Journal of Gynecological Research and Obstetrics
Body Mass Index Impact and Predictability on Preeclamptic Toxemia
Khaled M AbdAllah1*, Ahmed Abdelhamid1 and Omar Abd El Fattah Ahmed Nagy2
2Researcher at Department of Reproductive Health and Family Planning, National Research Centre, Cairo, Egypt
Cite this as
AbdAllah KM, Abdelhamid A, El Fattah Ahmed Nagy OA (2018) Body Mass Index Impact and Predictability on Preeclamptic Toxemia. J Gynecol Res Obstet 4(2): 009-014. DOI: 10.17352/jgro.000050Background: PET is a systemic disorder of vascular endothelial dysfunction and vasospasm that occurs after 20 weeks’ gestational age and can present as late as 4-6 weeks postpartum. It is clinically defined by hypertension and proteinuria, with or without pathologic edema. Maternal obesity is a prominent hazardous risk factor for the pathological development of PET.
Aim: To determine the correlation between the raised body mass index and the risk of PET.
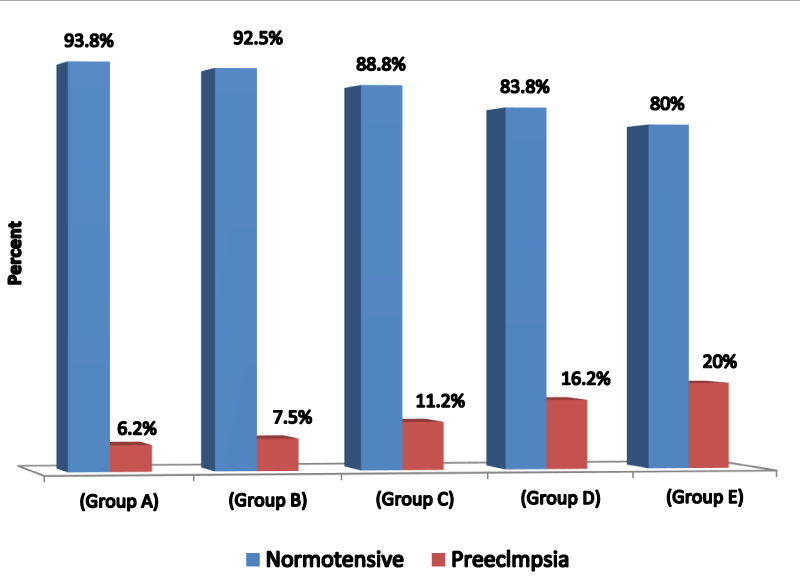
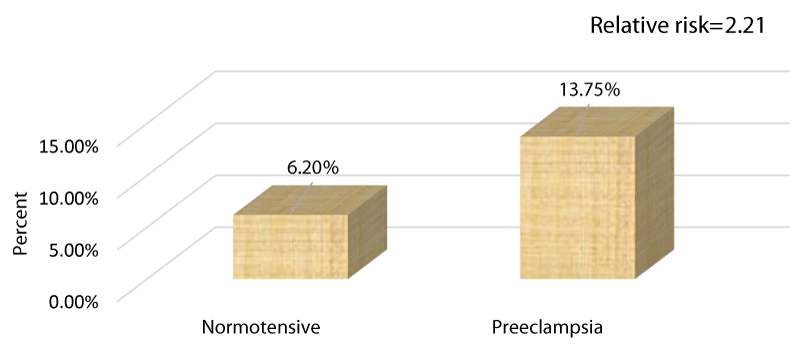
Methods: Research study performed on 400 recruited cases attending the antenatal care unit of Al-Azhar University Hospital (Assiut) they were recruited at 20 weeks of gestation and follow up was performed at 28 and 36 weeks of gestation. They were categorized into 5 research groups (each n=80) according to the selectivity criteria of their Body Mass Index BMI (kg/m2) at 20 weeks of gestational age. Research group A (n=80): normal BMI (18.50-24.99 kg/m2) research group B (n=80): overweight BMI (25-29.99 kg/m2) Group C (n=80): obese class I BMI (30-34.99 kg/m2) research group D (n=80): obese class II BMI (35-39.99 kg/m2) Research group E (n=80): obese class III BMI (>40 kg/m2). Follow up for PET development was performed to analyze the correlation between BMI and PET development. Developed in 5 cases in the group A (6.25%), 6 cases in the group B (7.5%), 9 cases in the group C (11.2%), 13 cases in the group D (16.2%), 16 cases in the group E (20%). Among these 11 cases developed severe preeclampsia in groups B, C, D, and E. It has been evident in the present study that the incidence of preeclampsia (either mild or severe) in cases of increased BMI (groups B, C, D, and E) was 13.75%. While the incidence of preeclampsia in the general population (group A) is cited to be 6.25%. Relative risk to general population=incidence of preeclampsia in people exposed (13.75%) / incidence in general population (6.25%) =2.2.
Introduction
Preeclampsia is a widespread vascular endothelial dysfunction and vasospasm that occurs after 20 weeks’ gestational age and could clinically present as late as 4-6 weeks postpartum. It is clinically defined by hypertension and proteinuria, with or without pathologic edema. The global incidence of PET has been expected TO be around 5-14% of all gestations [1-5]. Hypertensive disorders of pregnancy, involving PET, affect up to 10% of gestations worldwide, forming one of the chief causes of maternal and perinatal morbidity and mortality worldwide. Hypertensive disorders of gestation are major contributing factors to prematurity [6-10]. Preeclampsia is a well-known risk factor for future development of cardiovascular disease and metabolic disease in females.
In spite of extensive research performed worldwide, the etiology of pre-eclampsia stays vague. In the previous decade, tremendous updates in the knowledge and understanding of PET pathophysiological pathways as well as raised efforts to acquire evidence to advance management protocols have emerged. On the other hand, this knowledge acquired has not been applied into advanced clinical practice [11,12]. New guidelines are required to guide obstetricans to care of cases with all clinical types of PET and hypertension that exist during gestation, especially females suffering acute severe hypertension and superimposed PET. A system for continually updating these guidelines is also required and integrating them into daily clinical practice. Proper clinical Identification of cases with severe Clinical forms of PET is a great challenge for clinicians. Enhanced patient education and counseling protocols are required to manage more efficiently the hazards of PET and hypertension and the significance of early detection and predictability to females with varying levels of health literacy. Research studies for PET and other hypertensive disorders of gestation in both the laboratory and clinical regions require continued prominence and funding [13-15].
Obesity is a chief epidemic globally , obesity in pregnancy was displayed to raise the risk of gestational DM, hypertension, PET, cesarean delivery, postpartum weight retention, pretermature delivery, still birth, congenital anomalies involving neural tube defects, spontaneous abortion, recurrent miscarriage, macrosomia, birth injury, difficulties related to anesthesia management, and emergency cesarean delivery, postpartum haemorrhage, the delivery of large-for-dates babies, and stillbirth. Maternal obesity is a distinguished risk factor for the pathological development of PET [16-20].
The relationship that obesity raises the risk of PET has been reported for several populations all over the world signifying that this is not a phenomenon limited to western communities. It is also evident that this relationship is not limited to obese and overweight women because increases in BMI in the normal range is also correlated with a raised hazardous risk of developing PET [21-23].
The research study was performed on 400 recruited cases attending the antenatal care unit of Al-Azhar University Hospital (Assiut) they were recruited at 20 weeks of gestation and follow up was performed at 28 and 36 weeks of gestation. They were divided into 5 research groups (each n=80) according to the selectivity criteria of their Body Mass Index BMI (kg/m2) at 20 weeks of gestational age. Research group A (n=80): normal BMI (18.50-24.99 kg/m2) research group B (n=80): overweight BMI (25-29.99 kg/m2)Group C (n=80): obese class I BMI (30-34.99 kg/m2) research group D (n=80): obese class II BMI (35-39.99 kg/m2) Research group E (n=80): obese class III BMI (>40 kg/m2). All the recruited study subjects undergone the following: Complete history taking involving personal history, history of present illness, past history, menstrual history, obstetric history, medical history and family history to confirm exclusion and inclusion criteria. At 20 weeks of gestational age: Complete history taking, Examination with particular emphasis as regards: Body Mass Index BMI.
, the patients were categorized into the five research groups (each n=80) according to BMI.
Arterial Blood pressure estimation was performed by measuring systolic blood pressure and diastolic blood pressure using a simple mercury sphygmomanometer on right arm in a comfortable sitting position after 10 minutes of rest. Obstetric ultrasound was done to confirm fetal viability, gestational age using biparietal diameter (BPD) and femoral length (FL), and to exclude multiple gestation. Full Laboratory investigations. Follow-up visits: Re-evaluation of the following parameters was done at twenty eight weeks and thirty six weeks of gestation: Body mass index (kg/m2), arterial blood pressure estimation, full Laboratory investigations. If the systolic BP is ≥ 140 mmHg or the diastolic BP is ≥ 90mmHg re-evaluation was performed after 6 hours to confirm elevated blood pressure. Cases of preeclampsia were diagnosed according to the following criteria: Systolic BP ≥ 140 mmHg or diastolic BP ≥ 90mmHg on two occasions 6 hours apart after 20 weeks of gestation in a woman known not to have chronic hypertension prior to the pregnancy, proteinuria ≥ 0.3 g in a 24-hour urine collection or 2+ proteinuria on qualitative examination or urinalysis, edema and excessive weight gain may be present in preeclampsia but are no longer necessary for the diagnosis of preeclampsia.
Results
Table 1 demonstrates demographic data of studied population showing mean and range of age of studied population, residence and socioeconomic level
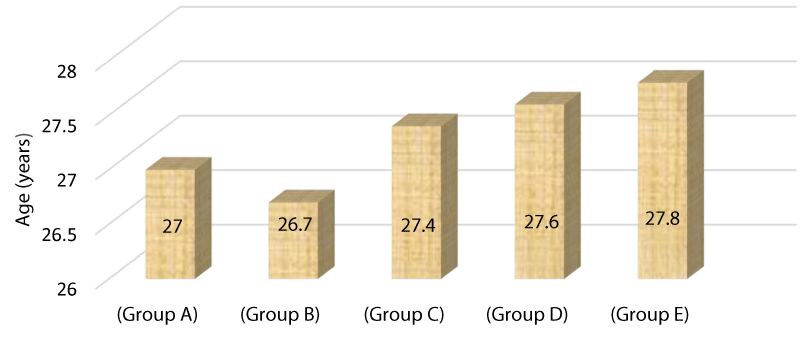
Table 2 compares the mean of age among research groups showing no statistical significant difference as p value =>0.5.
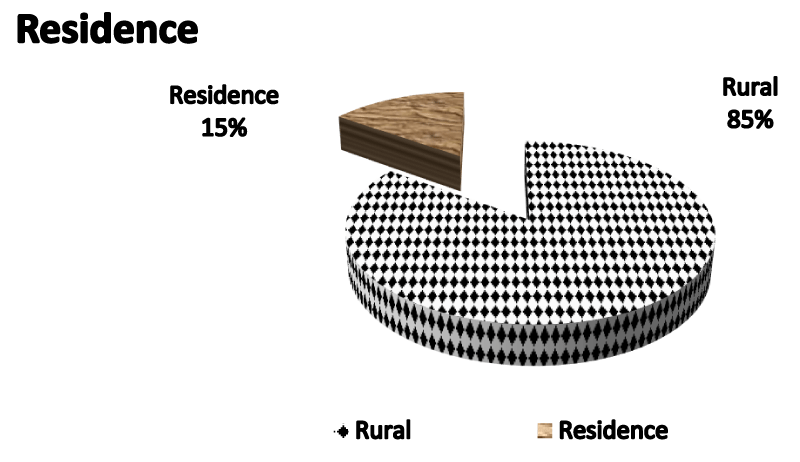
Figure 1 Pie chart demonstrating residence of studied population being 85 % from rural areas.
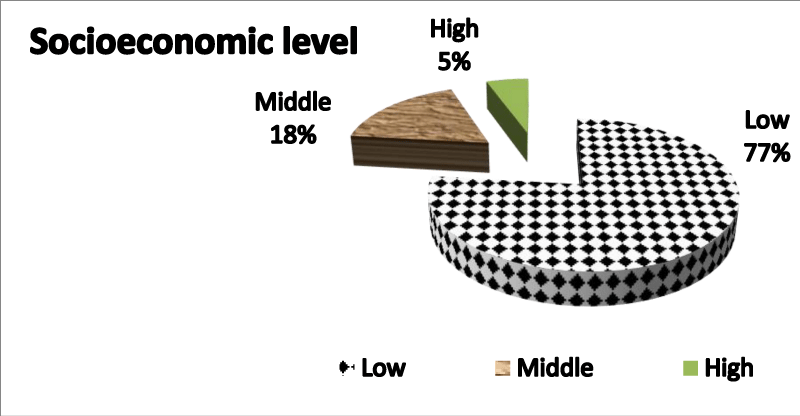
Figure 2 Pie chart demonstrating socio economic level showing low socioeconomic level 77% to be most of the studied population.
Figure 3 Bar chart demonstrating the mean age of different study groups Group A=27 years, Group B=26.7 years, Group C=27.4 years, Group D=27.6 years, Group E=27.8 years
Table 3 demonstrates clearly no statistically significant difference as regards residence among study groups as p value=0.67
Table 4 demonstrates clearly the difference between various research groups as regards socioeconomic levels showing no statistical significant difference p value=0.76 .
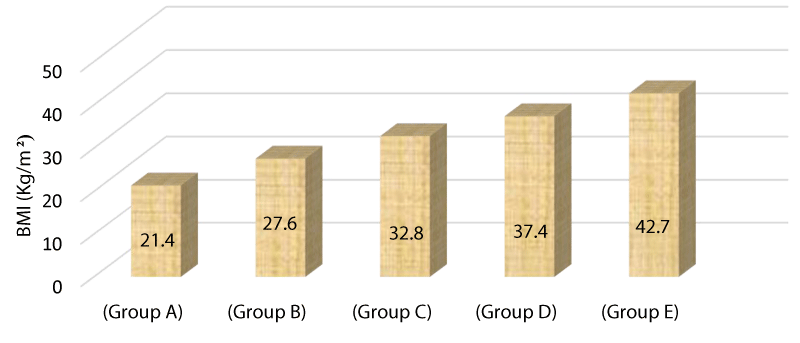
Table 5 displays the BMI in a comparative manner between various research groups showing statistically significant difference among the groups with a p value <0.001.
Figure 4 The bar chart above demonstrates the mean BMI among the study groups as group A=21.4, group B=27.6, group C=32.8, group D=37.4, group E=42.7.
Table 6 displays and demonstrates clearly the incidence of preeclampsia among the study groups showing a statistically significant difference between groups as incidence of PET increases as BMI increases with a p value =0.035.
Figure 5 bar chart displays the incidence of PET among various study groups group A=6.2%, group B=7.5%, group C=11.2%, group D=16.2%, group E=20%.
Table 7 shows that the relative risk is 2.21 i.e that the risk of PET nearly doubles with increased BMI.
Figure 6 bar chart compares the percentage of preeclampsia and normotensive cases among the study population regarding cases with increased BMI normotensive =6.2 %, preeclampsia =13.75%.
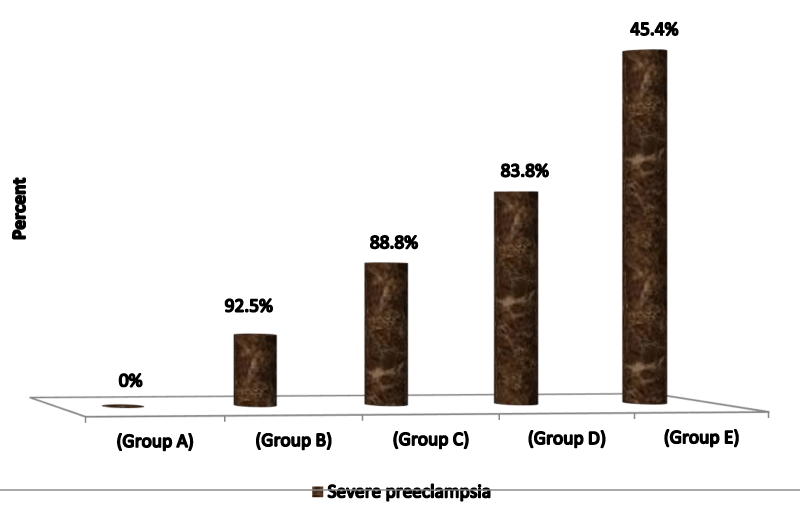
Table 8 demonstrates clearly the frequency of severe preeclampsia among various research groups as group A= 0%, group B=9.1%, group C=18.2%, group D=27.3%, group E=45.4%.
Figure 7 The bar chart above demonstrates the frequency of PET among various research groups.
Discussion
Our research group displayed in the current research study that PET developed in 5 cases in the research group A (6.25%), 6 cases in the research group B (7.5%), 9 cases in the research group C (11.2%), 13 cases in the research group D (16.2%), 16 cases in the research group E (20%). Among these 11 cases developed severe PET in groups B, C, D, and E. It has been evident in the current research study that the incidence of PET (either mild or severe) in cases of raised levels of BMI (groups B, C, D, E) was 13.75%. While the incidence of PET in the normal BMI (group A) is displayed to be 6.25%. Relative risk to general population=incidence of preeclampsia in people exposed (13.75%) / incidence in general population (6.25%) = 2.2.Therefore, it is rational to conclude that raised BMI in this research study was linked with 2.2 fold increase in risk of PET development than the general population. This risk is in harmony with that obtained from similar research studies. On the other hand we discovered that not all the obese gestations developed preeclampsia (86.25% of obese cases) which means that there are unknown multi factorial elements impact the internal clinical settings of gestation so they interact with the stressful clinical situation in a manner that guard against the development of PET and if it develops it is either mild or severe. Hypothetically these factors are capabilities of the mother to clear oxygen free radicals or the antioxidant competence of maternal physiology. Lifestyle factors e.g. inadequate dietary intake of antioxidants, calcium and vitamins (especially vitamin E) or physical inactivity during gestation may contribute to the coexistence and correlation of obesity and PET additionally, through the metabolic disordered pathways (i.e., by raised oxidative stress) [24-30].
A previously performed case-control research study of 55 PET gestations and 165 control gestations at 30 weeks of gestational age it was displayed raised body mass index was correlated with 1.7-fold raise in PET risk which is in harmony with findings of the current research study which displayed 2.2 fold raise in risk of PET in gestations with raised BMI. They demonstrated that around one third of the total effect of body mass index on preeclampsia risk is mediated via inflammation and triglyceride serum levels. They emphasized additionally that inflammation was a more crucial mediator than triglycerides by analyzing serum levels of C-reactive protein and triglycerides [1,3,7].
Another research prospective study displayed that the risk of PET development rose dramatically from a pre-conception BMI of 15 kg/m2 to a BMI of 35 kg/m2.The risk of PET development was roughly doubled at a BMI of 26 kg/m2, tripled at a BMI of 30 kg/m2, and halved at a BMI of 18 kg/m2 .A previously conducted research in a retrospective manner the BMI was calculated in 1067 women with a history of PET and 1063 control cases. After verification of exclusion criteria and matching for confounding factors, 687 gestations with a history of preeclampsia and 601 controls remained for statistical analyses [10,13,15]. They concluded and displayed that the rise in BMI was correlated with a raised risk of developing PET as the overweight women (BMI ≥25 and <30 kg/m2) had a 2-fold risk and the obese women (BMI ≥30 kg/m2) had a 3.2-fold risk of developing preeclampsia when compared with women of normal weight (BMI ≥15.5 and <25 kg/m2) [16,17,20].
Additionally another research concluded that the risk of PET doubled with each 5-7 kg/m2 rise in pre-coception body mass index Another research group performed a prospective population-based research cohort study of 3,480 women with morbid obesity (BMI greater than 40 kg/m2) and 12,698 women with a BMI between 35.1 kg/m2 and 40 kg/m2 had compared them with normal-weight women (BMI 19.8-26 kg/m2). They displayed that the morbidly obese mothers when compared to the normal-weight mothers had an increased risk of PET development and the correlations were similar for women with BMI between 35.1and 40 but to a lesser degree [21,22,25].
In a research published in the AJOG 2009 Wolk R et al., observed the pregnancies of 385 women from the UK and the Netherlands of all the females who were first time mothers and were obese. The research group displayed that obese first-time mothers had an 11.7 % risk of developing PET. In comparison, healthy-weight mothers have a 2 % risk of developing PET. In a research previously conducted of some atherogenic markers in PET had displayed that serum triglyceride, total cholesterol, low density lipoprotein cholesterol and Apo lipoprotein B were statistically significantly higher in PET research group than the normally pregnant group and non-pregnant cases. So a possible correlation might exist between the dyslipidaemia and the occurrence of PET which could agree with our research taking into account the fact that obesity is usually correlated with dyslipidaemia. A population-based research study of 159,072 singleton births in U.S.A. reveled that not only obese women (pre pregnancy body mass index [BMI] ≥ 30.0), but also overweight women (preconception BMI=25.0–29.9) were at a significantly higher risk for preeclampsia (Odds ratio 2.0 and 3.3, respectively) than women with a preconception BMI of less than 20.0 [26,27].
A British population-based research study of 287,213 births demonstrated that the incidence of PET was statistically significantly higher in obese women (pre pregnancy BMI≥30.0; Odd ratio 2.14)as well as overweight women (pre pregnancy BMI=25.0–29.9; Odd ratio 1.44) than in women with a pre pregnancy BMI of 20.0-24.9 .A population-based research of 972,806 births in Sweden clearly demonstrated that obese women (pre pregnancy BMI 29.1-30.0, pre pregnancy BMI 35.1-40.0, pre pregnancy BMI >40) were at a significantly higher risk of developing PET (Odds ratio 2.62, 3.90 and 4.82, respectively) than women with a pre coception BMI of 19.8-26.0 .In multivariable research analysis, obese females (BMI > 29.0 kg/m(2)) had 2.5 times the risk of hypertensive gestation (95% confidence interval [CI], 1.3-4.8) and 2.7 times the risk of PET development(95% CI, 1.2-5.8), in comparison with women whose BMI was 19.8 to 26.0 kg/m(2). Women with excessive gestational weight gain had a 3-fold increased risk of a hypertensive disorder of pregnancy (95% CI, 1.1-7.2) and a 4-fold risk of preeclampsia (95% CI, 1.2-14.5), in comparison with women achieving optimal weight gain guidelines [28-30].
Conclusion
Our research group concluded that increased BMI in this research was correlated with 2.2 fold increased risk of PET than the general population. This risk is confirmed by that reported in other research studies.
- Austin AM, Hill AG, Fawzi WW (2013) Maternal obesity trends in Egypt 1995-2005. Matern Child Nutr 9: 167-179. Link: https://bit.ly/2Ip16Eu
- Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, et al. (2014) Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med 6: 245ra92. Link: https://bit.ly/2rPZqss
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, et al. (2010) Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 116: 402–414. Link: https://goo.gl/yzMMWH
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2011) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473-482. Link: https://goo.gl/CaFjgi
- Chen CW, Jaffe IZ, Karumanchi SA (2014) Pre-eclampsia and cardiovascular disease. Cardiovasc Res 101: 579-586. Link: https://goo.gl/N84272
- Cindrova-Davies T, Herrera EA, Niu Y, KingdomJ, Giussani DA, et al. (2013) Reduced cystathionine g-lyase and increased miR- 21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am J Pathol 182: 1448–1458. Link: https://goo.gl/dmYVPA
- Dai B, Liu T, Zhang B, Zhang X, Wang Z (2013) The polymorphism for endothelial nitric oxide synthase gene, the level of nitric oxide and the risk for pre-eclampsia: A meta-analysis. Gene 519: 187–193. Link: https://goo.gl/LY58Yu
- de Jesus GR, de Jesus NR, Levy RA, Klumb EM (2014) The use of angiogenic and antiangiogenic factors in the differential diagnosis of pre-eclampsia, antiphospholipid syndrome nephropathy and lupus nephritis. Lupus 23: 1299–1301. Link: https://goo.gl/zE3wj3
- Fox S (2013) Early- and Late-Onset Preeclampsia: 2 Different Entities?. Medscape Medical News. Link: https://goo.gl/WbXEBo
- Hawkins TLA, Roberts JM, Mangos GJ, Davis GK, Roberts LM, et al. (2012) Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG 119: 484-492. Link: https://goo.gl/r1tk1J
- Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, et al. (2014) Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 25: 717-725. Link: https://goo.gl/Em5Hyr
- Keiser SD, Boyd KW, Rehberg JF, Elkins S, Owens MY, et al. (2012) A high LDH to AST ratio helps to differentiate pregnancy-associated thrombotic thrombocytopenic purpura (TTP) from HELLP syndrome. J Matern Fetal Neonatal Med 25: 1059-1063. Link: https://goo.gl/ycY4kB
- Kulandavelu S, Whiteley KJ, Qu Dawei, Mu Junwu, Bainbridge SA, et al. (2012) Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension 60: 231–238. Link: https://goo.gl/wtoz8J
- Linzke N, Schumacher A, Woidacki K, Croy BA, Zenclussen AC (2014) Carbon monoxide promotes proliferation of uterine natural killer cells and remodeling of spiral arteries in pregnant hypertensive heme oxygenase-1 mutant mice. Hypertension 63: 580–588, Link: https://goo.gl/CQNQqo
- Lisonkova S, Joseph KS (2013) Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 209: 544. Link: https://goo.gl/F2CJGH
- Peacock WF, Hilleman DE, Levy PD, Rhoney DH, Varon J (2012) A systematic review of nicardipine vs labetalol for the management of hypertensive crises. Am J Emerg Med 30: 981-993. Link: https://goo.gl/Wa8yA1
- Quillon A, Fromy B, Debret R (2015) Endothelium microenvironment sensing leading to nitric oxide mediated vasodilation: A review of nervous and biomechanical signals. Nitric Oxide 45: 20-26. Link: https://goo.gl/PBteUp
- Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, et al. (2012) Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125: 911-919. Link: https://goo.gl/BRihQm
- Rana S, Schnettler WT, Powe C, Wenger J, Salahuddin S, et al. (2013) Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 32: 189-201. Link: https://goo.gl/bY7Gqw
- Thilaganathan B (2016) Placental syndromes: getting to the heart of the matter. Ultrasound Obstet Gynecol 49: 7-9. Link: https://goo.gl/2DTGhw
- Osol G, Bernstein I (2014) Preeclampsia and maternal cardiovascular disease: consequence or predisposition? J Vasc Res 51: 290-304. Link: https://goo.gl/fTvHhM
- Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M (2013) Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update 19: 391–405. Link: https://goo.gl/CA4Qio
- Kliman HJ, Sammar M, Grimpel YI (2012) Placental Protein 13 and decidual zones of necrosis: an immunologic diversion that may be linked to preeclampsia. Reprod Sci 19: 16-30. Link: https://goo.gl/u6hXbY
- Than NG, Romero R, Balogh A, Karpati E, Mastrolia SA, et al. (2015) Galectins: double edged swords in the crossroads of pregnancy complications and female reproductive tract inflammation and neoplasia. J Pathol Transl Med 49: 181-208. Link: https://goo.gl/ZxMcSs
- Gizurarson S, Sigurdardottir ER, Meiri H, Huppertz B, Sammar M, et al. (2016) Placental Protein 13 administration to pregnant rats lowers blood pressure and augments fetal growth and venous remodeling. Fetal Diagn Ther 39: 56–63. Link: https://goo.gl/2AwoJP
- Than NG, Romero R, Xu Y, Erez O, Xu Z, et al. (2014) Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta 35: 855-865. Link: https://goo.gl/EsDtQ6
- Hahn S (2015) Preeclampsia - will orphan drug status facilitate innovative biological therapies?. Front Surg 2: 1-4. Link: https://goo.gl/t2qQsN
- Bogacz A, Procyk D, Bartkowiak-Wieczorek J, Majchrzycki M, Dziekan K (2016) Analysis of the gene polymorphism of aldosterone synthase (CYP11B2) and atrial natriuretic peptide (ANP) in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 197: 11-15. Link: https://goo.gl/R4xZ7T
- O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, et al. (2016) Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol 214: 1-103. Link: https://goo.gl/agQSRL
- Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, et al. (2016) Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med 374: 434-443. Link: https://goo.gl/2pK2ZL
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
