Journal of Gynecological Research and Obstetrics
Distribution and immunoexpression of LOX, PCP and Myofibroblasts in Vaginal Tissue of Postmenopausal Women with and without Pelvic Organ Prolapse
Christos Kleanthis1*, Kondi-Pafiti A2, Liapis E1, Lambrinoudaki Ε1 and Vakas P1
2Department of Aretaieion University Hospital, 76 Vasilissis Sofias Avenue, 11528 Athens, Greece
Cite this as
Kleanthis C, Kondi-Pafiti A, Liapis E, Lambrinoudaki Ε, Vakas P (2017) Distribution and immunoexpression of LOX, PCP and Myofibroblasts in Vaginal Tissue of Postmenopausal Women with and without Pelvic Organ Prolapse. J Gynecol Res Obstet 3(2): 056-060. DOI: 10.17352/jgro.000039Aim: of the study was to investigate the difference of distribution and expression of the lysyl oxidase enzymes (LOX), the pro-collagen C proteinase (PCP) and the myofibroblasts in the vaginal tissue of post-menopausal women, with and without prolapse.
Materials and Methods: This was a prospective closely matched controlled clinicopathological study. Samples were obtained from the anterior vaginal wall of postmenopausal patients that underwent hysterectomy for benign conditions. The immunohistochemical study of the samples was performed on paraffin embedded specimens with the semi-automatic Ventana method according to the manufacturer’s instructions. We have also examined the correlation between prolapse and parity, age and body weight in the same population.
Results: We have examined vaginal tissue from 44 postmenopausal patients: 21 with prolapse and 23 without (control group). LOX enzymes were observed in all samples, but they were strongly expressed only in the control group (P<0.005). Similarly PCP and myofibroblasts were positive in all tissues but no statistically important difference in their expression was observed between the two groups. Linear correlation between older age and higher parity with prolapse, was observed. There was no correlation between prolapse and body weight.
Conclusion: LOX enzymes are strongly expressed in the vaginal tissue of postmenopausal women without prolapse and may play an important role in the pathophysiology of POP. There is no statististical difference in the expression of PCP and fibroblasts between the control group and the patients with prolapse. Higher parity and older age are associated with prolapse, but the same doesn’t apply for higher body weight.
Introduction
Pelvic floor dysfunction and the resulting pelvic organ prolapse (POP) remain a global health problem, affecting the quality of life of female patients of all ages [1-3]. Prolapse is currently one the most frequently met indications for a gynecological operation, especially during the menopausal and postmenopausal period of life. Unfortunately up to 30% of patients that undergo surgery for POP return to their physician with recurrent symptoms [3]. Despite its high incidence, the precise pathophysiology of POP is still not entirely clear. It appears that multiparity, older age, higher body weight, obstructive lung disease and chronic conditions that result to the augmentation of the intra-abdominal cavity are some of the risk factors that lead to the appearance of prolapse [4,5].
On the other hand prolapse in nulliparous individuals as well as in patients with none of the aforementioned risk factors may lead us to speculate that there might be a genetic background to its apperence [6-8]. This speculation can be further supported by the higher incidence of prolapse in women whose mother (3.2 times higher) or sister (2.4 times higher) presented this condition [9]. Higher incidence of prolapse has also been shown in patients with hip disorders [10]. Hence it is probable that other factors affecting the connective tissue structure might also be involved.
Jackson was the first to introduce the above hypothesis. He has studied the content and structure of collagen fibers in the connective tissue from pelvic floor samples of patients with and without POP [11]. The pelvic floor is the lower border of the abdomen and it refrains the pelvic organs from prolapsing, via two main mechanisms: pelvic floor muscle contraction and the vaginal ligaments that are anchored on the bony pelvis. The collaboration of these two confers the normal function to the pelvic floor during coitus, labour, urination and defecation [12].
Vaginal wall consists of four layers: a superficial stratified squamous, a sub-epithelial layer of dense connective tissue containing mainly elastic and collagen fibers, a layer of smooth muscle fibers and a layer of loose connective tissue. The dense connective tissue and the smooth muscles form the two main layers of support of the vagina. The dense connective tissue apart from some fat cells and some histiocytes, mainly contains myofibroblasts that are responsible for the production of the extracellular matrix. The later consists of collagen and elastic fibers organized in a bed of glycoproteins, proteoglycans, hyaluronic acid and smooth muscle fibers [13].
Elastin and collagen fibers appear to have an important role in the support mechanism of the vagina as well. The quantity and quality of these fibers constantly change and they depend on the frequency of their production, remodeling and decomposition. Many factors are enrolled in their biogenesis, three of which are the Lysyl Oxidase family enzymes (LOX), the Procollagen C Proteinase (PCP) and the myofibroblasts. LOX are responsible for the conversion of the elastin and collagen immature forms to tropoelastin and tropocollagen [14]. PCP take over the remodeling of collagen in the cytoplasm [15]. Myofibroblasts finally, depending on their concentration in the tissues and their potential to produce collagen, affect the resilience of the vaginal tissue to tensile force [16]. There appears to be an association between estrogens and reduced function of myofibroblasts, leading to reduced production of collagen [17].
Aim of this study was to investigate the differences of distribution and expression of LOX, PCP and Actin, corresponding to myofibroblasts, in the vaginal tissue of postmenopausal women with and without POP.
Materials and Methods
Our study has been reviewed and approved by the research ethics committee of the Aretaieion University Hospital in Athens, Greece (protocol number M-3/09-11-2010). We have recruited a total of 44 postmenopausal patients, 21 with prolapse and 23 without prolapse. Patients without prolapse constituted group 1 (control group) and patients with prolapse group 2.
All our patients have undergone hysterectomy because of benign disease. Exclusion criteria were: malignant disease, hormone replacement therapy, pelvic radiation and pelvic inflammatory disease. The menopausal status of our patients has been defined as one year of amenorrhoea above the age of 50. None of the patients in either groups have used a vaginal pessary or ring before surgery.
Demographics like body weight and age and details from the obstetric history and the gynecological examination were retrieved from the Aretaieion Hospital Patients Archives. All patients were given code numbers so that their personal information data were kept safe.
Tissue samples were obtained from the anterior vaginal wall of all patients, after resection with Metzembaum scissors. The width of the samples varied between 0.5 and 1cm and the thickness was around 1cm. All samples were studied at the Aretaieion University Hospital Pathology Laboratory.
The immunohistochemical study was performed with the semi-automatic Ventana method, on paraffin embedded tissue sections, measuring 3-5 μμ. Antibodies Anti-Lox Polyclonal, Novus Biological (Rabitt, 1/200), PCP anti-BMP-1 (Abcam BMP1-1/1000) and Smooth Muscle Actin polyclonal Ab, Rabitt, clone 1A4 (Genemed, 1/50) were used to study LOX, PCP and myofibroblasts respectively. Microscopic examination was conducted with an Olympus CX-1 microscope. A semi-quantitative method has been used to classify the immunostain, as positive or negative.
LOX were considered to be positive (+) when there was a diffuse positive reaction observed in the connective tissue layer of the tissues. LOX was also found to be positive in the nuclei of squamous cells.
PCP was considered to be positive (+) when there was a diffuse positive reaction observed in the connective tissue or the muscle layer of the tissues.
The presence of myofibroblasts was evaluated according to the positive nuclear/cytoplasmatic immunohistochemical reaction of actin, in the cells of the connective tissue or the muscle layer of the tissues.
Results
Group 1 included both nulliparas and multiparas, of age between 55 and 75 years old and of body weight between 59 and 81 kilograms.
Respectively group 2 included both nulliparas and multiparas, of age between 54 and 80 years old and of body weight between 56 and 80 kilograms.
Correlation of age, body weight and parity with prolapse was also investigated. Separate groups of: age 50 to 59, 60 to 69, 70 to 79 and 80 to 89 years old, body weigh 50 to 59, 60 to 69, 70 to 79 and 80 to 89 kilograms and parity including nulliparas, patients with one previous vaginal delivery and patients with two or more vaginal deliveries, were studied.
Statistical analysis was performed with Pearson Chi-Square test and the Contingency Coefficient.
The results of the immunohistochemical study are shown below
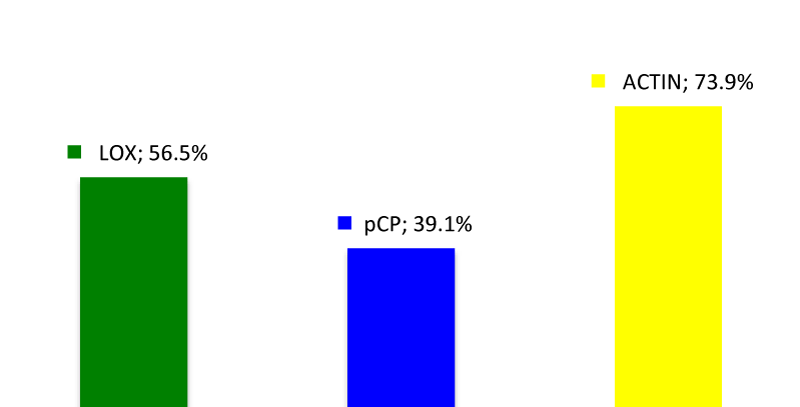
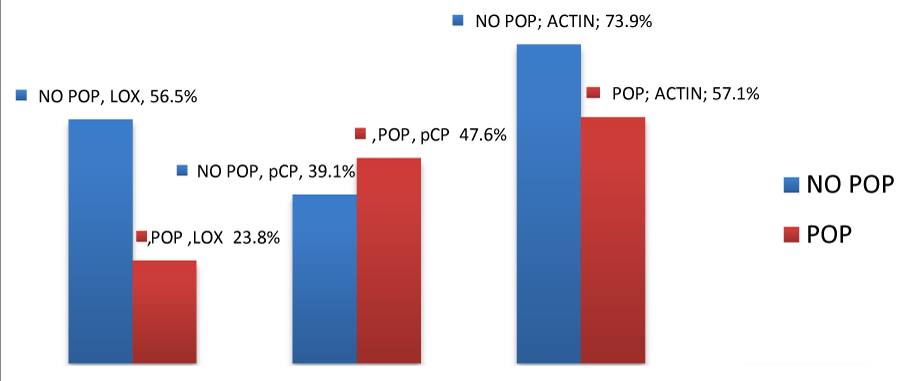
Group 1: The expression of LOX was positive in 13 out of the 23 samples (56.5%). PCP were found to be positive in 9 out of the 23 samples (39.1%) and actin was positive in 17 out of the 23 samples (73.9%).
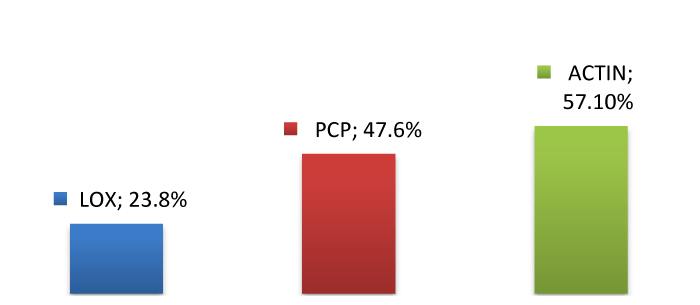
Group 2: The expression of LOX in the subcutaneous layer was positive in 5 out of 21 samples (23.8%). PCP reaction was positive in 10 out of 21 samples (47.6%). The later was observed only in the subcutaneous layer and not in the epithelial or the smooth muscle layer. Actin expression was positive in 12 out of 21 samples (57.1%) and it was observed in the muscle layer as well as around blood vessels.
The above results are shown in figures 1-3.
Statistical methodology
The results of the study underwent statistical analysis with the Pearson Chi Square Test and the Contingency Coefficient was used to analyze correlations between qualitative variables. Statistical significance was set at P<0.05. The methodology was conducted using IBM SPSS v 15.0. Results are shown in table 1.
LOX expression in the vaginal tissue of samples from the control group was found to be statistically significant (p=0.027) compared to the expression of PCP and Actin (both p>0.05).
From the qualitative variables it has been shown that age (p=0.011) and parity (p=0.016) appear to have statistically significant linear correlation, meaning that as age advances and the number of deliveries becomes higher, the risk of prolapse in a patient becomes greater. On the other hand weight did not appear to affect the above risk (p=0.346>0.05).
Discussion
Pelvic organ prolapse remains one of the most frequently met indications for gynecological operations since it reduces the quality of life of the patients with this condition [1-3]. Although POP is quite common among the female population, its pathophysiology is yet to be clarified. Many of the recognized risk factors that lead to the appearance of POP are not always identified in the group of patients suffering from it, therefore leading us to hypothesize the existence of a genetic predisposition or other factors arising from the connective tissue [4-10]. The later consists of elastin and collagen fibers, components that play a vital role in the support mechanism of the tissues [13].
The contraction of the pelvic floor muscles and the resilience of the vaginal ligaments render the pelvic floor a very good support mechanism for the pelvic viscera [12]. Vaginal connective tissue contains collagen fibers, whose metabolism depends on several factors of which LOX, PCP and myofibroblasts have been identified. Disruption of the normal function of these components may lead to inadequate quantitative or qualitative production of collagen in the pelvic floor connective tissue and consequently reduced support of pelvic viscera and prolapse [14,15].
In our study the expression of LOX enzymes was positive for both groups of patients with (23.8%) and without prolapse (56.5%), but it was statistically important only in the control group (p=0.027). Similar results were drawn from other studies like the one from M. Alarab et al., which was conducted on vaginal tissue samples from premenopausal patients with and without prolapse [18]. In other studies the concentration of LOX mRNA in the Cardinal and the Uterosacral ligaments of patients with prolapse appeared to be reduced compared to the control group [18,19]. Additionally, M. Bortolini et al., as well as H Kufaishi et al., drew similar results regarding the expression of LOX in vaginal tissue, in their studies [20-23]. Finally studies on mice have proven a strong correlation between LOX disorder and connective tissue defective function [24,25].
PCP and Actin expression was also apparent in both groups of patients, but none of the two gave a statistically important reaction in any of the two groups.
PCP was more strongly expressed in the group of patients with prolapse compared to the control group. This result contradicts results from other studies, like the one conducted by M. Bortolini et al., where PCP reaction was stronger in the control group [21,22,26]. Nevertheless the menopausal status alone could explain the overall reduction of PCP expression, as M. Bortolini et al., conclude [22,26,27].
The expression of Actin was present in both patient groups with no statistically important difference, as previously mentioned. Nonetheless Actin expression in the control group is as high as 73,9%, percentage much higher than the one met in the prolapse group 57.1%. This result is in accordance with the one drawn from Kocku et al., in their study regarding the reduction of myofibroblast population in the prolapse group of patients [28].
In our study we have also investigated the correlation of age, parity and body weight with the appearance of prolapse. There was a statistically important linear correlation between advancing age and higher parity with prolapse. Weight did not appear to be associated with POP contradicting results from other studies [4,5].
We identified as limitation of our study, the absence of details regarding the findings of the gynecological examination, like the classification and grading of pelvic organ prolapse, the improvement of which could lead to higher quality results.
Conclusion
We have studied the expression of LOX, PCP and Actin in 44 postmenopausal patients, 21 with prolapse, 23 without prolapse. We also tried to identify any correlation between age, parity and body weight and the appearance of pelvic organ prolapse in the above population.
The conclusions drawn are as below:
1. Prolapse is associated with higher parity and advancing age but not with body weight
2. There is a statistically important positive immunohistochemical reaction of the LOX enzymes in patients without prolapse
3. There is no statistically important immunohistochemical reaction of PCP and Actin in patients with or without prolapse.
Improvement in the more detailed description of findings from the gynecological examination of patients will confer a better categorization of our data, which will lead to a higher quality study results.
Further investigation not only of the cellular population of myofibroblasts but also of their contractile properties and their ability to produce components of the extracellular matrix remains a field for future research, that will lead to a better understanding of the nature and potential of these cells as well as their role in pelvic organ prolapse [12,28-30].
- Bump RC, Norton PA (1998) Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am 25: 723–746. Link: https://goo.gl/4CLVMP
- Dietz HP, Haylen BT, Vancaillie TG (2002) Female pelvic organ prolapse and voiding function. Int Urogynecol J Pelvic FloorDysfunct 13: 284–288. Link: https://goo.gl/OKXvks
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse andurinary incontinence. Obstet Gynecol 89: 501–506. Link: https://goo.gl/3bwhjO
- Bai SW, Choe BH, Kim JY, Park KH (2002) Pelvic organprolapse and connective tissue abnormalities in Korean women.J Reprod Med 47: 231–234. Link: https://goo.gl/soIDF9
- Mant J, Painter R, Vessey M (1997) Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol 104: 579–585. Link: https://goo.gl/E7kBWv
- Buchsbaum GM, Duecy EE, Kerr LA, Huang LS, Perevich M, et al. (2006) Pelvic organ prolapse in nulliparous women and their parous sisters. Obstet Gynecol 108: 1388–1393. Link: https://goo.gl/j0H2dk
- Nikolova G, Lee H, Berkovitz S, Nelson S, Sinsheimer J, et al (2007) Sequence variant in the laminin gamma 1 (LAMC1) gene associated with familial pelvic organ prolapse. Human Genetics 120: 847–856. Link: https://goo.gl/Y5XS3N
- Twiss C, Triaca V, Rodriguez LV (2007) Familial transmission of urogenital prolapse and incontinence. Curr Opin Obstet Gynecol 19: 464–468. Link: https://goo.gl/gF9Rba
- Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, et al (1999) Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol 82: 63–67. Link: https://goo.gl/BEq3BR
- Νorton PA, Baker JE, Sharp HC, Warenski JC (1995) Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol 85:225–28. Link: https://goo.gl/v8poHu
- Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, et al. (1996) Changes in metabolism of collagen in genitourinary prolapse. Lancet 347: 1658–1661. Link: https://goo.gl/nq8vSt
- Kerkhof MH, Hendriks L (2009) Brölmann HAM Changes in connective tissue in patients with pelvic organ prolapse – a review of the current literature. Int Urogynecol J 20: 461-474. Link: https://goo.gl/0fHsmt
- Goh JT (2003) Biomechanical and biochemical assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol 15: 391–394. Link: https://goo.gl/14wxzH
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, et al. (2004) Elastic fiber homeostasis requires Lysyl oxidase-like protein. Nat Genet 36: 178-182. Link: https://goo.gl/PIv4DB
- Greenspan DS (2005) Biosynthetic processing of collagen molecules. Top. Curr. Chem. 247: 149-183. Link: https://goo.gl/KaGxwt
- Kokcu A, Yanik F, Cetinkaya M, Alper T, Kandemir B, et al. (2002) Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet 2002 Apr 266: 75-78. Link: https://goo.gl/WIvopv
- Liu YM, Choy KW, Lui WT, Pang MW, Wong YF, et al. (2006) 17-beta estradiol suppresses proliferation of fibroblasts derived from cardinal ligaments in patients with or without pelvic organ prolapse. Hum. Reprod 21: 303-308. Link: https://goo.gl/MRVWaF
- May Alarab, Maria AT Bortolini, Harold Drutz, Stephen Lye, Oksana Shynlova (2010) LOX family enzymes expression in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Int Urogynecol J Nov 21: 1397-404. Link: https://goo.gl/nHlSgx
- Kobak W, Lu J, Hardart A, Zhang C, Stanczyk F, et al. (2005) Expression of lysyl oxidase and transforming growth factor B2 in women with severe pelvic organ prolapse. J Rep Med 50: 827-831. Link: https://goo.gl/PRpYmZ
- Zhang SQ, Zhang LL, Yu H (2008) Expression of elastin lysyl oxidase and elafin in the cardinal ligament of women with pelvic organ prolapse. Zonghua Fu Chan Ke Za Zhi 43: 675-679. Link: https://goo.gl/VTq9NA
- Bortolini MA, Shynlova O, Oleksiv N, Drutz H, Lye S, et al. (2010) Procollagen C Proteinase and LOX enzymes expression in vaginal tissue of women with and without pelvic organ prolapse.
- Bortolini M, Synlova O, Oleksiv N, Drutz H, Lye S, et al. (2011) Expression of enzymes regulating extracellular matrix biogenesis in vaginal tissue of women with and without pelvic organ prolapse. Link: https://goo.gl/i5DlIO
- Kufaishi H, Alarab M, Drutz H, Lye S, Shynlova O (2016) Comparative Characterization of Vaginal Cells Derived From Premenopausal Women with and Without Severe Pelvic Organ Prolapse. Reprod Sci Jul; 23: 931-943. Link: https://goo.gl/2sGgqH
- Liu X, Zhao Y, Pawlyk B, Damaser M, Li T (2006) Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol 168: 519-528. Link: https://goo.gl/5yhTSV
- Alperin M, Debes K, Abramowitch S Meyn L, Moalli PA (2008) LOXL1 deficiency negatively impacts the biomechanical properties of the mouse vagina and supportive tissues. Int Urogynecol J pelvic Floor Dysfunct 19: 977-986. Link: https://goo.gl/SZ5EIL
- Bortolini MA, Shynlova O, Drutz H, Girao MJBC, Castro R, et al. (2011) Expression of Bone Morhogenetic Protein-1 in vaginal tissue of women with severe pelvic organ prolapse. Am J Obstet Gynecol 204: 1-8. Link: https://goo.gl/ZQb4vw
- Synlova O, Bortolini M, Alarab M (2013) Genes responsible for vaginal extracellular matrix metabolism are modulated by women’s reproductive cycle and menopause. Int Braz J Urol 39: 257-67. Link: https://goo.gl/DwRqVd
- Kokcu A, Yanik F, Cetinkaya M, Alper T, Kandemir B, et al. (2002) Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol 344 Obstet 266: 75–78. https://goo.gl/xb9nzT
- Wu MP. (2010) Regulation of Extracellular Matrix remodeling associated with Pelvic Organ Prolapse. J Exp Clin Med 2: 11-16. Link: https://goo.gl/CrQd77
- Vetuschi A, D’Alfonso A, Sferra R, Zanelli D, Pompili S, et al. (2016) Changes in muscularis propria of anterior vaginal wall in women with pelvic organ prolapse. Eur J Histochem. Link: https://goo.gl/EgZGHe
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
