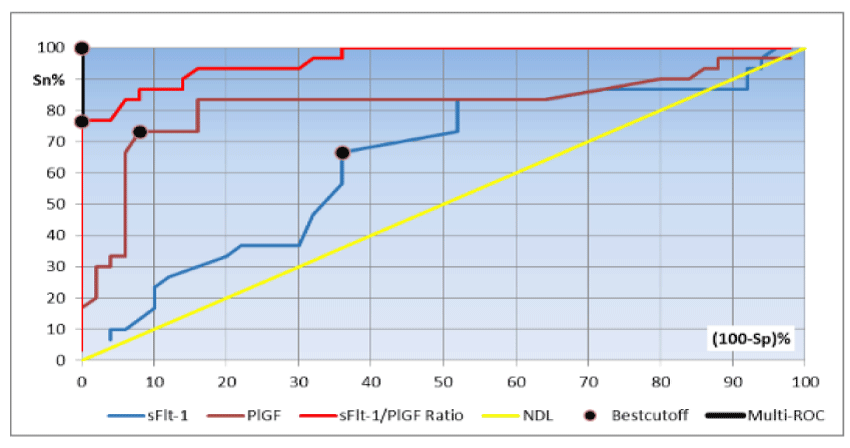
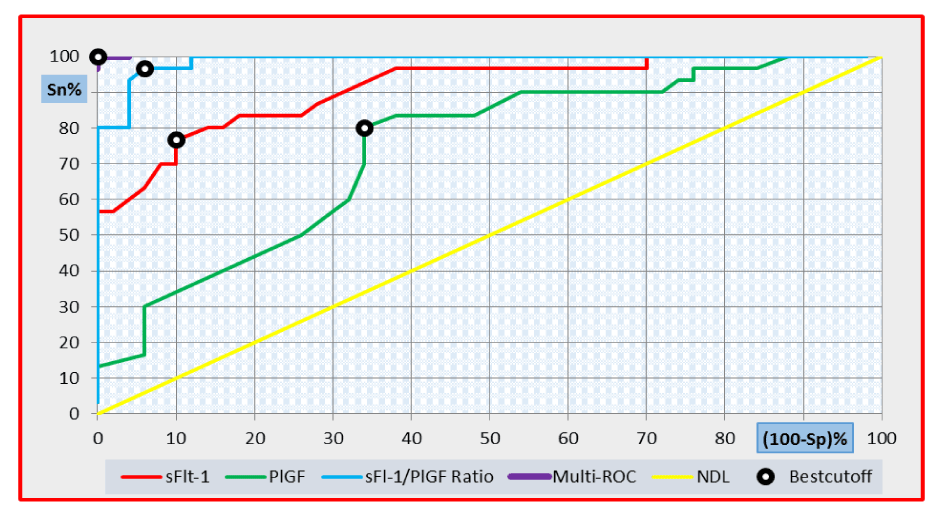
The diagnostic utility of serum sFlt-1, PlGF and sFlt-1/PlGF ratio in discriminating pre-eclamptic from healthy women during the second and third trimesters using ROC curve analysis are shown in tables 4,5 and figures 1, 2.
Multi-ROC curve analysis of the combined use of PlGF and sFlt-1/PlGF ratio during the second trimester revealed that the best cut-off value of PlGF was 328 pg/mL while that of the sFlt-1/PlGF ratio was 40. At these cut-off values, the diagnostic sensitivity, diagnostic specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic efficiency were 100%, respectively (Figure 1).
Multi-ROC curve analysis of the combined use of sFlt-1 and sFlt-1/PlGF ratio during the third trimester revealed that the best cut-off value of sFlt-1 was 4,840 pg/mL with a sFlt-1/PlGF ratio of 51. At these cut-off values the diagnostic sensitivity, diagnostic specificity, PPV, NPV and diagnostic efficiency were 100%, respectively (Figure 2).
In an attempt to determine the Odds ratio for the occurrence of pre-eclampsia and not just to discriminate between pre-eclamptic and healthy women, all pregnant women included in our study during the second trimester were distributed into four quartiles according to their serum levels of sFlt-1, PIGF and sFlt-1/PlGF ratio. Table 2 demonstrates the values quartiles (Q1, Q2, Q3 and Q4). Serum levels of sFlt-1 as well as sFlt-1/ PlGF ratio increase & the serum level of PlGF decreases with the occurrence of pre-eclampsia, thus pregnant women in “Q1” had the lowest values of sFlt-1 and sFlt-1/PlGF ratio, respectively and the highest values of PlGF. On the other hand, those in “Q4” had the highest values of sFlt-1 and sFlt-1/PlGF ratio, respectively and the lowest values of PlGF.
Table 6 demonstrates the Odds ratio for the possible occurrence of pre-eclampsia during the second trimester. It revealed that pregnant women in “Q4” had a 5.46 higher risk for the development of pre-eclampsia than pregnant women in “Q3”. Pregnant women in “Q3” had a 4.05 higher risk for the development of pre-eclampsia than pregnant women in “Q2”. Finally, there was no significant difference as regards the Odds risk between “Q2” and “Q1”.
Discussion
The recent screening studies for pre-eclampsia using sFlt-1 and PlGF were performed during the third trimester [11-13]. Meanwhile, conducting our study in the second trimester might be an additive privilege, which enlights the main value of this current study in early prediction of the disease and hence early follow up and proper management could be done.
Our study revealed significantly higher serum levels of sFlt-1 and sFlt-1/PIGF ratio in pre-eclamptic women in early second trimester as compared to the normotensive pregnant women, these being associated with significantly lower PlGF levels. This is in agreement with the findings of a prospective longitudinal study carried out from 15 weeks’ gestation onward [14]. Similarly, another prospective study measured serum sFlt-1 and PlGF levels at 10, 18, 26 and 35 weeks of gestation [15]. Both confirmed that increased serum sFlt-1 levels and decreased serum PlGF levels, early in pregnancy, are associated with a higher risk of pre-eclampsia.
The connection between circulating sFlt-1 and PlGF in pre-eclampsia was explored by previous studies suggesting that up-regulation of the anti-angiogenic sFlt-1 in pre-eclampsia leads to binding of the angiogenic factor PlGF, with a consequent decrease in circulating free PlGF levels [6,16].
Our study revealed a persistent highly significant increase in serum sFlt-1 levels with lowering of PlGF levels and raised sFlt-1/PIGF ratio among the studied pre-eclamptic patients during the third trimester of pregnancy. This highlights the potential role of sFlt-1, PlGF and sFlt-1/PIGF ratio as promising biomarkers for diagnosis of pre-eclampsia. Similar results recorded that pre-eclamptic women had higher serum levels of both sFlt-1 and sFlt-1/PlGF ratio and lower serum levels of PlGF compared to the values found in control women [17]. In this respect, sFlt-1 and PlGF seem to be important etiological factors in the pathogenesis of pre-eclampsia, as the elevated serum levels of sFlt-1 modify endothelial integrity of blood vessels, hence causing hepatic edema as well as the hypertension and proteinuria encountered in pre-eclamptic patients. Also, blood–brain–barrier damage may occur leading to brain edema. This aberrant angiogenesis and hypertension are the hallmarks of pre-eclampsia [16].
In context with the scenario of multi-system affection in pre-eclampsia, our current study revealed statistically significantly higher serum levels of AST, ALT, BUN, and creatinine in pre-eclamptic patients compared to controls during the third trimester. It was postulated that elevated serum transaminases might be attributed to the profound systemic vasoconstriction with generalized damage to the endothelium of the liver, which likely occurs following the release of toxic factors from the diseased placenta, namely von Willebrand antigen, cellular fibronectin, soluble tissue factor, platelet-derived growth factor and endothelin [18,19]. Moreover, the elevated serum levels of sFlt1 modify the endothelial integrity of blood vessels, causing hepatic edema which may also contribute to the elevation of hepatic transaminases. Concerning the elevated BUN and creatinine levels, this could be explained by the damage inflicted on the kidneys by excess of anti-angiogenic factors such as sFlt-1 over angiogenic mediators such as VEGF and PlGF in the systemic circulation [16]. A parallel imbalance in the renal circulation would be expected, thus affecting renal function [18,19].
Our second trimester results revealed that sFlt-1 at a cut-off level of 2,330 pg/mL had a relatively low diagnostic sensitivity of 66.7% and 64% diagnostic specificity in discriminating pre-eclamptic subjects from the healthy pregnant women. Meanwhile, PlGF showed a better diagnostic performance at a cut-off level of 92 pg/mL, with a diagnostic sensitivity of 83.3%, diagnostic specificity 84%. These findings are comparable with the results of a previous study showing that serum sFlt-1 at a cut-off value of 3,198 pg/mL could discriminate pre-eclamptic women from healthy pregnant women with 88% diagnostic sensitivity and 83.6% diagnostic specificity. At a cut-off value of 138 pg/mL, PlGF could predict pre-eclampsia with a diagnostic sensitivity of 85.5% and a diagnostic specificity of 77.2% [9].
The sFlt-1/PlGF ratio was the best tool for discriminating pre-eclamptic women from the healthy pregnant women early in their second trimester in our study. Our ratio’s best cut-off (32.6) had 93.3% diagnostic sensitivity and 84% diagnostic specificity. This is much close to the reported cut-off value of sFlt-1/PIGF ratio at 38.46 that predicted pre-eclampsia with a diagnostic sensitivity and specificity of 88.5%, respectively, during the second trimester (24-28 weeks of gestation) [6]. This highlights the clinical value of second trimester sFlt-1/PlGF ratio in improvement of the clinical outcome of the disease, suggesting that pregnant women with a sFlt-1/PlGF ratio 32.6 are not likely to develop preeclampsia [17].
Concerning the diagnostic performance of sFlt-1 in discriminating pre-eclamptic from healthy pregnant women during the third trimester, our chosen cut-off value of 6,400 pg/mL had a diagnostic sensitivity of 86.7% and diagnostic specificity 72%. As regards PlGF, the best cut-off level was 86 pg/mL, with a diagnostic sensitivity of 80%, and diagnostic specificity 66%. McElrath et al. (2012) measured serum levels of sFlt-1 and PlGF at 35 weeks of gestation in 153 pre-eclamptic patients in comparison to 1,951 matched controls, however they reported a different best cut-off value of 1,312 pg/mL for sFlt-1, with a diagnostic sensitivity of 62.7% and diagnostic specificity 68.9%. As regards PIGF, their chosen cut-off value of 242 pg/mL had a diagnostic sensitivity of 67.8% and diagnostic specificity 64.8% [20].
Concerning the diagnostic performance of sFlt-1/PlGF ratio during the third trimester, our best cutoff value of 51 was more valuable in discriminating the pre-eclamptic from healthy women with a diagnostic sensitivity of 96.7% and a diagnostic specificity of 94%. Our results agree with an earlier study, suggesting that sFlt-1/PIGF ratio at a cut-off value 45 could diagnose pre-eclampsia during the third trimester with a diagnostic sensitivity of 97% and a diagnostic specificity of 95% [21].
Our results were also compared to a recent study which suggested two cut-off values for sFlt-1/PlGF ratio in the diagnosis of pre-eclampsia at 20-37 weeks of gestation; the first cut-off value focusing on high diagnostic sensitivity at 20-33 weeks of gestation and the second one focusing on high diagnostic specificity at 34 weeks of gestation till delivery. These cut-offs were ≤33 and ≥85 with yielded diagnostic sensitivity/specificity of 95%/94% and 88%/99.5%, respectively [22,23].
In our study, the combined use of sFlt-1/PlGF ratio at a cut-off value 40 together with PlGF at a cutoff value of 328 pg/mL during the second trimester improved the diagnostic sensitivity, specificity, PPV, NPV and diagnostic efficiency to reach 100%, respectively in multi-ROC curve analysis performed in pre-eclamptic patients versus healthy controls. Similarly, the combined use of sFlt1/PlGF ratio at a cut-off value of 51 and sFlt-1 at a cut-off value of 4,840 pg/mL during the third trimester also made the same achievement in discriminating pre-eclamptic patients from healthy controls. Our results support the conclusion reporting that combinations of markers generally led to an increase in sensitivity and/or specificity compared with single markers [24].
The Odds ratio for the occurrence of pre-eclampsia was 5.4 in Q4 versus Q3 women and 4.05 higher in Q3 versus Q2 women. Women in Q2 and Q1 had similar risk for developing pre-eclampsia.
The Odds ratio of sFlt-1 and PlGF was evaluated separately in a previous study, assuming that the Odds ratio for developing pre-eclampsia increased progressively among pregnant women in the quartiles with high serum levels of sFlt-1 and sFlt-1/PlGF ratio or with low serum levels of PlGF. The researchers highlighted that the prediction of pre-eclampsia using standard diagnostic criteria as hypertension and proteinuria may misdiagnose a significant number of cases and they added that women whose rate of change in sFlt-1 and PlGF was in the most abnormal quartile had greater Odds of developing pre-eclampsia [15,25].
In conclusion, serum sFlt-1, PlGF and sFlt-1/PlGF ratio can be considered promising biomarkers for prediction of pr-eclampsia. The excellent diagnostic performance (100% diagnostic efficiency) of the combined use of PlGF and sFlt-1/PlGF ratio using their multi-ROC cut-offs (328 pg/mL and a ratio of 40, respectively) calls for their inclusion in the laboratory work-up of pregnant females, especially high-risk ones, for early prediction of the disease during the second trimester. Meanwhile, the combined use of sFlt-1 and sFlt-1/PlGF ratio at cut-offs 4,840 pg/mL and 51, respectively, is 100% diagnostic of the disease during the third trimester.
- Young BC, Levine RJ, Karumanchi SA (2010) Pathogenesis of pre-eclampsia. Annual Review of Pathology: Mechanisms of Disease. 5: 173-192 Link: https://tinyurl.com/y7p776ym
- Dalzell JR, Jackson CE, Chong KS, McDonagh TA, Gardner RS (2014) Do plasma concentrations of apelin predict prognosis in patients with advanced heart failure? Biomarkers in medicine 8: 807-813. Link: https://tinyurl.com/y87rnyub
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P (2014) Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 4: 105–145. Link: https://tinyurl.com/y8ok6jka
- Vaisbuch E, Whitty JE, Hassan SS, Romero R, Kusanovic JP, et al. (2011) Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol 204:152.e1-9. Link: https://tinyurl.com/yco44x9m
- Cerdeira AS, Karumanchi SA (2012) Angiogenic factors in pre-eclampsia and related disorders. Cold Spring Harb Perspect Med 2: 1-17. Link: https://tinyurl.com/ydajybvm
- Lapaire O, Shennan A, Stepan H (2010) The pre-eclampsia biomarkers soluble fms-like tyrosine kinase1 and placental growth factor: current knowledge, clinical implications and future application. Eur J Obstet Gynecol Reprod Biol 151: 122–129. Link: https://tinyurl.com/yb7hg6vj
- Barleon B, Reusch P, Totzke F, Herzog C, Keck C, et al. (2001) Soluble VEGFR1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 4: 143–154. Link: https://tinyurl.com/ybfv4563
- American College of Obstetricians and Gynecologists (2013) Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on hypertension in pregnancy. Obstet Gynecol 122: 1122–1131. Link: https://tinyurl.com/y9b4lrzh
- Hassan M, Rund NMA, Salama AH (2013) An elevated maternal plasma soluble fms-like tyrosine kinase-1 to placental growth factor ratio at midtrimester is a useful predictor for pre-eclampsia. Obstet Gynecol Int 1–8. Link: https://tinyurl.com/y7qxkt2n
- Li J, Fine J (2004) On sample size for sensitivity and specificity in prospective diagnostic accuracy studies. Statistics in Medicine 23: 2537-2550. Link: https://tinyurl.com/ycxd5oab
- Dragan I, Georgiou T, Prodan N, Akolekar R, Nicolaides KH (2017) Screening for pre-eclampsia using sFlt-1/PlGF ratio cut-off of 38 at 30-37 weeks' gestation. Ultrasound Obstet Gynecol 49: 7377. Link: https://tinyurl.com/y7nc37wn
- Tan MY, Wright D, Koutoulas L, Akolekar R, Nicolaides KH (2017) Comparison of screening for preeclampsia at 31-34 weeks' gestation by sFlt-1/PlGF ratio and a method combining maternal factors with sFlt-1 and PlGF. Ultrasound Obstet Gynecol 49: 201-208. Link: https://tinyurl.com/y82lyrtj
- Wright D, Dragan I, Syngelaki A, Akolekar R, Nicolaides KH (2017) Proposed clinical management of pregnancies after combined screening for pre-eclampsia at 30–34 weeks’ gestation. Ultrasound Obstet Gynecol 49: 194–200. Link: https://tinyurl.com/ybhqbojv
- Khalil A, Maiz N, Garcia-Mandujano R, Penco JM, Nicolaides KH (2016) Longitudinal changes in maternal serum placental growth factor and soluble fms-like tyrosine kinase-1 in women at increased risk of pre-eclampsia. Ultrasound Obstet Gynecol 47: 324–331. Link: https://tinyurl.com/yaw6o32u
- Honigberg MC, Cantonwine DE, Thomas AM, Lim KH, Parry SI, et al. (2016) Analysis of changes in maternal circulating angiogenic factors throughout pregnancy for the prediction of preeclampsia. J Perinatol 36:172-177. Link: https://tinyurl.com/yarcjxof
- Gurnadi JI, Mose J, Handono B, Satari MH, Anwar AD, et al. (2015) Difference of concentration of placental soluble fms-like tyrosine kinase-1(sFlt-1), placental growth factor (PlGF), and sFlt-1/PlGF ratio in severe pre-eclampsia and normal pregnancy. BMC Res Notes 8: 534. Link: https://tinyurl.com/ya6bg55m
- Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, et al. (2015) Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 45: 241–246. Link: https://tinyurl.com/y94y4ano
- Li Z, Zhang Y, Ying M J, Kapoun A M, Shao Q, et al. (2007) Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692. Link: https://tinyurl.com/ycqgjzsk
- Sircar M, Thadhani R, Karumanchi SA (2015) Pathogenesis of pre-eclampsia. Curr Opin Nephrol Hypertens 24: 131-138. Link: https://tinyurl.com/ycsysttc
- McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, et al. (2012) Longitudinal evaluation of predictive value for pre-eclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 207: 407.e1-7. Link: https://tinyurl.com/yaahshru
- Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, et al. (2010) Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt1 and PlGF levels in women with pre-eclampsia. Hypertens Res 33: 422–427. Link: https://tinyurl.com/ybk4nrj3
- Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, et al. (2014) New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for pre-eclampsia. Hypertension 63: 346–352. Link: https://tinyurl.com/y96v4tee
- Zhao M, Zhu Z, Liu C, Zhang Z (2017) Dual-cutoff of sFlt-1/PlGF ratio in the stratification of preeclampsia: a systematic review and meta-analysis. Arch Gynecol Obstet 295:1079– 1087. Link: https://tinyurl.com/y7clox38
- Youssef A, Righetti F, Morano D, Rizzo N, Farina A (2011) Uterine artery Doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11+0 to 13+6 weeks in the prediction of late (> 34 weeks) pre-eclampsia. Prenat Diagn 31: 1141–1146. Link: https://tinyurl.com/y7dg4hc6
- Leaños-Miranda A, Campos-Galicia I, Ramírez-Valenzuela KL, Chinolla-Arellano ZL, IsordiaSalas I (2013) Circulating angiogenic fac- tors and urinary prolactin as predictors of adverse outcomes in women with pre-eclampsia. Hypertension 61: 1118–1125. Link: https://tinyurl.com/yc2loo6z
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
