Journal of Gynecological Research and Obstetrics
Follicular Variant of Papillary Thyroid Carcinoma Arising in a Mature Cystic Teratoma of the Ovary
Ozlen Emekci Ozay1, Ali Cenk Ozay1*, Berrin Acar2, Meral Koyuncuoglu3 and Abdurrahman Comlekci4
2Dokuz Eylul University Department of Obstetrics and Gynecology, Izmir, Turkey
3Dokuz Eylul University Department of Pathology, Izmir, Turkey
4Dokuz Eylul University Department of Endocrinology and Metabolism, Izmir, Turkey
Cite this as
Ozay OE, Ozay AC, Acar B, Koyuncuoglu M, Comlekci A (2016) Follicular Variant of Papillary Thyroid Carcinoma Arising in a Mature Cystic Teratoma of the Ovary. J Gynecol Res Obstet 2(1): 078-080. DOI: 10.17352/jgro.000026Mature cystic teratomas form approximately 20% of all ovarian tumours. Of these, approximately 15% include benign thyroid tissue. When thyroid tissue comprises more than 50% of the ovarian teratoma, it is termed ‘‘struma ovarii’’. The exact incidence of malignancy in struma ovarii is hard to evaluate, because of its uncommon nature. The aim of this study is to report a rare case of follicular variant of papillary thyroid carcinoma arising in mature cystic teratoma of the right ovary. A 43-year-old premenopausal female, gravida 3, para 2 was admitted to Dokuz Eylul University Hospital with an incidental ultrasonographic finding of a complex solid echogenic right ovarian mass during her annual gynecological examination. The preoperative diagnosis was dermoid cyst. The patient underwent laparoscopic surgery. At exploration, the left ovary, the left fallopian tube, and the uterus were normal. The right ovary was enlarged, white and smooth ovarian semisolid mass was noted. Histopathologic evalutation revealed a tumor of follicular variant of papillary thyroid carcinoma arising in a mature cystic teratoma. The case was discussed at the multidisciplinary oncology meeting. Postoperatively, the thyroid function tests, thyroid gland sonogram were requested. The results were normal. A total body scanning with I131 was done. The test revealed no residual intrabdominal/pelvic carcinoma. Total abdominal hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, bilateral pelvic-paraaortic lymphadenectomy, partial omentectomy and peritoneal biopsies were performed. In conclusion, the treatment modalities for malignancy in struma ovarii or mature cystic teratoma depend on the stage of the disease and fertility desire.
Introduction
Mature cystic teratomas form approximately 20% of all ovarian tumours. Of these, approximately 15% include benign thyroid tissue. When thyroid tissue comprises more than 50% of the ovarian teratoma, it is termed ‘‘struma ovarii’’. The exact incidence of malignancy in struma ovarii is hard to evaluate, because of its uncommon nature [1,2].
The peak age of malignant transformation in struma ovarii is the fifth decade [3]. In addition to the usual signs and symptoms of a pelvic mass, clinical signs of hyperthyroidism are seen in 5-8% of cases. Thyrotoxicosis appears in 5–15% of cases. Ascites (17%) and Meigs’ syndrome (5%) can be seen [1-4].
Most cases of malignant struma ovarii are subclinical. Imaging studies generally diagnose an ovarian mass. After the operation, the pathology reveales the diagnosis and it is usually incidental [5,6].
The aim of this study is to report a rare case of follicular variant of papillary thyroid carcinoma arising in mature cystic teratoma of the right ovary.
Case Report
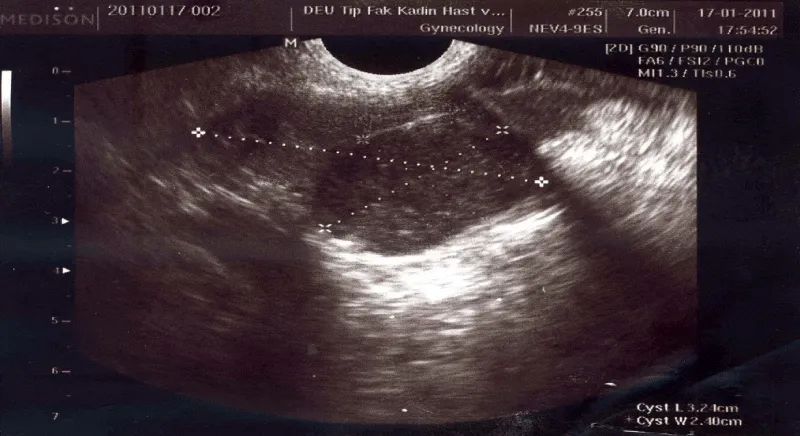
A 43-year-old premenopausal female, gravida 3, para 2 was admitted to Dokuz Eylul University Hospital with an incidental ultrasonographic finding of a complex solid echogenic right ovarian mass during her annual gynecological examination. She had a history of a laparoscopic left ovarian cystectomy for an endometrioma. In patient’s family history, her mother had endometrial cancer. No history of thyroid disease was found. Vaginal ultrasonography confirmed a 3.24 x 2.40 cm complex solid echogenic mass with a clearly demarcated border in the right ovary (Figure 1). No ascites was detected in the pouch of Douglas. All laboratory tests including thyroid function tests as well as tumor markers were normal. The preoperative diagnosis was dermoid cyst. The patient underwent laparoscopic surgery. At exploration, the left ovary, the left and the right fallopian tube, and the uterus were normal. The right ovary was enlarged, white and smooth ovarian semisolid mass was noted. There were no visible pathologic findings in the pelvic and abdominal cavity. The cystic mass was excised. The postoperative recovery was good and she was discharged the next day.
Histopathology
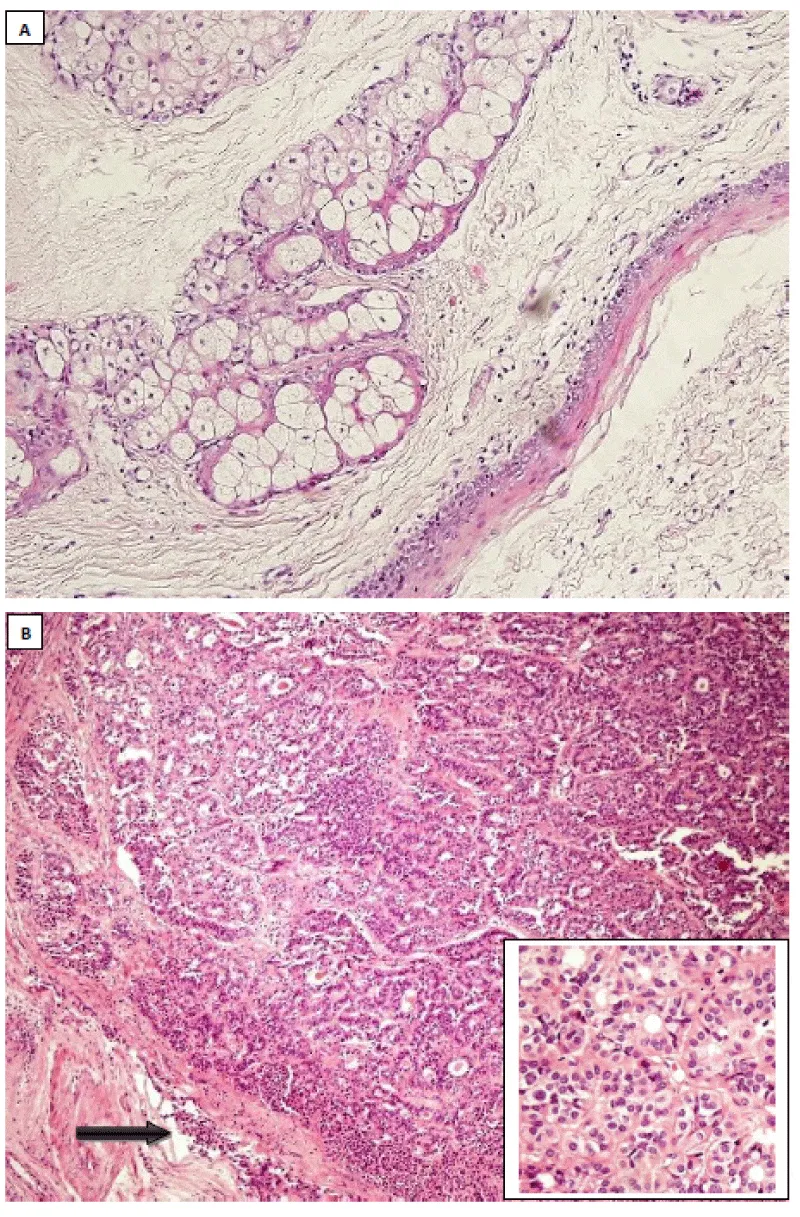
Macroscopically, the specimen consisted of membranous tissues measuring between 3x2x0.5 cm and 5x4x2 mm consistent with fragments of a cystic mass lesion’s wall, as well as a nodular, well-demarcated gray-white tissue with a diameter of approximately 1 cm. Histological evaluation of the specimen revealed mature teratomatous areas (Figure 2A). Macroscopically described nodular area consisted of malignant thyroid tissue, showing features of follicular variant of papillary thyroid carcinoma (Figure 2B). The tumor was not well-circumscribed, with focal capsular invasion. Immunohistochemical staining revealed thyroid transcription factor-1 (TTF-1), thyroglobulin, CK7, CK19, and focal high molecular weight cytokeratin (HMWCK) positivity. The chromogranine A was negative. The tumor was considered as follicular variant of papillary thyroid carcinoma arising in a mature cystic teratoma.
The case was discussed at the multidisciplinary oncology board. Postoperatively, the thyroid function tests, thyroid gland sonogram were requested. The results were normal. A total body scanning with I131 was done. The test revealed no residual intrabdominal/pelvic carcinoma. Total abdominal hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, bilateral pelvic-paraaortic lymphadenectomy, partial omentectomy and peritoneal biopsies were performed. Normal findings were reported by histopathology. Total thyroidectomy was performed to exclude a primary source. Thyroid gland was evaluated as normal in histology. Three months later, evaluation of pelvic magnetic resonance imaging was normal. Eight months after gynecological operation, radioactive I131 ablation treatment was performed by a dose of 100mCi. Thyroglobulin level was followed to evaluate the recurrence at 3-month intervals. The patient was on follow- up for 30 months without recurrence.
Discussion
Struma ovarii is a significantly specific patern, monodermal ovarian teratoma in which thyroid tissue is the predominant element [6]. It is found in about 2.7% of ovarian teratomas. Malignant transformation of struma ovarii is an extremely rare condition [1]. In literature, most of the data about this condition is collected from individual case reports, case series [1,2,4]. As presented in this case, the majority of cases are incidentally diagnosed [5,6-9]. Malignant struma ovarii often occurs in the fifth decade of life [3]. Patients predominantly presented with a pelvic mass (45%), abdominal pain (40%). Several patients presented with menstrual irregularities (9%), hyperthyroidism (5–8%), ascites (17%), deep vein thrombosis (4%) [2,4]. Only 5-8% of the patients with malignant struma ovarii present with clinical hyperthyroidism [1,3,4]. Intra-operative frozen section samples usually reveal a mature cyctic teratoma or benign thyroid tissue [6].
The differential diagnosis of struma ovarii includes the follicular type of granulosa cell tumor, carcinoid tumor, papillary cystadenoma, papillary cystadenocarcinoma [10]. Immunohistochemical stains for thyroglobulin, chromogranin A are necessary for the differential diagnosis. In our case, a follicular variant of papillary thyroid carcinoma with capsular invasion was found. We found a limited area with typical histological characteristics for a follicular variant of papillary thyroid carcinoma as well as positive thyroglobulin and negative chromogranin A on immunohistochemical analysis. Thus, it is considered a follicular variant of papillary thyroid carcinoma with capsular invasion arising in an ovarian mature cystic teratoma. Metastases to the ovary from a primary thyroid cancer should be excluded by clinical thyroid examination, thyroid functional tests and thyroid ultrasonography [10].
Metastases are indicated only in 5–6% of patients with malignant struma ovarii [8]. The tumor can disseminate with regional lymphatics to regional pelvic, para-aortic lymph nodes. Direct spread to the peritoneal cavity, the omentum , the contralateral ovary is also possible, as well as hematologic dissemination to the bone, lung, liver, and brain [8].
The management of cases of ovarian mature cystic teratoma with thyroid type malignancy is based on small cases series [10]. For this reason, the treatment of malignant struma ovarii remains controversial. The standard surgical treatment of a patient with thyroid malignancy in struma ovarii is ranged from total abdominal hysterectomy plus bilateral salpingo-oophorectomy, peritoneal washings, pelvic-paraaortic lymphadenectomy and partial omentectomy to conservative surgery. There is no gold standard and direct comparisons between treatment approaches. Following surgical staging, some authors recommend thyroidectomy and I131 radioablation [4,9].
Devaney et al. [6], reviewed 54 cases of struma ovarii, in which 13 cases were malignant struma ovarii. 11 of the 13 were papillary carcinomas of thyroid type, whereas 2 were follicular carcinoma. None of the patients received adjuvant therapy. On follow-up examination (mean follow-up interval 7.3 years), one patient had persistent disease with peritoneal involvement, but none of the patients had clinical evidence of recurrent disease.
Yassa et al. [8], suggested that treatment after surgery should be according to the aggressive activity of the tumor. Low risk (≤2 cm, confined to ovary, no worrisome histologic findings) should be managed with thyroxine therapy, pelvic imaging, and periodic measurements of serum thyroglobulin. High risk (larger carcinomas, disease outside the struma ovarii and with more aggressive histologic features) should be treated with total thyroidectomy, radioactive iodine ablation and levothyroxine suppression therapy [8].
Lara et al. [11], reported a case aged 36 years old with malignant struma ovarii in 2016. They performed a left salpingoopherectomy with omentectomy. In addition, total thyroidectomy was done. There are no consensus guidelines for the management of struma ovarii. In this case who wanted to preserve fertility, unilateral salpingo-oophorectomy, along with total thyroidectomy and radioactive iodine ablation was performed.
In our case, focus of follicular variant of papillary thyroid carcinoma was not large but there was a capsular invasion, according to the decision of our multidisciplinary board, and upon acceptance by our patient, radical gynecological surgery, total thyroidectomy with radioactive iodine ablation were performed.
In conclusion, the treatment modalities for malignancy in struma ovarii or mature cystic teratoma depend on the stage of the disease and fertility desire. The initial surgery options include unilateral oophorectomy or cystectomy; total hysterectomy, bilateral salpingooophorectomy with omentectomy and bilateral pelvic-paraaortic lymphadenectomy. The adjuvant treatment options include thyroxine, total thyroidectomy with radioactive iodine ablation or no adjuvant treatment. Long-term follow-up is recommended in all cases.
No financial relationship with the any organization. Authors have full control of all primary data. The case report was presented in FIGO World Congress, Rome 2012 as a poster presentation. All authors contributed significantly and they are in agreement with the content of the manuscript. No conflict of interest.
Disclosure
No financial relationship with the any organization. Authors have full control of all primary data. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
- Matsuda K, Maehama T, Kanazawa K (2001) Malignant struma ovarii with thyrotoxicosis. Gynecol Oncol 82: 575– 577. Link: https://goo.gl/EbupSz
- Makani S, Kim S, Gaba AR (2004) Struma ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol Oncol 94: 835–839. Link: https://goo.gl/xou0lU
- Scully RE, Young RH, Clement PB (1996) Atlas of tumor pathology—tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. 3rd series, fasicle 23, pp. 169–188. Armed Forces Institute of Pathology, Washington, D.C.
- DeSimone CP, Lele SM, Modesitt SC (2003) Malignant struma ovarii: a case report and analysis of case reported in the literature with focus on survival and 131I therapy. Gynecol Oncol 89: 543–548. Link: https://goo.gl/RkmBQl
- Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, et al. (2008) Clinical characteristics of struma ovarii. J Gynecol Oncol 19: 135–138. Link: https://goo.gl/89s5LA
- Devaney K, Snyder R, Norris HJ, Tavassoli FA (1993) Proliferative and histologically malignant struma ovarii: a clinico-pathologic study of 54 cases. Int J Gynecol Pathol 2: 333–343. Link: https://goo.gl/Lwm7ev
- Rose PG, Arafah B, Abdul-Karim FW (1998) Malignant struma ovarii: recurrence and response to treatment monitored by thyroglobulin levels. Gynecol Oncol 70: 425–427. Link: https://goo.gl/dNMBim
- Yassa L, Sadow P, Marqusee E (2008) Malignant struma ovarii. Nat Clin Pract Endocrinol Metab 4: 469–472. Link: https://goo.gl/wXnofw
- Ciccarelli A, Valdes-Socin H, Parma J, Khoo SK, Schoumans J, et al. (2004) Thyrotoxic adenoma followed by a typical hyperthyroidism due to struma ovarii: clinical and genetic studies. Eur J Endocrinol 150: 431–437. Link: https://goo.gl/EO4Ida
- Altaras M, Goldberg GL, Levin W, Darge L, Bloch B, et al. (1986) The value of cancer antigen-125 as a tumor marker in malignant germ cell tumors of the ovary. Gynecol Oncol 25: 150–159. Link: https://goo.gl/dDyZ7s
- Lara C, Cuenca D, Salame L, Padilla-Longoria R, Mercado M (2016) A Hormonally Active Malignant Struma Ovarii. Case Rep Oncol Med 2016: 2643470. Link: https://goo.gl/SuP5fR
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
