International Journal of Clinical Endocrinology and Metabolism
Diabetic Retinopathy: Focus on Minority Populations
Arpine Barsegian1, Boleslav Kotlyar1, Justin Lee2, Moro O Salifu2 and Samy I McFarlane2*
2Department of Medicine, SUNY-Downstate Medical Center and Kings County Hospital, Brooklyn, NY 11203, USA
Cite this as
Barsegian A, Kotlyar B, Lee J, Salifu MO, McFarlane SI (2017) Diabetic Retinopathy: Focus on Minority Populations. Int J Clin Endocrinol Metab 3(1): 034-045. DOI: 10.17352/ijcem.000027Diabetic retinopathy is a major cause of blindness in the United States. With rise of the epidemic of obesity and diabetes in the USA and around the globe, serious and common diabetic complications are evolving as a major public health problem, particularly among minority populations. These populations are disproportionately affected by diabetes and 2-3 times more likely to develop visually significant complications. In this highly illustrated review article, we discuss the diabetic epidemic, highlighting the biology and the pathophysiologic mechanisms of this disorder on the anatomy of the eye. We also discuss the risk factors and the implications for minority populations. For the health care providers, we provide cutting edge information and imminently relevant information to help evaluate, manage, and know when to refer their patients to a specialist in ophthalmology to quell the tide of the epidemic.
Introduction
Diabetes Mellitus types 1 and 2 can cause diabetic retinopathy and blindness. Diabetic retinopathy (DR) is the 3rd leading source of overall blindness in the United States and the leading cause of new cases of legal blindness in working age Americans [1]. The total number of individuals worldwide with diabetes is expected to increase from 171 million in 2000 to 366 million in 2030 [2], and the concurrent rise in preventable visual dysfunction and quality adjusted life years is lamentable. In the United States, an estimated 25.6 million people aged 20 or older have either been diagnosed or remain undiagnosed with diabetes mellitus (11% of people in this age group) [3]. The prevalence rate for retinopathy for all adults with diabetes over 40 years of age in the United States is 28.5% (4.2 million people), and worldwide it has been estimated at 34.6% (93 million people). Assuming similar prevalence of diabetes mellitus, 6 million Americans are projected to have diabetic retinopathy by the year 2020 [4,5].
The diabetes epidemic already disproportionately affects racial and ethnic minorities, who have higher rates of macro and microvascular complications of diabetes [6], and are more susceptible to known risk factors of DR [7-9]. The same groups may undergo screening less often than white patients for a variety of factors (including patient, provider, and system factors [10]); for this reason, it is important to understand the classification scheme for both categories of DR, as each stage of disease necessitates a different screening, monitoring, and possibly, treatment schedule [11]. As the minority fraction of the total US population continues to grow, this will continue to evolve into a larger problem.
Biology
Diabetic retinopathy occurs through injury to the retinal microvasculature due to extended exposure to the metabolic modifications caused by diabetes. Without appropriate intervention, it progresses in a predictable fashion from mild to more severe stages. Non-proliferative diabetic retinopathy (NPDR) is characterized by superficial and deep retinal hemorrhages, microaneurysms, venous dilation, and cotton-wool spots (signs of focal retinal nerve fiber layer ischemia). Increased vascular permeability may occur at any stage of the disease, resulting in retinal thickening (edema) and lipid deposits (hard exudates). With progression of disease, there is continuing impairment of retinal perfusion and increasing signs of generalized ischemia with more significant venous abnormalities (such as dilation, beading, and loops), peripheral capillary nonperfusion, and formation of intraretinal microvascular abnormalities (IRMA) with remodeling of pre-existing intraretinal vessels. Ensuing proliferative diabetic retinopathy (PDR) occurs with the formation of new fragile blood vessels at the inner surface of the retina, and often growth into vitreous cavity. These vessels are prone to breaking, leading to vitreous hemorrhages, fibrosis and scarring of the retina and vitreous. These processes ultimately cause contraction resulting in tears, or purely tractional retinal detachments and blindness. The abnormal proliferation of blood vessels may also occur in the drainage angle of the eye, obstructing outflow and leading to persistent eye pressure elevations, otherwise known as neovascular glaucoma. Cataracts also occur at an earlier age, 2-5 times more frequently in patient with diabetes, compounding the issue of significant visual decline [12,13].
Pathogenesis
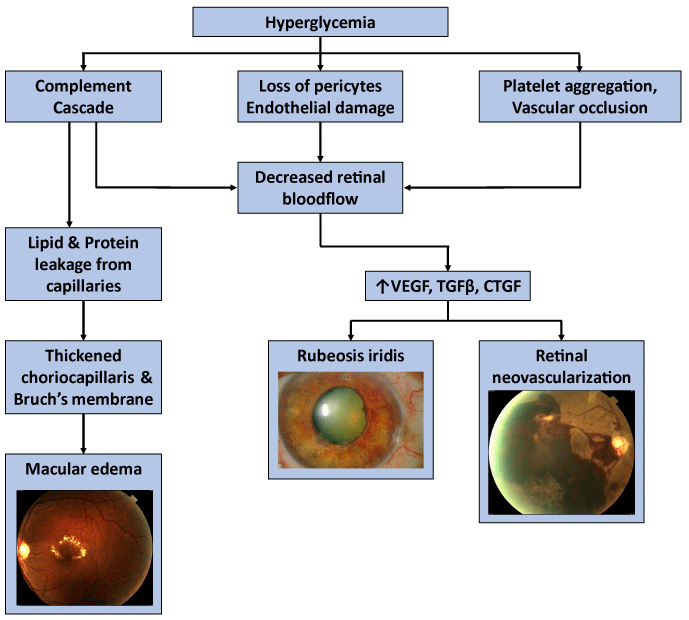
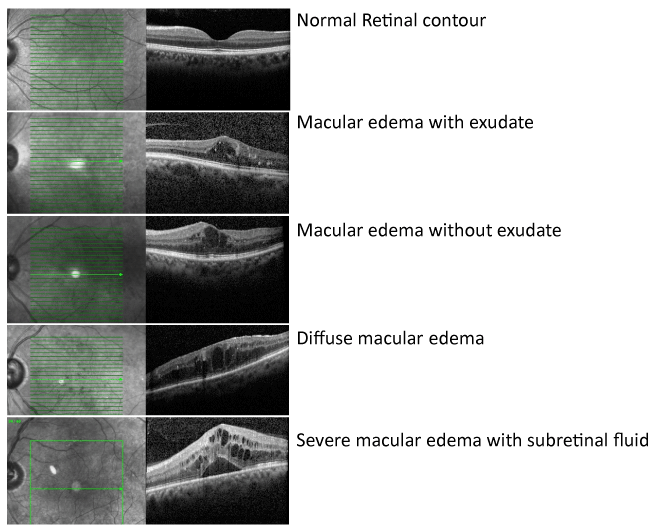
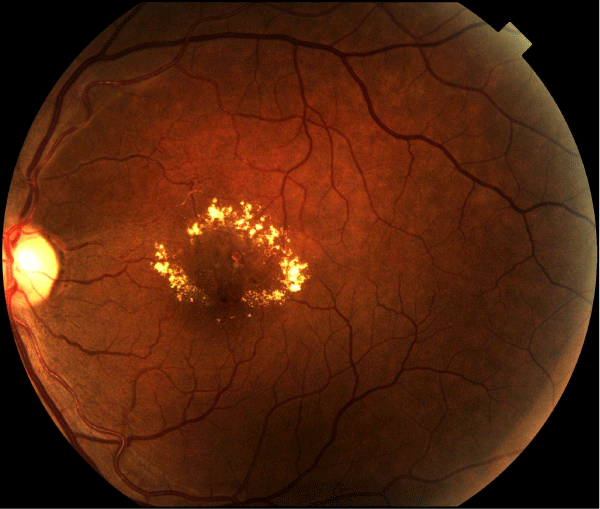
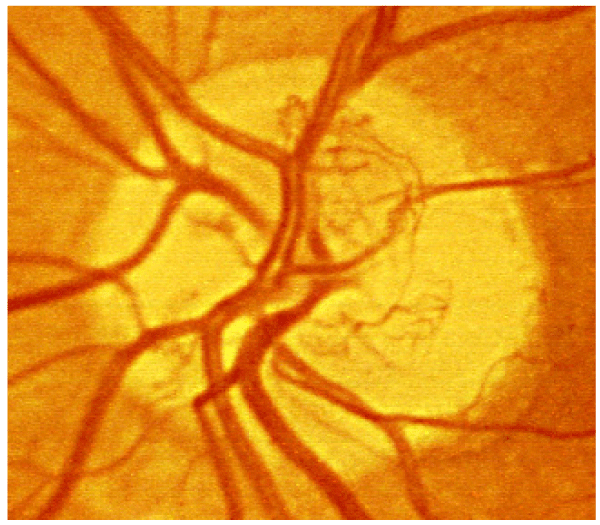
The pathogenesis of diabetic retinopathy is thought to entail three factors: neovascularization caused by damage to blood vessels, inflammation, and platelet abnormalities. Diabetic retinopathy is characterized as a microangiopathy and its development is due to glucose injury to the microvasculature leading to increased permeability of the blood retinal barrier, with loss of pericytes and endothelial cells, and eventual occlusion of capillaries, vascular basement membrane thickening, and retinal neuronal and glial damage [14] (Figure 1). In response to this injury and to relative focal areas of retinal ischemia, the retina and retinal pigment epithelium exude vasoproliferative factors, such as vascular endothelial growth factor (VEGF), which result in neovascularization. These effects also contribute to the formation of diabetic macular edema (DME). DME is the most common cause of vision loss in patients suffering from diabetes [15], and is characterized by cystic swelling of the most visually critical area of retina as a result of increased vascular permeability (Figure 2), and is often associated with increased lipid deposition (hard exudates) in the area (Figure 3). Randomized controlled trials of local (ophthalmic) VEGF inhibition have resulted in an entire class of effective drugs having become quite popular in the treatment of diabetic retinopathy and its associated complications [16-21].
Diabetic retinopathy also has an inflammatory element that is caused by macrophage and complement activation. Deposition of complement factors 5b-9 and fibronectin have been described in the connective tissue matrix of the choriocapillaris. Complement activation causes increased neutrophil activation, and eventually endothelial injury. Damaged capillaries leak lipids and proteins, and the complement cascade influences nearby cells, causing a thickened choriocapillaris and Bruch’s membrane. Therefore, inflammation also exacerbates macular edema; in fact, it is believed that long-standing DME may be related to inflammation more than to neovascularization and responds significantly to steroids [22].
Lastly, platelet abnormalities and increased viscosity also lead to focal capillary occlusion and focal regions of ischemia in the retina, further aggravating diabetic retinopathy.
The Classification and Treatment of Diabetic Retinopathy
Diabetic retinopathy is classically divided into nonproliferative and proliferative based on the absence or presence of neovascularization and the nature and quantity of specific lesions that predispose to their development. The Early Treatment Diabetic Retinopathy Study (ETDRS) in 1999 created a classification system for diabetic retinopathy disease severity level based on a standard series of stereo fundus photographs [11]. NPDR can be further sub-classified (Table 1) into very mild, mild, moderate (more than ETDRS photograph 2A), severe, and very severe. In general, treatment of NPDR consists of managing systemic risk factors, and improving blood glucose control, while maintaining close follow-up with ophthalmology to catch progression of disease into the proliferative stage. The Diabetes Control and Complications Trial (DCCT) showed that patients with type 1 diabetes who tightly controlled their blood glucose level (with four measurements daily) had a 76% reduction in the rate of development of any retinopathy, and a 54% reduction in progression of pre-existing retinopathy [23]. In fact, DCCT showed that for every 1% decrease in HbA1C level, the incidence of DR decreased by 28%. The Epidemiology of Diabetes Intervention and Complications observational study of the DCCT cohort showed continued benefit for the tight glucose control cohort over conventional treatment (once daily blood glucose measurement) despite normalization of glucose control even after seven years of follow-up [24]. Intensive management of glycemic level and blood pressure in people with type 2 diabetes was explored in the 1998 United Kingdom Prospective Diabetes Study (UKPDS), revealing 21% reduction in the one year rate of progression of retinopathy [25]; this highlights the decrease in need for intensive treatment of diabetic retinopathy. There was no evidence of glycemic threshold for any of the microvascular complications above normal glucose levels (HbA1C > 6.2%).
Proliferative Diabetic Retinopathy is defined as any of the following: retinal neovascularization of the disc (within 1 disc diameter of the optic disc), neovascularization elsewhere (further than 1 disc diameter from the optic disc), vitreous hemorrhage, and/or fibrous tissue proliferation. High-risk PDR is defined as at least 1/3 disc area of disc neovascularization (greater than ETDRS standard photograph 10A) in the absence of vitreous hemorrhage, any disc neovascularization with vitreous hemorrhage, or at least ½ disc area of retinal neovascularization elsewhere with vitreous hemorrhage [11]. At this stage, tight glucose control alone is no longer adequate in preventing progression to vision loss, and DCCT was halted early after 6.5 years for this reason. Therapy at this point has historically been administered through panretinal photocoagulation (PRP). The initial Diabetic Retinopathy Study (DRS), which enrolled over 1700 patients consisting of almost equal amounts of juvenile and adult onset diabetes patients between 1972 and 1975, had a principal endpoint of severe visual loss; defined to be less than 5/200 at two or more consecutively completed 4-month follow-up visits. One eye of each patient was randomly assigned to immediate photocoagulation, and the other to follow-up without treatment. After an average of only 15 months of follow-up, the 2 year incidence of blindness was 16.3% in untreated eyes, but only 6.4% in treated eyes, showing that photocoagulation reduced the 2 year risk of blindness by about 60%. On the basis of the high statistical significance, the study design was changed more than 3 years prior to the planned termination date of the DRS, and photocoagulation was used on initially untreated eyes which at that time point or anytime in the future would fulfill the characteristics of what is now called high risk PDR, as defined above. Furthermore, although the principal goal of PRP was to prevent visual loss, not to improve vision, some eyes showed evidence of recovery (vision improving to better than 5/200). The percentage of eyes with evidence of recovery at post-laser month 4, 8, and 12 were 28.6%, 12.2%, and 7.7% in untreated eyes, compared to 48.8%, 28.6%, and 20.8% in treated eyes, respectively. This seems to show that visual acuity recovered more frequently in treated eyes [26]. Benefits of treatment of proliferative diabetic retinopathy were also illustrated by ETDRS, which showed that after 7 years of follow-up, 25% of eyes that received PRP developed high risk PDR as compared to 75% in which PRP was deferred until high risk PDR features developed.
PRP is achieved through 360 degree photocoagulation of ischemic retina with a few thousand noncontiguous small burns, generally peripheral to the major retinal blood vessels. Laser energy is absorbed and converted to thermal energy causing denaturation of tissue proteins leading to local retinal cell death and coagulative necrosis. Over time, these damaged tissues form heavily pigmented laser scars at the level of the retinal pigment epithelium. By destroying the largely unused, ischemic extramacular retina, PRP reduces total production of vasoproliferative factors in the eye and the impetus for neovascularization [27]. As with any treatment, the benefits must always be balanced against the risk of therapy. Currently, most commonly followed treatment paradigms maintain close patient follow-up with systemic diabetic control, and reserve the use of PRP until eyes reach proliferative or even high-risk PDR stages due to the laser’s damaging permanent effects on peripheral vision, overall decrease in light sensitivity (affecting night vision most notably), and similar effects on fine color vision differentiation. The procedure itself may also be uncomfortable and is usually broken up into multiple sessions until sufficient areas of retina are covered with laser marks. Other common therapies directly target and aim to decrease the level of vasoproliferative factors in the eyes, most significantly with the anti-VEGF class of intravitreal injectable medicines, and corticosteroids. Despite the demonstrated improvements in vision and degree of retinopathy, the intravitreal injections frequently require repeat administration, as often as every month, increasing the burden on both patients and healthcare systems.
When diabetic retinopathy damages the macula, it is called diabetic maculopathy. The vascular seepage and eventual swelling of the macula is called diabetic macular edema. Although DME can develop at any stage during the progression of retinopathy, it is most commonly seen during the later phases, after advanced vascular and neural injury [28,29]. This maculopathy may be further classified as central or non-central, depending on the involvement of the fovea; focal or diffuse, depending on the extent of the edema, and ischemic or non-ischemic, depending on the extent of occlusion of the perifoveal capillary network [14]. Additionally, clinically significant macular edema (CSME), is a distinct entity that requires particularly aggressive treatment. It was defined by the ETDRS as one or more of the following: retinal thickening within 500 microns of the center of the macula, exudates within 500 microns of the center of the macula (if associated with retinal thickening, the thickening itself may be outside the 500 microns), or retinal thickening 1 disc area (1500 microns) or larger, any part of which is within 1 disc diameter of the center of the macula [11]. Furthermore, diabetic maculopathy may be named tractional or non-tractional, depending on whether traction from an incompletely detached posterior hyaloid or pre-retinal neovascularization is involved.
Diabetic Retinopathy in Minorities
Many studies have investigated disparities in the incidence, prevalence, screening, and treatment of diabetic retinopathy in minorities. In the Salisbury Eye evaluation, African Americans had a 4 fold risk of visual loss; Dr. Muñoz, et al reported that DR causes 17% of visual loss in African Americans as compared to 8% in non-Hispanic whites [30-32]. Furthermore, even with limited studies, the reported prevalence of diabetic retinopathy in some Native American tribes is as high as 45.3% [30,33,34].
The Hispanic population makes up the largest ethnic or racial minority in the United States, and is growing as a fraction of the US total. As of July 1, 2015, there were 56.6 million Americans of Hispanic descent, which constituted 17.6% of the nation’s total population [35]. For adults over 18 years of age, the age adjusted prevalence of diagnosed diabetes in 2006 was 10.5% for Hispanic or Latino individuals compared with 6.8% for non-Hispanic whites [36].
The Los Angeles Latino Eye Study (LALES) is the most extensive epidemiological study of visual loss in Latinos performed in the United States. Over 6000 Latinos (mostly Mexican Americans) over the age of 40 underwent a thorough medical interview and physical examination. The study enrolled patients from Feb 2000 to May 2003 and investigated their risk factors for eye disease and calculated health-related and vision-related quality of life. LALES researchers found that over the four-year interval, Latinos developed visual impairment and blindness (from any reason) at the highest rate of any ethnic group in the country, when compared with estimates from other U.S. population-based studies. Overall, nearly 3% of Latinos developed visual impairment and 0.3% developed blindness in both eyes, with older adults impacted more frequently. Of Latinos age 80 and older, 19.4% became visually impaired, and 3.8% became blind in both eyes. U.S. Latinos were also more likely to develop diabetic retinopathy than non-Hispanic whites. One in 5 patients with diabetes was newly diagnosed during the intake physical exam; twenty-five percent of these newly diagnosed individuals already had signs of retinopathy at their first exam. Almost half (46.9%) of the 1064 Latinos with diabetes who were included in analyses had some DR and 6.1% showed signs of proliferative disease. Over the four-year enrollment period, 34% of Latinos who had diabetes developed DR, with Latinos aged 40 to 59 having the highest rate. Though increasing age did not play a role, Latinos with a longer duration of diabetes were more likely to develop the disease. In fact, 42% of Latinos with diabetes for more than 15 years developed diabetic retinopathy. Also, among participants who had diabetic retinopathy at the beginning of the study, 39% showed worsening of the disease four years later [37].
Proyecto VER (Vision Evaluation and Research) also highlighted these disparities; this was a diabetic retinopathy prevalence study of 4774 Hispanic adults over the age of 40 living in Arizona; the prevalence of diabetes in this population was 22%, with 15% of these being made up of patients undiagnosed prior to study enrollment. Of the newly diagnosed patients, 23% had some retinopathy, with 9% already in moderate to severe stages. The prevalence of diabetic retinopathy in Hispanics was 48%, which was almost twice that of Caucasians [38], and very similar to the 46.9% prevalence rate seen in LALES.
The disparities in prevalence of DR may be associated with delayed diagnosis, access to care, comorbidities such as hypertension, and inequality of screening for diabetic retinopathy. Not surprisingly, inadequate access to healthcare in low socioeconomic populations is frequently cited as a reason for health-related disparities in minority groups. For example, the LALES showed that Latinos with less education and without health insurance were less probable to be screened than those with more education and health insurance [39].
Interestingly, the severity of DR, rather than its presence, was found to aggregate in type 2 diabetes afflicted families in a study of Mexican-American siblings of individuals with DR [40]; this implies a possible genetic component to the severity of DR in Mexican-Americans [41].
Risk Factors
Duration of diabetes is a major risk factor associated with development of diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (enrollment period between 1980 and 1982) is an ongoing prospective population-based cohort study following individuals with diabetes diagnosed at either younger or older than 30 years of age. Of the almost 1000 presumed patients with type 1 diabetes (by age of diagnosis), approximately 25% developed retinopathy after 5 years, with those numbers rising to almost 60% by 10 years, 80% by 15 years and leveling off at 83% at 25 years. The rate of PDR in this group was 42% after 25 years [15,42-46] (Table 2).
In the 1370 patients presumed to have type 2 diabetes, 40% of those taking insulin and 24% of those not taking insulin had retinopathy, when known duration of diabetes was less than 5 years. These rates increased to 84% and 53%, respectively, when the duration of diabetes had been documented for up to 19 years. PDR developed in 2% of type 2 patients who had diabetes less than 5 years, and in 25% of patients who had diabetes for 25 years or more.
The rate of development of diabetic retinopathy in a patient with no previous retinopathy on exam one year prior is reported to be between 5 and 10%.
LALES and Proyecto VER showed that 18% of patients with diabetes of more than 15 years duration had PDR, with no difference in percentage of PDR in type 1 vs type 2 diabetes [34,38].
Risk Factors for Proliferative Diabetic Retinopathy in African Americans and Latinos
In the US, African Americans not only have a high prevalence of type 2 diabetes, but they also seem to be at high risk for consequent microvascular disease, including nephropathy and retinopathy [47]. Some studies have concluded that African Americans have an increased risk of developing DR when compared with Caucasians [7,48-51]. As an example, the prevalence of moderate NPDR or worse was elevated for African American veterans over Caucasian veterans in the Veterans Affairs Diabetes Trial of type 2 diabetes even after adjusting for clinical risk factors [51].
In general, the following risk factors have been consistently found to be associated with DR: longer diabetes duration, hyperglycemia, hyperlipidemia, and hypertension [52]. In regards to hyperlipidemia, there is evidence that total cholesterol and low-density lipoprotein cholesterol are associated with the presence of hard exudates, a finding in the primary stages of DR; furthermore, the severity of DR is inversely associated with systemic apolipoprotein A1, and positively associated with ApoB and the ApoB-to-ApoA1 ratio; thus, the role of lipid-lowering medication in the control of DR should not be underestimated [53]. Risk factors for progression to proliferative diabetic retinopathy are especially valuable for selected subsets, such as African Americans, due to the risk of life-changing vision loss in these patients. A prospective, non-interventional, cross sectional case control study by Penman et al. analyzed 380 African Americans with type 2 diabetes [54]. PDR was associated with increased systolic blood pressure (OR 1.65, P<0.001), insulin use (OR 6.65, P<0.001), and a prolonged duration of diabetes (odds ratio OR 1.62, P<0.001); surprisingly, HbA1c was not a significant risk factor in the multivariate analysis, though it did achieve statistical significance in the univariate analysis. This exact same result was seen in a parallel PDR study in a Latino patient population [55]. The correlation between HbA1c and DR may decline with more severe retinopathy and explain differences in results of this study compared to UKPDS and DCCT. One possible reason for this is that patients with PDR may become inspired to improve their glycemic control, lowering their HbA1c which is no longer reflective of their glycemic control for most of the duration of their diabetes. There is also contradictory evidence as to whether retinopathy starts to appear at lower HbA1c levels in African Americans [56,57]. This may be because these population-based trials on cultural changes in HbA1c have concentrated on prevalence of any DR, as opposed to advanced DR. It is still unclear as to whether or not these ethnic differences influence the relationship between HbA1c and PDR in African-Americans.
The association with insulin may be due to the fact that participants with poorly controlled diabetes are more likely to use more insulin. It is important to note that although HbA1c was not found to be significant in the multivariate analysis, the HbA1c was based on a 1 time draw of blood, not on the average levels over an extended period of time. In this Penman cohort, African Americans had a 65% increased chance of progressing to PDR for every 10 mmHg increase in systolic blood pressure. This further highlights the need for patient education among African Americans regarding the value of hypertension management in preventing diabetes related visual loss. Importantly, prospective randomized controlled trials in patients with type 2 diabetes have demonstrated the advantage of blood pressure control on development of retinopathy in general [25,58]. Even though these trials were carried out in mostly Caucasian patients, one study included 30% non-white patients, including African Americans and other non-white ethnic minorities [59]. Another study performed in African Americans with type 2 diabetes also noted that hypertension, renal disease, and poor glycemic control are associated with PDR [59]. The following factors have not been noted to influence progression of PDR: age, sex, diastolic blood pressure, serum lipid levels, BMI, waist circumference, or smoking status. Importantly, randomized clinical trials in patients with type 2 diabetes have established that glycemic control is vital to decreasing the risk of DR development [25,58].
A parallel prospective case control study conducted in a Latino American patient population by Nittala et al. also found the following risk factors to be associated with an increased risk of PDR in a multivariate analysis (p<0.05): male gender, insulin treatment, high creatinine levels, and hypertension. Surprisingly, obesity, total cholesterol, and HbA1c were not associated with PDR; if anything, obese patients were less likely to have PDR. Those with a history of smoking also demonstrated a decreased risk of developing PDR, although the risk of PDR was greater in those who smoked a higher number of cigarettes daily. Patients with a greater than 25 year diagnosis of DM were at increased risk when compared to patients with a 10 to 15 year diagnosis of diabetes mellitus [55].
The Importance of Blood Pressure
Many studies have shown the protective influences of blood pressure control on the progression of diabetic retinopathy. The United Kingdom Prospective Diabetes Study showed that patients with tighter control of blood pressure were much less likely to experience advancement of their DR [60,61]. In addition, the META-EYE study demonstrated that patients with normal blood pressure were less likely to demonstrate DR development compared to those with hypertension (BP >140/90 mmHg) or those already taking anti-hypertensive drugs [52]. A study by Silva et al. even suggested that episodes of systemic hypertension can aggravate the initiation and development of retinopathy [62]. For this reason, patients with diabetes may benefit from the earlier use of anti-hypertensive medications such as angiotensin-converting enzyme inhibitors and angiotensin-2 receptor blockers [63]. Hypertension as a risk factor in diabetic retinopathy progression was likewise noted in studies specifically looking at African American and Hispanic-American populations [54,55]. Thus, prompt and aggressive treatment of hypertension likely helps in management of diabetic retinopathy.
Macular Edema in Minorities
A comparative study between ethnic groups showed that Hispanic and Latino Americans have higher risks of clinically significant macular edema [4]. Prevalence of CSME was 8.63% in the Beaver Dam Eye Study, twice that reported in the white population [64-66]. LALES reported over 10% of participants with diabetes had macular edema, 60% of these warranting laser therapy.
Similarly, the Veterans Affair Diabetes Trial reported that 15.6% of African Americans had CSME, compared to 6.3% of Non-Hispanic Whites [51] and Multi-Ethnic Study of Atherosclerosis showed that the risk of CSME is approximately 5 times higher in African-Americans than in white patients (11.1 % vs 2.7%) [9]. It is unclear why minority populations have a higher risk of CSME; it may be due to delayed detection and screening, varieties in the pathogenesis of diabetic retinopathy in these patients, or simply a vulnerability to CSME. Regardless, given the increased risk of permanent vision loss in these patients, it is important to diagnose and treat them in a timely fashion.
Disparities in Diabetic Retinopathy Screening
Benefits of early detection and treatment: The value of timely detection and screening for proliferative retinopathy and macular edema have been well recognized by the Diabetic Retinopathy Study [68], and the Early Treatment of Diabetic Retinopathy Study [69]. In addition to helping to preserve vision for patients, there is also a financial benefit to the healthcare system and “the economy as a whole,” in early disease assessment and intervention. A yearly eye exam for diabetes patients actually saves 2162 dollars per year of vision gained [70-73]. Previous cost estimates per patient of retinopathy screening by a PCP was 31 dollars, which can significantly discount the 14,296 dollars per person yearly cost to the federal government for a patient with diabetes below age 65 who develops blindness. A full screening exam for diabetic retinopathy should include visual acuity, pupils, intraocular pressure, and a retinal exam with a slit lamp and indirect ophthalmoscope to look for macular edema and neovascularization. Patients with type 1 DM should have yearly exams after a 3-5 year length of disease [74-76]. Patients with type 2 DM should have an exam at diagnosis and then yearly [74]. However, only 33-68% of adults with diabetes in the US have annual dilated retinal exams [77-80]. The healthcare effectiveness data and information set (HEDIS) is a group of standardized measures related to various public health issues often used by America’s health plans to measure performance of care and service. Measures related to diabetes include annual eye exams, cholesterol screening and control, nephropathy monitoring, and HbA1c testing. In 2006, only 54.8% of diabetic patients with commercial insurance, 48.6% with Medicaid, and 66.5% with Medicare had dilated eye exams. While all 3 of these groups showed increases from 2000, there is still a large variance from the standard of care [81]. Moreover, 5 of 19 studies in a 2008 qualitative review of various minority groups, including African Americans, American Indians, and Hispanics, showed statistically significant decreased rates of eye examinations [82].
CDC data for 2010 showed that 64% of both African Americans and Non-Hispanic whites with diabetes had received a dilated eye exam in the past year, as compared to 55% of Hispanic subjects [83]. Sloan and Orr have both noted that African Americans are less probable to be screened for DR than whites [77,84]. A recent observational study also showed lower rates of dilated eye examinations among ethnic minorities compared with non-Hispanic white patients with diabetes [85,86].
Barriers to Screening
Patient factors: These include a lack of understanding of diabetic retinopathy and the importance and accessibility to treatment [78,80,87]. The following have all been mentioned as obstacles to routine screening: economic issues, spirituality, absence of time and transportation, and no desire to make follow-up appointments [26,78,80,84,87-89]. LALES reported that patients with lower socioeconomic status were at higher risk of not obtaining preventive care; those with no health care were twice as likely to not have had an eye exam or a visit to the doctor in the prior year [34]. In addition, health literacy, the extent to which patients can receive, process, and comprehend the simple health information and services they require to make proper health decisions [90], has been demonstrated to affect diabetes management, results, and knowledge [91,92]. Language barriers for non-English-speaking Hispanic patients, lack of access to specialty physicians, and a lack of health insurance have also been described as impediments to screening [82]. Other factors that may play a role are rejection of services, lack of timely care, and a general distrust of the medical system [79,82]. Walker et al. conducted a cross sectional study through telephone interview to assess the knowledge and health beliefs preventing African-Americans from undergoing regular screening for DR. In this patient group, “retinopathy” had the lowest level of perceived risk (30%), and only 21% of the sample believed there were efficacious treatments for retinopathy. 87% believed that “diabetic eye problems have symptoms.” 36% of the sample had heard of retinopathy, and of these, only 8% described it accurately. Obstacles mentioned by patients included: fear, spirituality (faith and hope), economic or logistical factors, and priorities [80].
Interventions concentrated on patients can help improve diabetic retinopathy screening. Culturally specific screening efforts [93], patient reminders [94], and educating patients about the importance of DR screening [80,87] have all demonstrated positive effect in some settings [78,80,87,89,94-96]. In addition, personalized follow-up and patient reminders, and diabetes management programs that enhance self-management skills can all lead to improved screening rates. Language barriers and health literacy can be addressed by interventions to mend any disparities in patient-provider communication [92,97-99].
Provider factors: These include insufficient patient education [79,80], patient-physician interaction [87], and time limitations [100], all of which prevent patients from completing proper screening exams in suggested timeframes. Mukamel et al. inspected a number of obstacles to compliance with diabetic screening standards; in his study, the most important limitation was the average number of primary care physician (PCP) visits each patient had. This implies that an increase in contact may allow more time for proper education and interaction with the patient [97,101]. Even an increase of 1 monthly visit to the PCP escalated the odds of screening by 28% [10].
Provider-level interventions such as educating primary care providers about screening guidelines and diabetic retinopathy may improve screening rates [102]. Also, training to aid providers in increasing their quality of screening and helping them track their adherence to guidelines may also be efficacious [103,104]. In addition, cultural competency can also play a role in increasing the number of ethnic minority patients who are screened [105,106].
System factors: These include: an extended waiting time for appointments, problems scheduling appointments, and a lack of insurance [26,87]. A cross-sectional analysis by Kirk JK, et al. showed that physicians treating African-American patients had greater difficulty acquiring subspecialty care and diagnostic imaging for their patients. Insufficient numbers of optometrists and ophthalmologists in addition to a high turnover also contribute to lack of screening [101].
System-level interventions can be undertaken to improve screening rates as well. These include patient registries and diabetes collaboratives to improve the quality of diabetes care [102-104,107], provider reminders (such as prompts in electronic medical records) [108,109], to aid providers in tracking which patients have missed screening appointments, population-based screening programs [110], diabetes management programs, including those that have been undertaken by HMOs [93,111,112], and community based diabetes management and DR screening programs [93]. Telemedicine [113,114], mobile screening [114-121], and the placement of diabetic retinopathy screening equipment in primary care offices may also help decrease the number of cases of DR that progress to vision-threatening complications; this is particularly valid in populations with limited health care access, including rural Native American tribes and various other rural communities.
Importantly, retinal imaging in primary care offices has been quite successful in eradicating some of the obstacles that impede diabetic patients from undergoing diabetic retinopathy screening [113,122,123,125,126]. Patients with diabetic retinopathy (or ungradeable disease) noted on photographs may be referred to ophthalmologists for further evaluation and treatment; this may eventually lower the number of overall screening referrals to ophthalmology for disease not warranting treatment, thus allowing more resources for evaluation of patients with ungradeable pathology on photos or treatment of those with clearly noted disease [124]. The ubiquitous nature of smartphones and their ever increasing quality of cameras also opens new avenues for screening without the need for expensive equipment. Building databases that integrate machine learning algorithms can further decrease the amount of photos and exams that need to be performed by an ophthalmologist [127]. International efforts to combat vision loss from diabetic retinopathy are beginning to make a difference. Between 2003 and 2008, England and Wales introduced nationwide diabetic retinopathy screening services, which annually screen almost 2 million people. A comparison of reasons for blindness certifications in England and Wales in working age adults for 2009-2010 showed diabetic retinopathy dropping to second place, for the first time in over fifty years [128].
Conclusion
Diabetic retinopathy is a significant public health issue that is projected to only increase in impact in the coming years. Minority populations are particularly vulnerable to macro and microvascular complications of diabetes, particularly retinopathy. Their vulnerability combined with insufficient screening is a recipe for many vision-threatening complications. The authors seek to raise awareness and provide a review of many aspects of diabetic retinopathy with reminders of the social implications of DR, especially in regard to minority populations, focusing on pathogenesis, risk factors, classification, and treatment options for this condition. With greater awareness, increased screening, and encouragement, we are hopeful that diabetic retinopathy will no longer be the number one cause of new cases of blindness in adults in the United States.
This work is sponsored in part by the Brooklyn Health Disparities Center NIH grant #P20 MD006875.
- Morello CM (2007) Etiology and natural history of diabetic retinopathy: an overview. American journal of health-system pharmacy: AJHP official journal of the American Society of Health-System Pharmacists 64(17 Suppl 12): S3-S7. Link: https://goo.gl/txJZq8
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care27: 1047-1053. Link: https://goo.gl/q26ZfH
- (2011) US Department of Health and Human Services CfDCaP. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Accessed May 5, 2017. Link: https://goo.gl/W37cc9
- Kempen JH, O'Colmain BJ, Leske MC, Steven M Haffner, Ronald Klein, et al. (2004) The prevalence of diabetic retinopathy among adults in the United States. Archives of ophthalmology.122: 552-563. Link: https://goo.gl/GMfP6y
- Zhang X, Saaddine JB, Chou CF, Mary Frances Cotch, Yiling J. Cheng, et al. (2010) Prevalence of diabetic retinopathy in the United States, 2005-2008. Jama304: 649-656. Link: https://goo.gl/YwJ6b7
- Harris EL, Feldman S, Robinson CR, Sherman S, Georgopoulos A (1993) Racial differences in the relationship between blood pressure and risk of retinopathy among individuals with NIDDM. Diabetes care 16: 748-754. Link: https://goo.gl/itF6GS
- Harris EL, Sherman SH, Georgopoulos A (1999) Black-white differences in risk of developing retinopathy among individuals with type 2 diabetes. Diabetes care22: 779-783. Link: https://goo.gl/mxqX7g
- Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD (1998) Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes care 21: 1230-1235. Link: https://goo.gl/f7XU66
- Wong TY, Klein R, Islam FM, Mary Frances Cotch, Aaron R Folsom, et al. (2006) Diabetic retinopathy in a multi-ethnic cohort in the United States. American journal of ophthalmology 141: 446-455. Link: https://goo.gl/hV5SzV
- Nsiah-Kumi P, Ortmeier SR, Brown AE (2009) Disparities in diabetic retinopathy screening and disease for racial and ethnic minority populations--a literature review. Journal of the National Medical Association101: 430-437. Link: https://goo.gl/2moVy7
- (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98(5 Suppl): 786-806. Link: https://goo.gl/KwE6SX
- Klein BE, Klein R, Wang Q, Moss SE (1995) Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic epidemiology2: 49-55. Link: https://goo.gl/RFzah3
- Klein BE, Klein R, Moss SE (1995) Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. American journal of ophthalmology119: 295-300. Link: https://goo.gl/XqG5Fo
- Stitt AW, Curtis TM, Chen M, Reinhold J. Medina, Gareth J. McKay, et al. (2016) The progress in understanding and treatment of diabetic retinopathy. Progress in retinal and eye research 51: 156-186. Link: https://goo.gl/28YcRw
- Duh E, Sun JK, Stitt AW (2017) Diabetic Retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2: e93751. Link: https://goo.gl/DPXnMn
- Korobelnik JF, Do DV, Schmidt-Erfurth U, David S. Boyer, Frank G. Holz, et al. (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology121: 2247-2254. Link: https://goo.gl/RGK33a
- Massin P, Bandello F, Garweg JG, Lutz L. Hansen, Simon P. Harding, et al. (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes care 33: 2399-2405. Link: https://goo.gl/gH9SKW
- Mitchell P, Bandello F, Schmidt-Erfurth U, Gabriele E. Lang, Pascale Massin, et al. (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118: 615-625. Link:
- Nguyen QD, Shah SM, Heier JS, et al. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175-2181 e2171. Link: https://goo.gl/5RUiE9
- Michaelides M, Kaines A, Hamilton RD, Samantha Fraser-Bell, Ranjan Rajendram, et al. (2010) A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 117: 1078-1086 e1072. Link: https://goo.gl/Fre9Mv
- Do DV, Schmidt-Erfurth U, Gonzalez VH, Carmelina M. Gordon, Michael Tolentino, et al. (2011) The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology118: 1819-1826. Link: https://goo.gl/K4S42L
- Yanoff Myron, DJS (2013) Ophthalmology: Expert Consult. Elsevier Health Sciences.
- Group DR (1988) Are continuing studies of metabolic control and microvascular complications in insulin-dependent diabetes mellitus justified? The Diabetes Control and Complications Trial. The New England journal of medicine 318: 246-250. Link: https://goo.gl/QGfgcR
- Epidemiology of Diabetes Interventions and Complications (EDIC) (1999) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes care 22: 99-111.
- Stratton IM, Kohner EM, Aldington SJ, R. C. Turner, R. R. Holman, et al. (2001) UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis.Diabetologia 44: 156-163. Link: https://goo.gl/LLA9EV
- Photocoagulation treatment of proliferative diabetic retinopathy (1981) Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 88: 583-600.
- Ophthalmology AAo. Link: https://goo.gl/wH5nhv
- Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. The New England journal of medicine366: 1227-1239. Link: https://goo.gl/H2j51d
- Bowling B (2015) Kanski's Clinical Ophthalmology: A Systematic Approach. 8 edition Saunders Ltd. Link: https://goo.gl/tsKxbm
- Lamoureux EL, Hassell JB, Keeffe JE (2004) The impact of diabetic retinopathy on participation in daily living. Archives of ophthalmology 122: 84-88. Link: https://goo.gl/ozH8mS
- Munoz B, O'Leary M, Fonseca-Becker F, Evelyn Rosario, Isabel Burguess, et al. (2008) Knowledge of diabetic eye disease and vision care guidelines among Hispanic individuals in Baltimore with and without diabetes. Archives of ophthalmology 126: 968-974. Link: https://goo.gl/WnEc4g
- Munoz B, West SK, Rubin GS, OD Schein, Harry A. Quigley, et al. (2000) Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Archives of ophthalmology 118: 819-825. Link: https://goo.gl/LTxfp7
- Coyne KS, Margolis MK, Kennedy-Martin T, Timothy M Baker, Ronald Klein, et al. (2004) The impact of diabetic retinopathy: perspectives from patient focus groups. Family practice 21: 447-453. Link: https://goo.gl/6jioZw
- Varma R, Torres M, Pena F, Klein R, Azen SP, et al. (2004) Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 111: 1298-1306. Link: https://goo.gl/x5aaLs
- (2017) FFF: Hispanic Heritage Month 2016. Link: https://goo.gl/A6f97Q
- (2006) Control CfD. Accessed May 5, 2017. Link: https://goo.gl/d3jcHA
- (2017) Insititute NE. Accessed May 5. Link: https://goo.gl/iWXcu6
- West SK, Klein R, Rodriguez J, Muñoz B, Broman AT, et al. (2001) Diabetes and diabetic retinopathy in a Mexican-American population: Proyecto VER. Diabetes care24: 1204-1209. Link: https://goo.gl/kx32AC
- Paz SH, Varma R, Klein R, Wu J, Azen SP, et al. (2006) Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus: the Los Angeles Latino Eye Study. Ophthalmology 113: 1372-1377. Link: https://goo.gl/h3C6g8
- Hallman DM, Huber JC Jr, Gonzalez VH, Klein BE, Klein R, et al. (2005) Familial aggregation of severity of diabetic retinopathy in Mexican Americans from Starr County, Texas. Diabetes care28: 1163-1168. Link: https://goo.gl/RnN9d7
- Jandeleit-Dahm K, Cooper ME (2006) Hypertension and diabetes: role of the renin-angiotensin system. Endocrinology and metabolism clinics of North America 35: 469-490. Link: https://goo.gl/nAEUoS
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1984) The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Archives of ophthalmology 102: 527-532. Link: https://goo.gl/a8BBV5
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1984) The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of ophthalmology 102: 520-526. Link: https://goo.gl/MLB6hf
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1989) The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Archives of ophthalmology 107: 244-249. Link: https://goo.gl/z1cLrH
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1989) The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of ophthalmology107: 237-243. Link: https://goo.gl/NnYz6S
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE (2008) The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 115: 1859-1868. Link: https://goo.gl/WRPPTT
- Zhang X, Cotch MF, Ryskulova A, Primo SA, Nair P, et al. (2012) Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. American journal of ophthalmology 154(6 Suppl): S53-S62. Link: https://goo.gl/jxmXUX
- Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, et al. (2002) The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. The British journal of ophthalmology 86: 84-90. Link: https://goo.gl/jMvMFH
- Leske MC, Wu SY, Hennis A, Nemesure B, Schachat AP, et al. (2006) Nine-year incidence of diabetic retinopathy in the Barbados Eye Studies. Archives of ophthalmology124: 250-255. Link: https://goo.gl/fj4Sti
- Emanuele N, Sacks J, Klein R, Reda D, Anderson R, et al. (2005) Ethnicity, race, and baseline retinopathy correlates in the veterans affairs diabetes trial. Diabetes care28: 1954-1958. Link: https://goo.gl/s4qq42
- Emanuele N, Moritz T, Klein R, Davis MD, Glander K, et al. (2009) Ethnicity, race, and clinically significant macular edema in the Veterans Affairs Diabetes Trial (VADT). Diabetes research and clinical practice86: 104-110. Link: https://goo.gl/1B8Ttr
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes care35: 556-564. Link: https://goo.gl/zKv6cs
- Chang YC, Wu WC (2013) Dyslipidemia and diabetic retinopathy. The review of diabetic studies RDS 10: 121-132. Link: https://goo.gl/nv6s44
- Penman A, Hancock H, Papavasileiou E, James M, Idowu O,et al. (2016) Risk Factors for Proliferative Diabetic Retinopathy in African Americans with Type 2 Diabetes. Ophthalmic epidemiology23: 88-93. Link: https://goo.gl/UnKtgb
- Nittala MG, Keane PA, Zhang K, Sadda SR (2014) Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina 34: 1594-1599. Link: https://goo.gl/r1GxZ4
- Bower JK, Brancati FL, Selvin E (2013) No ethnic differences in the association of glycated hemoglobin with retinopathy: the national health and nutrition examination survey 2005-2008. Diabetes care 36: 569-573. Link: https://goo.gl/ob7pCf
- Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC (2012) Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Annals of internal medicine157: 153-159. Link: https://goo.gl/xAeezN
- Group AS, Group AES, Chew EY, et al. (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. The New England journal of medicine363: 233-244. Link: https://goo.gl/PYQxEy
- Roy MS (2000) Diabetic retinopathy in African Americans with type 1 diabetes: The New Jersey 725: I. Methodology, population, frequency of retinopathy, and visual impairment. Archives of ophthalmology 118: 97-104. Link: https://goo.gl/Dc2cTT
- (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 854-865. Link: https://goo.gl/vBh4BF
- (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj 317: 703-713. Link: https://goo.gl/k8xAPj
- Silva PS, Cavallerano JD, Sun JK, Aiello LM, Aiello LP (2010) Effect of systemic medications on onset and progression of diabetic retinopathy. Nature reviews. Endocrinology6: 494-508. Link: https://goo.gl/ffJVo1
- Simo R, Hernandez C (2009) Advances in the medical treatment of diabetic retinopathy. Diabetes care 32: 1556-1562. Link: https://goo.gl/RXgrMw
- Klein R, Klein BE, Moss SE, Cruickshanks KJ (1995) The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology102: 7-16. Link: https://goo.gl/eZFhak
- Klein R, Klein BE, Moss SE, Linton KL (1992) The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology 99: 58-62. Link: https://goo.gl/yfr5ZD
- Leske MC, Wu SY, Hyman L, Li X, Hennis A, et al. (1999) Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology 106: 1893-1899. Link: https://goo.gl/kHjyZd
- Lim A, Stewart J, Chui TY, Lin M, Ray K, et al. (2008) Prevalence and risk factors of diabetic retinopathy in a multi-racial underserved population. Ophthalmic epidemiology15: 402-409. Link: https://goo.gl/kfFoy6
- (1979) Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. The Diabetic Retinopathy Study Research Group. Archives of ophthalmology97: 654-655. Link: https://goo.gl/kBBeBc
- (1987) Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report no. 4. The Early Treatment Diabetic Retinopathy Study Research Group. Int ophthalmol clin 27: 265-272. Link: https://goo.gl/nokgk8
- Javitt JC, Aiello LP (1996) Cost-effectiveness of detecting and treating diabetic retinopathy. Ann intern med 124: 164-169. Link: https://goo.gl/bMCHtD
- Griffith SP, Freeman WL, Shaw CJ, Mitchell WH, Olden CR, et al. (1993) Screening for diabetic retinopathy in a clinical setting: a comparison of direct ophthalmoscopy by primary care physicians with fundus photography. The Journal of family practice 37: 49-56. Link: https://goo.gl/ncu432
- Javitt JC, Aiello LP, Bassi LJ, Chiang YP, Canner JK (1991) Detecting and treating retinopathy in patients with type I diabetes mellitus. Savings associated with improved implementation of current guidelines. American Academy of Ophthalmology. Ophthalmology98: 1565-1573. Link: https://goo.gl/5myjLH
- Maberley D, Walker H, Koushik A, Cruess A (2003) Screening for diabetic retinopathy in James Bay, Ontario: a cost-effectiveness analysis. CMAJ 168: 160-164. Link: https://goo.gl/uEpZqh
- Standards of medical care in diabetes--2008. Diabetes care 31 Suppl. American Diabetes A 1: S12-54. Link: https://goo.gl/hyVkGH
- (1992) Screening guidelines for diabetic retinopathy. American College of Physicians, American Diabetes Association, and American Academy of Ophthalmology. Annals of internal medicine 116: 683-685. Link: https://goo.gl/BzwiUF
- Fong DS, Aiello LP, Ferris FL, 3rd, Klein R (2004) Diabetic retinopathy. Diabetes care 27: 2540-2553. Link: https://goo.gl/aDQdDu
- Orr P, Barron Y, Schein OD, Rubin GS, West SK (1999) Eye care utilization by older Americans: the SEE Project. Salisbury Eye Evaluation. Ophthalmology 106: 904-909. Link: https://goo.gl/D7p4cM
- Schoenfeld ER, Greene JM, Wu SY, Leske MC (2001) Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology 108: 563-571. Link: https://goo.gl/4Ptueh
- Moss SE, Klein R, Klein BE (1995) Factors associated with having eye examinations in persons with diabetes. Arch Fam Med 4: 529-534. Link: https://goo.gl/LN7D6V
- Walker EA, Basch CE, Howard CJ, Zybert PA, Kromholz WN, et al. (1997) Incentives and barriers to retinopathy screening among African-Americans with diabetes. J Diabetes Complications 11: 298-306. Link: https://goo.gl/xtAmRF
- Schoenbaum SC, Holmgren AL (2006) The National Committee for Quality Assurance’s The State of Health Care Quality 2006. Issue Brief 1: 1-6. Link: https://goo.gl/qaZ3Xs
- Kirk JK, Passmore LV, Bell RA, Narayan KM, D'Agostino RB, et al. (2008) Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes care 31: 240-246. Link: https://goo.gl/QW3DXd
- Hiroi S, Sugiura K, Matsuno K, Hirayama M, Kuriyama K, et al. (2013) A multicenter, phase III evaluation of the efficacy and safety of a new fixed-dose pioglitazone/glimepiride combination tablet in Japanese patients with type 2 diabetes. Diabetes technol ther 15: 158-165. Link: https://goo.gl/eCxdED
- Sloan FA, Brown DS, Carlisle ES, Picone GA, Lee PP (2004) Monitoring visual status: why patients do or do not comply with practice guidelines. Health serv Res 39: 1429-1448. Link: https://goo.gl/2SpWqn
- Shi Q, Zhao Y, Fonseca V, Krousel-Wood M, Shi L (2014) Racial disparity of eye examinations among the U.S. working-age population with diabetes: 2002-2009. Diabetes care 37: 1321-1328. Link: https://goo.gl/baA6cQ
- Tomar SL, Lester A (2000) Dental and other health care visits among U.S. adults with diabetes. Diabetes care 23: 1505-1510. Link: https://goo.gl/KhSfY8
- Hartnett ME, Key IJ, Loyacano NM, Horswell RL, Desalvo KB (2005) Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch ophthalmol 123: 387-391. Link: https://goo.gl/qweVPD
- Roy MS (2004) Eye care in African Americans with type 1 diabetes: the New Jersey 725. Ophthalmology 111: 914-920. Link: https://goo.gl/68m51y
- Anderson RM, Musch DC, Nwankwo RB, Wolf FM, Gillard ML, et al. (2003) Personalized follow-up increases return rate at urban eye disease screening clinics for African Americans with diabetes: results of a randomized trial. Ethnicity & disease 13: 40-46. Link: https://goo.gl/SKweUL
- Nielsen-Bohlman L, Panzer AM, Kindig DA, eds (2004) Health Literacy: A Prescription to End Confusion. Washington (DC). Link: https://goo.gl/pTZ38n
- Williams MV, Baker DW, Parker RM, Nurss JR (1998) Relationship of functional health literacy to patients' knowledge of their chronic disease. A study of patients with hypertension and diabetes. Arch inter med 158: 166-172. Link: https://goo.gl/aLjGmr
- Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, et al. (2003) closing the loop: physician communication with diabetic patients who have low health literacy. Archives of internal medicine 163: 83-90. Link: https://goo.gl/NNEvfy
- Anderson RM, Wolf FM, Musch DC, Fitzgerald JT, Johnson MW, et al. (2002) Conducting community-based, culturally specific, eye disease screening clinics for urban African Americans with diabetes. Ethnicity & disease 12: 404-410. Link: https://goo.gl/zazshC
- Halbert RJ, Leung KM, Nichol JM, Legorreta AP (1999) Effect of multiple patient reminders in improving diabetic retinopathy screening. A randomized trial. Diabetes care 22: 752-755. Link: https://goo.gl/YKArSb
- Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P (1999) the effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. American journal of public health 89: 1878-1882. Link: https://goo.gl/zdxzBR
- Lafata JE, Baker AM, Divine GW, McCarthy BD, Xi H (2002) the use of computerized birthday greeting reminders in the management of diabetes. Journal of general internal medicine 17: 521-530. Link: https://goo.gl/fgbXXZ
- Mukamel DB, Bresnick GH, Wang Q, Dickey CF (1999) Barriers to compliance with screening guidelines for diabetic retinopathy. Ophthalmic epidemiol 6: 61-72. Link: https://goo.gl/nuRzYz
- Piette JD, Schillinger D, Potter MB, Heisler M (2003) Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J gen intern med 18: 624-633. Link: https://goo.gl/9zdVKG
- Aikens JE, Bingham R, Piette JD (2005) Patient-provider communication and self-care behavior among type 2 diabetes patients. The Diabetes educator 31: 681-690. Link: https://goo.gl/7pWzh6
- Chin MH, Cook S, Jin L, Drum ML, Harrison JF, et al. (2001) Barriers to providing diabetes care in community health centers. Diabetes care 24: 268-274. Link: https://goo.gl/SiBNMg
- Silver K, Williams M, Macario E (2006) The National Eye Health Education Program: increasing awareness of diabetic eye disease among American Indians and Alaska Natives. Ethnicity & disease 16: 920-925. Link: https://goo.gl/AC99NB
- Gorman CA, Zimmerman BR, Smith SA, Dinneen SF, Knudsen JB, et al. (2000) DEMS - a second generation diabetes electronic management system. Computer methods and programs in biomedicine 62: 127-140. Link: https://goo.gl/DVb6Lo
- Chin MH, Cook S, Drum ML, Jin L, Guillen M, et al. (2004) Improving diabetes care in midwest community health centers with the health disparities collaborative. Diabetes care 27: 2-8. Link: https://goo.gl/4p8eij
- Chin MH, Drum ML, Guillen M, Rimington A, Levie JR, et al. (2007) Improving and sustaining diabetes care in community health centers with the health disparities collaboratives. Medical care 45: 1135-1143. Link: https://goo.gl/sWUtXW
- Anderson S, Broadbent DM, Swain JY, Vora JP, Harding SP (2003) Ambulatory photographic screening for diabetic retinopathy in nursing homes. Eye 17: 711-716. Link: https://goo.gl/YKBLiL
- Betancourt JR, Green AR, Carrillo JE, Park ER (2005) Cultural competence and health care disparities: key perspectives and trends. Health affairs 24: 499-505. Link: https://goo.gl/qnGmtC
- Gorman C, Looker J, Fisk T, Oelke W, Erickson D, et al. (1996) a clinically useful diabetes electronic medical record: lessons from the past; pointers toward the future. European journal of endocrinology 134: 31-42. Link: https://goo.gl/W1PcJn
- Baker RS, Bazargan M, Bazargan-Hejazi S, Calderon JL (2005) Access to vision care in an urban low-income multiethnic population. Ophthalmic epidemiology 12: 1-12. Link: https://goo.gl/Fj7gDx
- Baker SB, Vallbona C, Pavlik V, Fasser CE, Armbruster M et al. (1993) A diabetes control program in a public health care setting. Public health reports 108: 595-605. Link: https://goo.gl/LGg8uo
- McCulloch DK, Price MJ, Hindmarsh M, Wagner EH (1998) a population-based approach to diabetes management in a primary care setting: early results and lessons learned. Effective clinical practice: ECP 1: 12-22. Link: https://goo.gl/Zjti83
- Dinneen SF, Bjornsen SS, Bryant SC, Zimmerman BR, Gorman CA, et al. (2000) towards an optimal model for community-based diabetes care: design and baseline data from the Mayo Health System Diabetes Translation Project. Journal of evaluation in clinical practice 6: 421-429. Link: https://goo.gl/q1PdqY
- Montori VM, Dinneen SF, Gorman CA, Zimmerman BR, Rizza RA, et al. (2002) the impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes care25: 1952-1957. Link: https://goo.gl/v9FuEj
- Leese GP, Morris AD, Swaminathan K, Petrie JR, Sinharay R, et al. (2005) Implementation of national diabetes retinal screening programme is associated with a lower proportion of patients referred to ophthalmology. Diabetic medicine: a journal of the British Diabetic Association22: 1112-1115. Link: https://goo.gl/BY52w8
- Gillibrand W, Broadbent D, Harding S, Vora J (2004) The English national risk-reduction programme for preservation of sight in diabetes. Molecular and cellular biochemistry 261: 183-185. Link: https://goo.gl/ysbPkS
- Choremis J, Chow DR (2003) Use of telemedicine in screening for diabetic retinopathy. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie 38: 575-579. Link: https://goo.gl/ydmcvJ
- Sender Palacios MJ, Maseras Bover M, Vernet Vernet M, Larrosa Sáez P, de la Puente Martorellc ML,et al. (2003) Aplicacion de un metodo de deteccion precoz de retinopatia diabetica en la atencion primaria de salud. Rev Clin Esp 203: 224-229. Link: https://goo.gl/r8XQSY
- Massin P, Aubert JP, Erginay A, Bourovitch JC, Benmehidi A, et al. (2004) Screening for diabetic retinopathy: the first telemedical approach in a primary care setting in France. Diabetes & metab 30: 451-457. Link: https://goo.gl/7Uu8s8
- Massin P, Aubert JP, Eschwege E, Erginay A, Bourovitch JC, et al. (2005) Evaluation of a screening program for diabetic retinopathy in a primary care setting Dodia (Depistage ophtalmologique du diabete) study. Diabetes & metab 31: 153-162. Link: https://goo.gl/rcSiYY
- Stellingwerf C, Hardus PL, Hooymans JM (2001) Two-field photography can identify patients with vision-threatening diabetic retinopathy: a screening approach in the primary care setting. Diabetes care 24: 2086-2090.Link: https://goo.gl/zesMcC
- Chia DS, Yap EY (2004) Comparison of the effectiveness of detecting diabetic eye disease: diabetic retinal photography versus ophthalmic consultation. Singapore medical journal 45: 276-279. Link: https://goo.gl/GjLZsD
- Littenberg B, MacLean CD (2008) Mandated diabetes registries will benefit persons with diabetes. Archives of internal medicine 168: 797-799; discussion 802-793. Link: https://goo.gl/reSdVU
- Scanlon PH, Carter S, Foy C, Ratiram D, Harney B (2005) An evaluation of the change in activity and workload arising from diabetic ophthalmology referrals following the introduction of a community based digital retinal photographic screening programme. British journal of ophthalmology 89: 971-975. Link: https://goo.gl/aoc4vX
- Eiser JR, Eiser C, Riazi A, Taylor DJ, Hammersley S, et al. (2001) Screening for diabetic retinopathy is well received by patients and may improve self-management intentions. Diabetic medicine: a journal of the British Diabetic Association18: 835-841. Link: https://goo.gl/JsTUUD
- Carter JS, Pugh JA, Monterrosa A (1996) Non-insulin-dependent diabetes mellitus in minorities in the United States. Annals of internal medicine 125: 221-232. Link: https://goo.gl/D6gi5N
- Mansberger SL, Sheppler C, Barker G, Gardiner SK, Demirel S, et al. (2015) Long-term Comparative Effectiveness of Telemedicine in Providing Diabetic Retinopathy Screening Examinations: A Randomized Clinical Trial. JAMA Ophthalmology 133: 518-525. Link: https://goo.gl/UsQ6w9
- Bolster NM, Giardini ME, Bastawrous A (2015) the diabetic retinopathy screening workflow: Potential for smartphone imaging. J Diabetes Sci Technol 10: 318-324. Link: https://goo.gl/cEYDWT
- Wong TY, Bressler NM (2016) Artificial intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA 316: 2366-2367. Link: https://goo.gl/z34hdd
- Liew G, Michaelides M, Bunce C (2014) A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years), 1999-2000 with 2009-2010. BMJ Open4: e004015. Link: https://goo.gl/X3TyBf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
