Journal of Gynecological Research and Obstetrics
Dysbiosis as a Risk Factor for Endometriosis: A Synthesis of Systematic Reviews and Meta-analyses
Viviane Cavalcanti Lins1*, Gilvandro Lins2, Leticia Vanderlei1, Maria Beatriz Ferreira1, Larissa Azevedo1, Virginia Cavalcanti3 and Carolina Bandeira1
1Paraiba School of Public Health, Executive Secretariat of Health, Brazil
2Federal University of Paraiba, Brazil
3Afya Faculty of Medical Sciences of Paraiba, João Pessoa/PB, Brazil
Cite this as
Lins VC, Lins G, Vanderlei L, Ferreira MB, Azevedo L, Cavalcanti V, et al. Dysbiosis as a Risk Factor for Endometriosis: A Synthesis of Systematic Reviews and Meta-analyses. J Gynecol Res Obstet. 2025;11(1):001-005. Available from: 10.17352/jgro.000131Copyright License
© 2025 Lins VC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Endometriosis is a chronic inflammation that represents one of the most common benign gynecological diseases. It is a condition in which endometrial-like tissue grows outside the uterine cavity, implanting itself in tissues and organs, causing pelvic pain, dysmenorrhea and infertility. The increase in Escherichia coli and bacterial endotoxins are associated with the proliferation of endometriotic lesions by activating inflammatory factors. In the cervicovaginal microbiome, the dominance of Lactobacillus is associated with gynecological and reproductive health.
Objective: To perform a synthesis of systematic reviews and meta-analyses published on the association of dysbiosis with the development of endometriosis .
Methodology: Systematic literature search in the PubMed, BVS, Scielo and Cochrane library platforms, using the descriptors Dysbiosis OR “microbiota imbalance” OR “vaginal microbiome” OR “gut microbiota” AND Bacterial Vaginosis OR “bacterial vaginosis” OR “Gardnerella vaginalis” OR “vaginal dysbiosis” AND Endometriosis OR “endometriotic lesions” OR “chronic pelvic pain” OR “ectopic endometrium” and their combinations.
Results: A total of 383 productions were found in the searched platforms, after selection stages, two articles were selected, both of which are systematic reviews or meta-analyses that met the eligibility criteria.
Conclusion: The association between endometriosis and the presence of dysbiosis in samples collected from the intestine, vagina and peritoneal fluid of patients with endometriosis was shown to be significant.
Introduction
Endometriosis is a chronic, steroid-dependent condition and one of the most prevalent benign gynecological diseases. It is characterized by the ectopic growth of tissue similar to the endometrium—composed of glands and stroma—outside the uterine cavity. These implants can establish themselves in structures such as the fallopian tubes, ovaries, peritoneum, colon, rectovaginal area, and bladder [1], triggering symptoms such as pelvic pain, dysmenorrhea, and infertility, affecting approximately 40% of women with pelvic pain and 50% of those with infertility, although 16% may remain asymptomatic [2,3].
Various theories have been proposed to explain the origin and dissemination of endometriotic implants. The theory of retrograde menstruation, first described by Sampson, et al. in 1927, is the most widely accepted; however, considering that more than 90% of women of reproductive age experience retrograde menstruation without developing the disease, it is plausible that additional factors—such as genetic predisposition, immunological alterations, and environmental influences - play a crucial role in its pathogenesis [4].
The development of endometriosis is intrinsically linked to an intense inflammatory process. Cytokines such as IL-1, IL-6, IL-8, and Tumor Necrosis Factor (TNF) promote the infiltration of peritoneal leukocytes and facilitate both the implantation and progression of endometriotic lesions [5]. Studies have shown that affected tissues exhibit an increased expression of the enzyme cyclooxygenase-2 (COX-2), whose activity is associated with various pathologies of the reproductive tract -including carcinomas, menorrhagia, and dysmenorrhea—and plays a central role in both the inflammatory and tumorigenic processes [6].
Furthermore, endometrial samples from women with endometriosis reveal a higher expression of the COX-2 gene compared to tissues from women without the disease. This increase may promote cellular proliferation, inhibit apoptosis, enhance tissue invasion, and stimulate angiogenesis as well as aromatase activity, resulting in elevated estrogen levels—a key factor in the development and maintenance of endometriosis [7].
It is also observed that women with endometriosis exhibit higher quantities of Escherichia coli and elevated levels of bacterial endotoxin in menstrual blood compared to control groups. These elements may stimulate lesion proliferation through the activation of Toll-like receptor 4 (TLR-4), while gram-negative bacteria, by activating nuclear factor kappa B (NF-κB) and COX-2, contribute to the maintenance of a highly inflammatory environment [8].
Alterations in the microbiome also play a significant role in the pathogenesis of endometriosis. In the gut, for instance, estrogen deconjugation — mediated by the enzyme β-glucuronidase present in bacteria such as Escherichia coli, Bacteroides fragilis, and Streptococcus agalactiae — allows the reabsorption of active estrogen, creating an inflammatory environment that may disrupt circulating estrogen levels and predispose to the development of the disease through the promotion of inflammation, cellular proliferation, inhibition of apoptosis, increased oxidative stress, and angiogenesis [9].
In the cervicovaginal microbiome, the predominance of Lactobacillus is essential for maintaining gynecological health, as these microorganisms produce lactic acid, reducing the vaginal pH to 4.5 or lower and inhibiting pathogen growth. Additionally, Lactobacillus spp. contribute to homeostasis by preventing colonization by pathogenic agents and stimulating the production of anti-inflammatory cytokines, as well as strengthening the epithelial barrier. In contrast, bacterial vaginosis (BV)—characterized by a reduction in Lactobacillus and an overgrowth of anaerobic bacteria—has been associated with endometriosis, as it favors the formation of resistant polymicrobial biofilms in the cervicovaginal epithelium.
Comparative studies of the endometrial microbiota indicate that, in women with endometriosis, there is an increase in genera such as Streptococcus, Gardnerella, Escherichia, Shigella, and Ureaplasma, along with an absence of Atopobium, compared to control groups. These findings support the hypothesis that a reduction in Lactobacillus within the cervicovaginal microbiome may be associated with the presence of microorganisms commonly related to BV [9]. Similarly, analyses of the peritoneal fluid microbiota, collected during laparoscopic surgeries, have shown an enrichment of Prevotella, Veillonellaceae, Atopobium, and Comamonas in women with endometriosis, suggesting that dysbiotic bacteria may ascend to the upper genital tract and contribute to the development of the disease.
In summary, endometriosis is associated with dysbiosis characterized by a reduction in beneficial microorganisms and an increase in pathogens, resulting in an inflammatory environment and compromised immune response. This dysregulation favors the elevation of pro-inflammatory cytokines and the progression of endometriotic implants, highlighting the central role of inflammation in the disease’s pathogenesis [10].
Given this scenario, the present study proposes a synthesis of published systematic reviews and meta-analyses, aiming to elucidate the underlying mechanisms linking dysbiosis to the onset of endometriosis.
Methodology
A systematic literature search was conducted to evaluate the presence of dysbiosis in the microbiome of women with endometriosis and its relationship with the development of the disease. A systematic review involves identifying, selecting, critically appraising, compiling, and presenting published evidence on topics relevant to population health. To ensure methodological transparency and reproducibility , this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.
The search strategy included the following descriptors and their combinations: Dysbiosis OR “microbiota imbalance” OR “vaginal microbiome” OR “gut microbiota”; Bacterial Vaginosis OR “bacterial vaginosis” OR “Gardnerella vaginalis” OR “vaginal dysbiosis” AND Endometriosis OR “endometriotic lesions” OR “chronic pelvic pain” OR “ectopic endometrium”.
These terms were applied to the databases PubMed, BVS, SciELO, and Cochrane Library, as shown in Table 1 . The selection and analysis of articles were conducted independently by two researchers, and compiled the results for analysis.
Results
Studies included in the review focused on women over 18 years of age diagnosed with endometriosis and, additionally, with bacterial vaginosis confirmed either by microscopic evaluation (using the Nugent score or Amsel/Spiegel criteria) or by gene sequencing analysis. Only studies published in Portuguese or English from 2014 to 2024 were considered .
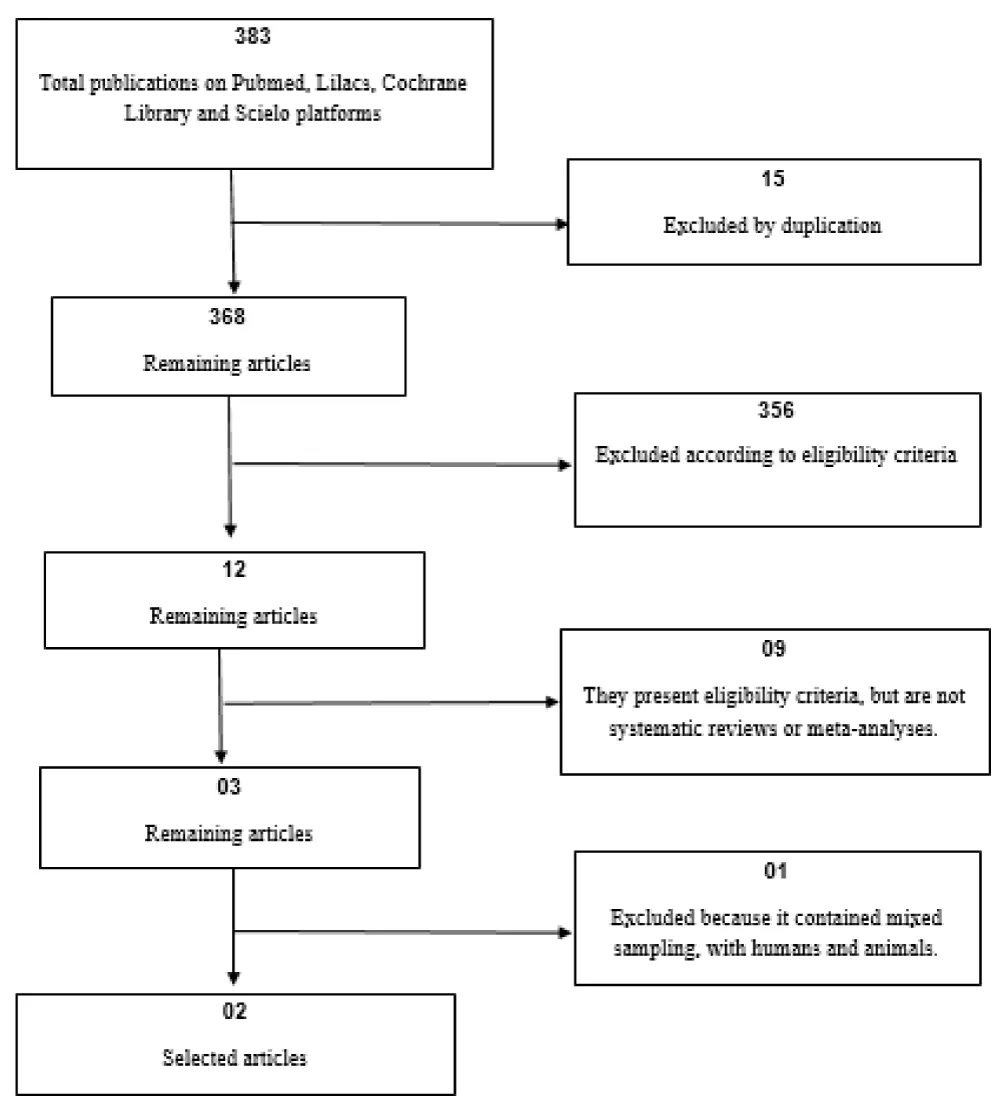
An initial search yielded 383 records , of which 15 duplicates were excluded, leaving 368 publications. Subsequently, a screening of titles and abstracts led to the exclusion of 356 records that were not related to the topic, leaving 12 studies for full-text evaluation. After a complete review of these texts, 3 studies were initially deemed eligible; however, following a thorough analysis, 2 articles were ultimately selected, both being systematic reviews or meta-analyses based exclusively on human samples (Figure 1, Table 2).
Discussion
Endometriosis is a complex condition involving physical and emotional symptoms, significantly impacting the personal and professional lives of those affected, as well as leading to considerable financial and economic implications—estimated to incur a global economic burden exceeding 80 million USD. As a public health issue, it is essential that clinical decisions are based on robust, evidence-based studies.
Research investigating the association between dysbiosis and endometriosis has relied on experimental results supporting the inflammatory pathophysiology of the disease . The advancement in understanding the human microbiome has led to the emergence of a new hypothesis proposing an infectious origin for endometriosis. According to this hypothesis, alterations in the healthy microbiota—whether from the gut, oral cavity, or female genital tract—could contribute to the risk of developing the disease, possibly as a result of a compromised immune response, leading to subclinical inflammation conducive to the formation of endometriotic implants [10].
Additionally, the bacterial contamination hypothesis suggests that the increased endotoxins produced by bacteria, in conjunction with tubal retrograde menstruation, could trigger inflammatory processes in the pelvic environment, promoting the growth and progression of endometriosis through the activation of inflammatory receptors. In this context, lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, may act as an initiating factor, either alone or in combination with ovarian steroids, contributing to the development of the disease [11].
Experimental studies in murine models have shown promising results. Protocols using Letrozole-a cytochrome P450 aromatase inhibitor—combined with Alpha-Linolenic Acid (ALA) supplementation have demonstrated a significant reduction in both the number and size of surgically induced endometriotic lesions. These treatments have been associated with decreased cellular proliferation , increased apoptosis, decreased angiogenesis, and improved gut microbiota composition. This resulted in a stronger intestinal barrier and reduced macrophage aggregation within the intestinal walls, mitigating the local inflammatory process [12-14].
Given the multifactorial nature of endometriosis, this systematic review and meta-analysis aimed to gather evidence on the association between dysbiosis and the development of the disease. Quing, et al. [15] performed a systematic review that identified a potential positive association between dysbiosis and endometriosis (OR 1.17 [0.81–1.70]), although without statistical significance. In contrast, the analysis of the relationship between a normal vaginal microbiota and endometriosis incidence revealed an inverse association (OR 0.90 [0.55–1.46]), suggesting a protective effect from a healthy vaginal microbiota, even though this association also did not reach statistical significance. The authors further highlighted the relationship between the absence of Lactobacillus and the proliferation of bacteria linked to bacterial vaginosis in the cervicovaginal microbiota and the association with endometriosis and infertility [15].
In line with this, Colonetti, et al. [16] reviewed 16 studies investigating dysbiosis in various samples—including material from the intestinal tract, vagina, and peritoneal fluid—and its association with endometriosis. Although the group of women with endometriosis showed higher levels of certain markers, no analysis achieved statistical significance. In one of the studies, it was observed that in more severe cases of endometriosis, the vaginal and cervical microbiota displayed an absence of Atopobium (Actinobacteria) and increased levels of Gardnerella, Streptococcus, Escherichia, Shigella, and Ureaplasma, potentially pathogenic organisms [16].
The studies analyzed indicated the prevalence of some genera such as Enterobacter, Streptococcus, Lactobacillus, Gardnerella, Veillonella and Prevotella in patients with endometriosis, highlighting the need for more specific investigations, focused on the identification of genera and species that may be implicated in the pathogenesis of endometriosis, and that trigger inflammatory changes arising from dysregulated states of the intestinal flora, allowing translocation of pathogenic microbial metabolites .
Experimental analyses have explored emerging therapies for endometriosis, such as antibiotics , Lactobacillus supplementation and microbial transplantation; preliminary results demonstrate a reduction in endometriotic lesions in animal models . And the use of probiotics, for oral supplementation of Lactobacillus , can alleviate pain and improve the quality of life of patients with endometriosis, albeit to a limited extent [17,18]. Such findings may contribute to more targeted and effective clinical management of these patients.
Finally, it is important to recognize that the microbiome is influenced by a variety of factors—including demographics, lifestyle, age, race/ethnicity, diet, medical history, pregnancy, childbirth, sexual and hygiene practices, genetic variation, cultural habits, menstrual cycle, hormone levels, and the general environment—thus making more accurate analyses of this study difficult. Therefore, standardizing sample collection methods and matching clinical factors, such as menstrual cycle phase and technique used, are essential to ensure consistency of results and correct interpretation of data.
Conclusion
Although current data do not demonstrate a statistically significant association between endometriosis and the presence of dysbiosis in intestinal, vaginal, and peritoneal fluid samples, considerable heterogeneity among the populations studied highlights the need for further investigations.
Future studies should investigate the most prevalent bacterial taxa in women with endometriosis and apply standardized methodologies for more robust and generalizable findings in order to validate these findings and enhance our understanding of the underlying mechanisms of the disease.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;1789–99. Available from: https://doi.org/10.1016/s0140-6736(04)17403-5
- Missmer AS, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. Available from: https://doi.org/10.1016/s0889-8545(02)00050-5
- Barbosa CP, Souza AM, Bianco B, Christofolini D, Bach FA, Lima GR. Frequency of endometriotic lesions in peritoneum samples from asymptomatic fertile women and correlation with CA 125 values. São Paulo Med J. 2009;127:342–345. Available from: https://doi.org/10.1590/s1516-31802009000600004
- Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. Available from: https://doi.org/10.1111/j.1749-6632.2001.tb03797.x
- Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the physiopathology of endometriosis. Reproduction. 2002;123:217–226. Available from: https://doi.org/10.1530/rep.0.1230217
- Cho S, Park SH, Choi YS, Seo SK, Kim HY, Park KH, et al. Expression of cyclooxygenase-2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecol Obstet Invest. 2010;69:93–100. Available from: https://doi.org/10.1159/000261017
- Cavalcanti V, Ponce TG, Mafra FA, Mendonça GA, Christofolini DM, Barbosa CP, et al. Evaluation of the frequency of G-765 polymorphism in the promoter region of the COX-2 gene and its correlation with the expression of this gene in the endometrium of women with endometriosis. Arch Gynecol Obstet. 2016;293:109–115. Available from: https://doi.org/10.1007/s00404-015-3808-9
- Kobayashi H. Similarities in pathogenetic mechanisms underlying the bidirectional relationship between endometriosis and pelvic inflammatory disease. Diagnostics. 2023;13:868. Available from: https://doi.org/10.3390/diagnostics13050868
- Salliss ME, Farland LV, Mahnertl ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2022;28:92–131. Available from: https://doi.org/10.1093/humupd/dmab035
- Jiang I, Yong PJ, Allaire C, Bedaiwy A. Intricate connections between the microbiota and endometriosis. Int J Mol Sci. 2021;22:5644. Available from: https://doi.org/10.3390/ijms22115644
- Romero B, Navarro AL, Fontes LM, Calderón FJ, Mozas J. Pelvic abscess after oocyte retrieval in women with endometriosis: a case series. Iran J Reprod. 2013;11:677–680. Available from: https://pubmed.ncbi.nlm.nih.gov/24639807/
- Chang CY, Chiang AJ, Lai MT, Yan MJ, Tseng CC, Lo LC, et al. A more diverse cervical microbiome associates with better clinical outcomes in patients with endometriosis: a pilot study. Biomedicine. 2022;10:174. Available from: https://doi.org/10.3390/biomedicines10010174
- Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125–133. Available from: https://doi.org/10.1002/rmb2.12083
- Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. Available from: https://doi.org/10.1016/j.chom.2011.10.003
- Quing X, Xie M, Liu P, Ou F, Leng H, Guo H, et al. Correlation between dysbiosis of vaginal microecology and endometriosis: a systematic review and meta-analysis. PLoS One. 2024;19:e0306780. Available from: https://doi.org/10.1371/journal.pone.0306780
- Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Denoni JC, Ceretta LB, et al. Gut and vaginal microbiota in endometriosis: systematic review and meta-analysis. Biomed Res Int. 2023;2675966. Available from: https://doi.org/10.1155/2023/2675966
- Hertz FB, Holm JB, Paeejá A, Björnsdóttir MK, Mikkelsen LS, Brandsborg E, et al. Vaginal microbiome following orally administered probiotic. APMIS. 2022;130(10):605-611. Available from: https://doi.org/10.1111/apm.13261
- Lu F, Wei J, Zhong Y, Feng Y, Ma B, Xiong Y, et al. Antibiotic therapy and vaginal microbiota transplantation reduce endometriosis disease progression in female mice via NF-kB signaling pathway. Front Med. 2022. Available from: https://doi.org/10.3389/fmed.2022.831115
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
