Journal of Gynecological Research and Obstetrics
Post burn and bilateral inflammatory breast cancer: Three case reports from one patient
Ashraf Nour1*, Hamdan Swellmein2, Hameed Jawad3 and Khaled Abdelhady4
2Department of Medical Oncology, Saad Specialist Hospital, AlMana Hospital, AlKhobar, Kingdom of Saudi Arabia
3Department of Pathology, Saad Specialist Hospital, AlMana Hospital, AlKhobar, Kingdom of Saudi Arabia
4Cairo University, Cairo, Egypt
Cite this as
Nour A, Swellmein H, Jawad H, Abdelhady K (2024) Post burn and bilateral inflammatory breast cancer: Three case reports from one patient. J Gynecol Res Obstet 10(1): 007-010. DOI: 10.17352/jgro.000124Copyright License
© 2024 Nour A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Inflammatory Breast Cancer (IBC) is the most aggressive form of primary breast cancer. IBC has an incidence of approximately 2.5 cases per 100,000 women.
Malignant neoplasms arising from burn scars are well-known but rarely encountered. The subject was reviewed in a comprehensive publication that reviewed the literature between 1923 and 2007 and found 412 well-documented cases of squamous cell carcinoma (71%), basal cell carcinoma (12%), melanoma (6%), sarcoma (5%), other neoplasms (4%), squamo-basal cell carcinoma (1%), and squamous cell-melanoma (1%). In 2008, two cases were reported by Losanof, et al. both female, who were diagnosed with advanced breast cancer many years after severe thermal injury to their breasts. Bilateral synchronous or metachronous breast cancer is well-known and documented except for IBC. To our knowledge, our search using Medline and other searches did not reveal any post-burn IBC or bilateral IBC cases reported in the literature that occurred till 2006. There are only 2 cases reported in 2010 and 2016.
We report our 3 case reports presented from one patient; who was diagnosed in 2006; a case of post-burn breast cancer, and to our knowledge the first case report of post-burn IBC type and a rare case report of bilateral metachronous IBC.
Introduction
Inflammatory Breast Cancer (IBC) is the most aggressive form of primary breast cancer. IBC has an incidence of approximately 2.5 cases per 100,000 women [1]. It is characterized by rapid tumor progression, early metastasis and poor survival.
IBC is a clinical diagnosis characterized by the presence of symptoms and signs such as erythema, tenderness, edema, pain, and rarely, ulceration that rapidly extends to the entire breast [2,3]. Pathologically, IBC is frequently diagnosed by the presence of cancer cells penetrating dermal lymphatic channels causing inflammatory signs [4].
Malignant neoplasms arising from burn scars are well-known but rarely encountered. The subject was reviewed in a comprehensive publication that reviewed the literature between 1923 and 2007 and found 412 well-documented cases of squamous cell carcinoma (71%), basal cell carcinoma (12%), melanoma (6%), sarcoma (5%), other neoplasms (4%), squamo-basal cell carcinoma (1%), and squamous cell-melanoma (1%) [5]. An extensive literature search, done by Losanof, et al. [6] using the Medline and Current Contents computerized databases located two previous reports of adenocarcinoma; one was a lung cancer metastasis to the arm [7] and the second a ductal or sweat gland carcinoma of the breast [8]. In 2008, two cases were reported by Losanof, et al. both female [6], who were diagnosed with advanced breast cancer many years after severe thermal injury to their breasts. Bilateral synchronous or metachronous breast cancer is well-known and documented except for IBC. To our knowledge, our search using Medline and other searches did not reveal any post-burn IBC or bilateral IBC cases reported in the literature that occurred till 2006. There are only 2 cases reported in 2010 and 2016 [9,10].
We report our 3 case reports presented from one patient; who was diagnosed in 2006; a case of post-burn breast cancer, and to our knowledge the first case report of post-burn IBC type and a rare case report of bilateral metachronous IBC.
Case reports
A 64-year-old lady presented to our breast clinic in December 2006 with right breast swelling and redness. Her past medical history is otherwise significant for peptic ulcer disease, hypertension, and chest asthma. A history of car accidents that led to bilateral shoulder bone fractures.
She does not smoke or drink. She has taken hormone replacement therapy for one year only.
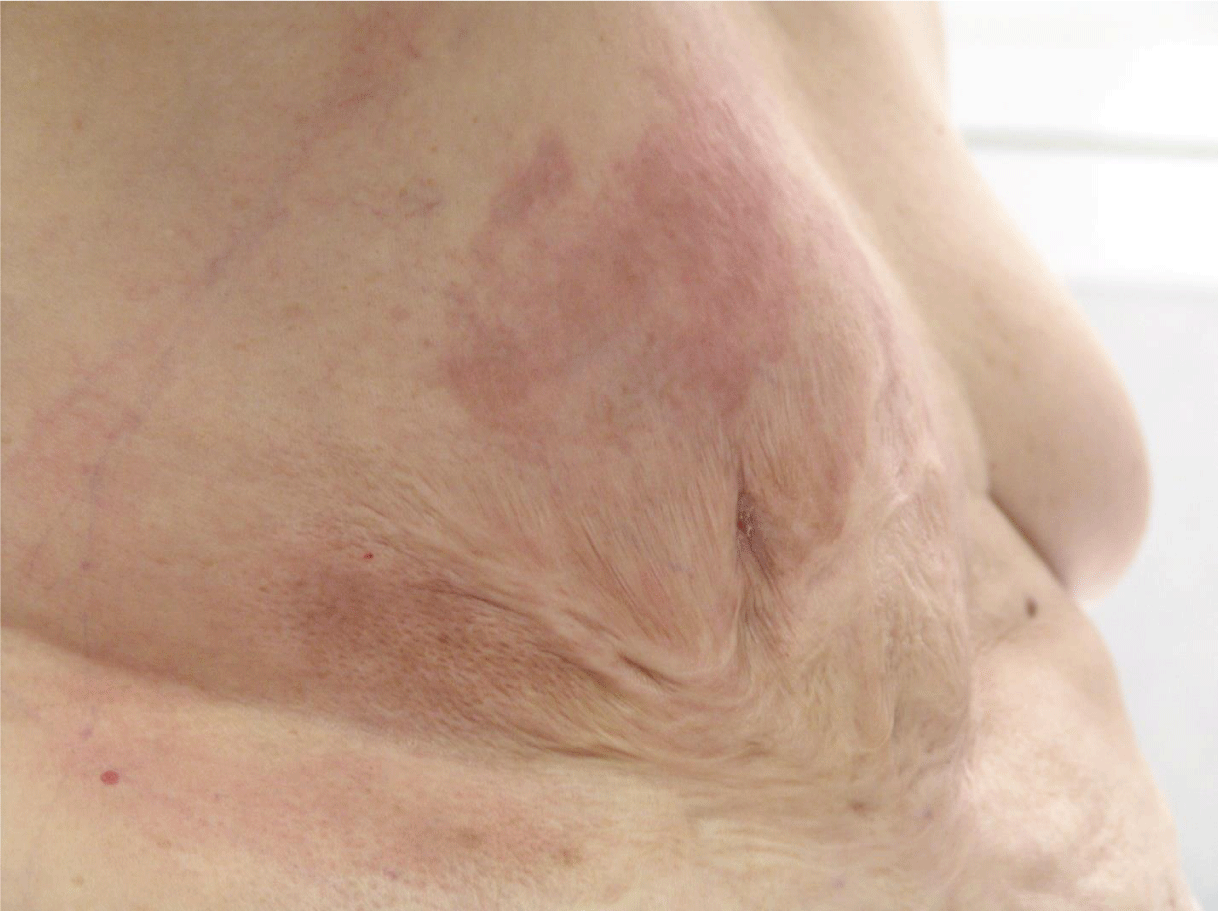
It is significant to mention that she had a deep and extensive burn affecting the anterior chest wall, especially on the right side involving the right breast at the age of 2 years. The burn was left to heal by secondary intention (Figure 1).
She has no family history of breast cancer.
One month before presenting to our hospital, she noted right breast pain with progressive and increasing redness, heaviness, and thickening. The redness involved the area below the right shoulder but not the abdominal wall. She was given oral antibiotics at a local hospital with partial improvement in redness that receded to the right breast only.
Physical exam revealed
A chubby lady who was slightly pale, with no localized bony tenderness, no ascites or organomegaly, or lower limb edema.
The right breast was deformed, and had an old post-burn fibrotic contracture scar, with no ulcerations, involving the right anterior chest and abdominal wall. The left breast had no abnormality upon clinical exam.
The right breast showed a full-blown picture of IBC. The redness involved most of it and extended to a small area of the lateral chest wall. There was a right nipple retraction with a dry blackish scab with no ulceration. There was generalized thickening of the breast with an ill-defined underlying mass, mobile on the chest wall and attached to the skin. There were matted nodes in the right axilla with a supraclavicular fossa node of < 1.5 cm, firm in consistency.
All required investigations were performed on the patient including bilateral mammograms, breast ultrasound, core biopsies, CT scans of the chest, abdomen, and pelvis, and whole body bone scans.
She was diagnosed with Right Breast Inflammatory Carcinoma (Stage IIIc: T4d N3 M0 ER and PR negative, Her2 neu positive).
She received pre-operative chemotherapy (Anthracyclin plus Taxane). Unfortunately, she relapsed locally on Taxol, thus Herceptin was added. The tumor responded dramatically to Herceptin (5 cycles).
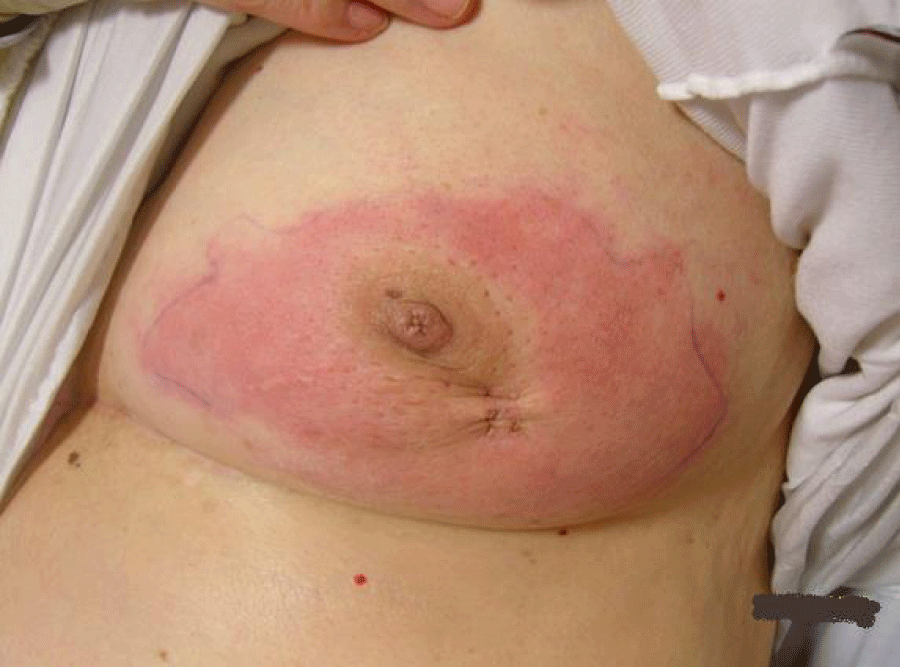
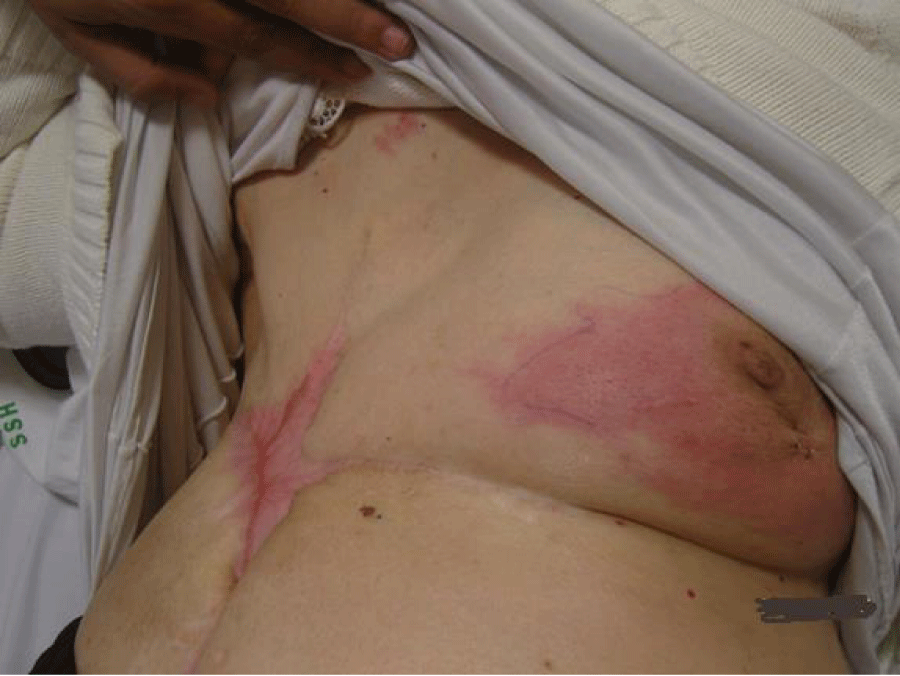
Right-modified radical mastectomy was performed on the patient after she responded to Herceptin as this was the optimal time to achieve local control by surgery. Generous skin margins were excised with the mastectomy specimen to ensure free safety margins. The post-operative course was smooth and the patient tolerated the operation well. On follow-up, on the 15th postoperative day, the patient developed wound infection and gaping of the wound, probably due to poor vascularity of the skin flaps from the previous burn and tension closure of the area after wide skin excision. The complication was managed by frequent dressing, and systemic antibiotics and was left to heal by secondary intention (Figures 2,3).
Complete healing was achieved after 4 weeks, after which post-operative chemotherapy was given to the patient.
On follow-up visits to the surgery clinic, 10 months after the initial surgery, the patient complained of redness, edema, and swollen left breast. Upon clinical examination, there was redness, firm ill-defined swelling occupying almost half of the left breast.
Suspicion was towards a metachronous IBC in the left breast. True-cut biopsies were taken from the skin, superficial and deep left breast tissues; and pathology came back to be IBC. The patient started another course of chemotherapy and underwent left modified radical mastectomy.
The patient died of her disease in 2009 from disseminated metastasis.
Discussion
The links between old thermal injuries and breast cancer are scantly published in the literature.
Cancers arising in old Burn Scars (BS) are rare. The average latent period for the development of a post-burn malignancy is 30 years [5,11].
Residual scar tissue after burn injury associated with a malignant lesion is typically extensive [12] as in the patients we reported. Literature from the 1950s and 1960s suggests that severe burns which are allowed to heal without grafting, as in our patient, are especially prone to the development of cancer [12]. Scar malignancies, however, have been described in both grafted and non-grafted burns [5,12,13], suggesting that other factors such as the location of the scar, repeat trauma, and failure to heal properly may play a role [13].
In a recent publication in 2024, a case report of infiltrating duct carcinoma was published showing the occurrence of invasive duct carcinoma in an old burn scar [14].
Our patients’ malignancy was discovered at advanced stage III. Detection was delayed; possible explanations include patient denial, difficulty in performing a breast exam resulting from altered breast structure by the scars, rapid tumor growth, lack of breast health awareness, or a combination of these.
Interestingly, both tumors were ER, PR negative and Her2 receptor strongly positive. It is consistent with aggressive tumors.
Theories put forward to explain the etiology of tumors occurring in burn scars include chronic irritation (Virchow), misplaced epithelial cells (Ribbet), and release of toxins by autolysis (Treves and Pack); however, the specific etiology of malignancy in burn scars remains not entirely clear [6,8]. Anatomically the cancer in this patient involved the skin in the previously burned area. Embryologically the breast is a modified sweat gland connected to the skin and is typically located close to the external skin surface. We, therefore, agree with the hypothesis that a thermal injury of the stem cells in the prepubertal breast predisposed these women to the late development of breast cancer.
For a tumor to qualify as a post-burn malignancy, it must fulfil ‘Ewing’s postulates’ that include five criteria: (a) evidence of a burn scar, (b) tumor within the boundaries of the scar, (c) no previous tumor in that location, (d) tumor histology compatible with the cell types found in the skin scar, (e) an adequate interval between burn injury and tumor development [2]. The present case fulfils these postulates and hence qualifies as a malignancy arising in a burn scar rather than just a coincidental finding. All these criteria apply to our case.
Our case is unique in that the patient developed bilateral metachronous IBC and IBC following a long-standing burn scar.
According to SEER rules, all metachronous cancers occurring 2 or more months after the first cancer diagnosis are considered independent primaries unless the medical records indicate that the tumor is a recurrent or metastatic disease [15].
Conclusion
Our experience, similar to the scant previously published data, reinforces prior conclusions that burn scars demand a high index of suspicion for the presence of an underlying malignant neoplasm. The literature and our study suggest that breast cancer may occur after severe thermal injury so thermal injury during childhood to the breast could be considered as a risk factor for subsequent development of breast cancer. Breast lesions in this or a similar clinical scenario should be considered malignant until proven otherwise, and a tissue diagnosis must be pursued without delay.
We also encourage the report and submission of any cases that developed thermal injury to the breast region with or without the subsequent development of breast cancer so as to estimate the incidence of post-burn breast cancer and consider burns as a risk factor for subsequent cancer development.
Ethical considerations
The husband and the son of the patient agreed and consented to publish the case as a case report for the benefit of studying breast cancer cases.
- Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (IBC). Breast Dis. 2005-2006;22:9-23. doi: 10.3233/bd-2006-22103. PMID: 16735783; PMCID: PMC2852616.
- Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992 Jun;10(6):1014-24. doi: 10.1200/JCO.1992.10.6.1014. PMID: 1588366.
- Tabbane F, Bahi J, Rahal K, el May A, Riahi M, Cammoun M, Hechiche M, Jaziri M, Mourali N. Inflammatory symptoms in breast cancer. Correlations with growth rate, clinicopathologic variables, and evolution. Cancer. 1989 Nov 15;64(10):2081-9. doi: 10.1002/1097-0142(19891115)64:10<2081::aid-cncr2820641019>3.0.co;2-7. PMID: 2804897.
- Taylor GMA. Inflammatory carcinoma of the breast. Am J Cancer 1938; 33:33-34.
- Kowal-Vern A, Criswell BK. Burn scar neoplasms: a literature review and statistical analysis. Burns. 2005 Jun;31(4):403-13. doi: 10.1016/j.burns.2005.02.015. Epub 2005 Apr 1. PMID: 15896501.
- Losanoff JE, Konrad A, Sauter ER. Breast cancer after severe burn injury: coincidence or consequence? Breast J. 2008 Jan-Feb;14(1):87-9. doi: 10.1111/j.1524-4741.2007.00526.x. PMID: 18186869.
- Balakrishnan C, Noorily MJ, Prasad JK, Wilson RF. Metastatic adenocarcinoma in a recent burn scar. Burns. 1994 Aug;20(4):371-2. doi: 10.1016/0305-4179(94)90073-6. PMID: 7945834.
- Vögelin E, Feichter G, Lüscher NJ. Breast cancer in previously burned skin: a postburn skin adnexal malignancy? Burns. 1997 Jun;23(4):366-8. doi: 10.1016/s0305-4179(96)00138-6. PMID: 9248650.
- Masannat YA, Peter M, Turton P, Shaaban AM. Case report of bilateral inflammatory breast cancer. Eur J Cancer Care (Engl). 2010 Jul;19(4):558-60. doi: 10.1111/j.1365-2354.2009.01075.x. Epub 2009 Aug 25. PMID: 19708943.
- Kurtz J, Halaharvi D, Sarwar S, Cripe M. A Case of a Bilateral Inflammatory Breast Cancer: A Case Report. Breast J. 2016 May;22(3):342-346. doi: 10.1111/tbj.12578. Epub 2016 Feb 21. PMID: 26899495.
- Huang CY, Feng CH, Hsiao YC, Chuang SS, Yang JY. Burn scar carcinoma. J Dermatolog Treat. 2010 Nov;21(6):350-6. doi: 10.3109/09546630903386580. PMID: 20438387.
- ENGLER HS, FERNANDEZ A, BLIVEN FE, MORETZ WH. CANCER ARISING IN SCARS OF OLD BURNS AND IN CHRONIC OSTEOMYELITIS, ULCERS, AND DRAINAGE SITES. Surgery. 1964 May;55:654-64. PMID: 14146525.
- Kaplan RP. Cancer complicating chronic ulcerative and scarifying mucocutaneous disorders. Adv Dermatol. 1987;2:19-46. PMID: 3079255.
- Singh N, Rao S, Jain S. Post-burn duct carcinoma breast: An unusual case report. J Cytol. 2013 Apr;30(2):139-41. doi: 10.4103/0970-9371.112660. PMID: 23833406; PMCID: PMC3701340.
- Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF. NIH Publ. No. 05–5302. National Cancer Institute; Bethesda, MD: 2006.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
