International Journal of Clinical Endocrinology and Metabolism
Protein S100A8/A9: A Potential New Biomarker for Pancreatic Diseases
Alexander T El Gammal1*, Jörn H Sturm1*, Hans O Pinnschmidt2, Bianca T Hofmann1, Eugen Bellon1, Tarik Ghadban1, Nathaniel Melling1, Kai Bachmann1, Jakob R Izbicki1, Maximilian Bockhorn1, Cenap Güngör1# and Daniel R Perez1#
2Department of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
*Shared first authorship
#Shared senior authorship
Cite this as
El Gammal AT, Sturm JH, Pinnschmidt HO, Hofmann BT, Bellon E, et al. (2017) Protein S100A8/A9: A Potential New Biomarker for Pancreatic Diseases. Int J Clin Endocrinol Metab 3(1): 023-028. DOI: 10.17352/ijcem.000025Objectives: S100A8/A9 expression has been linked to carcinogenesis and inflammation. We hypothesized that S100A8/A9 protein serum levels are a useful stratification marker for patients with pancreatic ductal adenocarcinoma (PDAC), intraductal papillary mucinous neoplasm (IPMN) or chronic pancreatitis (CP).
Methods: S100A8/A9 serum levels were analysed in PDAC, CP and IPMN patients and compared to S100A8/A9 healthy donor controls (HD) using ELISA. S100A8/A9 levels and clinical data were statistically analysed.
Results: Out of 134 patients included, 84 were diagnosed with PDAC (46%), 30 patients with IPMN (16%) and 20 patients with CP (11%), 50 patients were HD (27%). When compared to HD (343.3 ng/ml), S100A8/A9 serum concentration was elevated in PDAC (402.0 ng/ml, p = 0.001) and CP patients (426.51 ng/ml p < 0.001). Also, S100A8/A9 levels were elevated in PDAC compared to IPMN group (369.0 ng/ml p = 0.026) and in CP compared to IPMN group (p = 0.001). A multivariate model including age, gender, leukocyte levels, C-reactive protein (CrP), S100A8/A9 and CA 19-9 concentrations reported a diagnostic sensitivity of 74.8%.
Conclusion: S100A8/A9 serum levels are increased in patients with PDAC, CP, and IPMN and might be useful to distinguish malignant and inflammatory diseases from normal and non-malignant pathological conditions.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal cancers worldwide and is still substantiated by insufficient diagnostic tools and therapeutic options. The overall 5-year survival rate among PDAC patients is less than 5%, which is partially due to an almost symptomless progression. Today, PDAC is ranked as the 4th leading cause of cancer related death worldwide.
The S100 protein family is the largest subgroup of the Ca2+-binding EF-hand (helix E-loop-helix F) proteins [1]. First being identified by Moore in 1965 [2], S100 proteins are expressed in a wide range of tissues featuring multiple cellular functions such as contraction, motility, cell growth, differentiation, and secretion [1,3]. As a member of the S100 family, S100A8/A9 is composed of two subunits, S100A8 and S100A9, both forming heterogenous multimers. The S100A8/A9 protein expression is primarily found in cells of the innate immune response [4-6], as well as in inflammatory endothelial and epithelial cells [7,8]. Several autoimmune diseases are associated with S100A8/A9 expression such as rheumatoid arthritis, cystic fibrosis, or inflammatory bowel disease [5,9,10].
Initially, the S100A8/A9 protein has been described in neutrophils and macrophages and was shown to be involved in the regulation of innate immunity and inflammation [11], Known S100A8/A9 functions include regulation of phagocyte transmigration and extravasation [9-13], arachidonic acid transport in neutrophils [14], regulation of the NADPH oxidase complex [10,15,16] and NO transport [17]. Consequently, it was identified in serum of patients with acute pancreatitis [18].
More recently, an association of S100A8/A9 protein expression with adenocarcinoma in human has emerged [19-21]. Immunohistochemical investigations have shown that the protein is overexpressed in gastric [22,23], ovarian [24], colorectal [25-27], thyroid [28], bladder [29], hepatocellular [30], prostate cancer [31] and invasive ductal carcinomas of the breast [32,33]. In these tumors, elevated S100A8/A9 expression was correlated with poor differentiation or prognosis, respectively. In patients with ovarian carcinomas, S100A8/A9 was found to be enriched in cystic fluid and serum [24]. Consequently, S100A8/A9 has been examined in regard of its suitability as a possible biomarker for cancer diagnosis. Genomic profiling studies revealed overexpression of the protein in pancreatic cancer tissue, microdissections, pancreatic cyst fluid, and pancreatic juice [34-39]. To the best of our knowledge, sera of patients with neoplastic or inflammatory pancreatic lesions have not been screened for S100A8/A9 so far. Therefore, this study investigates S100A8/A9 protein serum levels in PDAC patients, intraductal papillary mucinous neoplasia (IPMN) patients and chronic pancreatitis (CP) patients in comparison to serum levels from healthy donors (HD) in order to evaluate its role as a potential biomarker.
Materials and Methods
Patient samples
Patient serum samples were obtained before surgery with approval of the institutional review board and after informed written consent. In total, 84 Patients were diagnosed with PDAC, 30 patients with IPMN or 20 patients CP (n=20) as well as 50 HD were included. Samples were immediately processed and stored at -80°C. Serum marker C-reactive protein (CrP), Carbohydrate-Antigen 19-9 (CA 19-9) and leukocyte serum levels were determined before surgery. Patients with neoadjuvant chemo - or radiotherapy were excluded.
All patients were treated at the Department of General, Visceral, and Thoracic Surgery, University Medical Center Hamburg-Eppendorf between 2008 and 2013. Tissue diagnosis was reviewed by an experienced hepato-pancreatico-biliary pathologist.
S100A8/A9 ELISA
S100A8/A9 (serum) protein levels were measured using Calprotectin Enzyme Linked Immunosorbent Assay (ELISA) kit (Hycultec; Beutelsbach, Germany) in accordance to the manufacturer’s protocol. The Tracer was incubated overnight at 4°C.
Statistical analysis
Metric variables were tested for normality via Kolgomorov-Smirnov and Shapiro-Wilk tests. Their distributions were also assessed via histograms and boxplots. Means, medians and 1st and 3rd quartiles (interquartile range; IQR) of metric variables were reported. The variables CrP and CA 19-9, which displayed heavily right-skewed distributions, were ln-transformed (ln[x+1]) prior to further analyses. The means of variable S100A8/A9 were compared across diagnostic groups using student’s t-test. Variables CA 19-9, CrP and leukocytes had about 27%, 8% and 6% missing values, respectively. Multiple imputations were therefore performed using the monotone method for data having a monotone pattern of missing and the Markov chain Monte Carlo method for data with non-monotone pattern of missing, thus yielding 10 imputed data sets. Diagnosis, as a dependent nominally scaled variable consisting of 3 categories (0= PDAC 1=IPMN and 2=CP), was then subjected to multinomial logistic regression modelling, using the variables ln(CA 19-9+1), S100A8/A9, ln(CrP+1), leukocytes, age and gender of the 10 imputed data sets as independent variables (covariates). All independent variables were tested in univariate models as well as in multivariate models. The multivariate approach started with an initial model containing all main effect terms of the independent variables plus all their two-way interaction terms. Non-significant terms were then removed, based on the p-values of likelihood-ratio tests (α = 0.05), following a stepwise hierarchical backward elimination procedure sensu Kleinbaum & Klein [40]. However, the main effect terms of all independent variables were kept in the final multivariate model, even if they were not significant. Model-predicted diagnosis was cross-tabulated versus observed diagnosis for comparison of models and Nagelkerke’s pseudo R2 was computed to indicate strength of association and model fit. All statistical analyses were done using SPSS version 22.
Results
Patient demographics are summarized in tables 1,2. A total of 184 individuals were included in the study. Of these, 81 males (44%) and 90 females (49%). In 13 patients (7%) no gender information was available. Mean age at operation was 66.0 years, with median age of 67.0 years and a range between 40 to 83 years of age. Out of 134 patients, 84 were histopathologically diagnosed with PDAC (63%), 30 patients featured IPMN (22%), 20 patients had CP (15%). Fifty healthy controls (HD) were included.
In the PDAC group, the 30-day mortality rate was 4.8% (4/84). Median overall survival in the PDAC group (n=84) was 6.9 months (31 to 1189 days). Most tumors showed moderate (n=54) or poor (n=21) histological differentiation. Only a minority (n=2) featured a well-differentiated histological phenotype. M-status was available for 21 patients; 14 patients featured M1 status and 7 patients M0 status. 24/84 patients were treated with a palliative approach only, due to advanced stage of disease. For seven PDAC patients, no histological data were available.
In 73% (61/84) of the investigated PDAC patients, the pT stage was recorded and the majority of the PDAC patients featured a pT3 stage tumor (85%). Lymph node metastases were present in 81% (50/62) of PDAC patients. A positive resection and circumferential surgical margin status was present in 59% (33/56). None of the PDAC patients received neo-adjuvant chemo-/radiotherapy.
CA19-9 serum concentrations are shown in table 1. CA19-9 data were heavily right-skewed. In 77% (53/69) of the PDAC patients, pre-operative serum CA19-9 values were increased (> 37 U/mL; (Table 1). Median CA 19-9 was 311.60 U/mL (1st and 3rd quartile: 40 and 1182.5 U/mL, respectively). 15 of the 84 (18%) PDAC patients did not feature pre-operative CA 19-9 data.
In the IPMN patient group, median pre-operative CA 19-9 levels were 11.15 U/mL (3.98 and 35.90 U/mL). In total, 39% (7/18) of IPMN patients had increased pre-operative CA 19-9 levels.
In the CP patients group, CA 19-9 data were available for 55% (11/20) of the CP patients with a median value of 11.20 U/ml (8.60 and 83.70 U/mL).
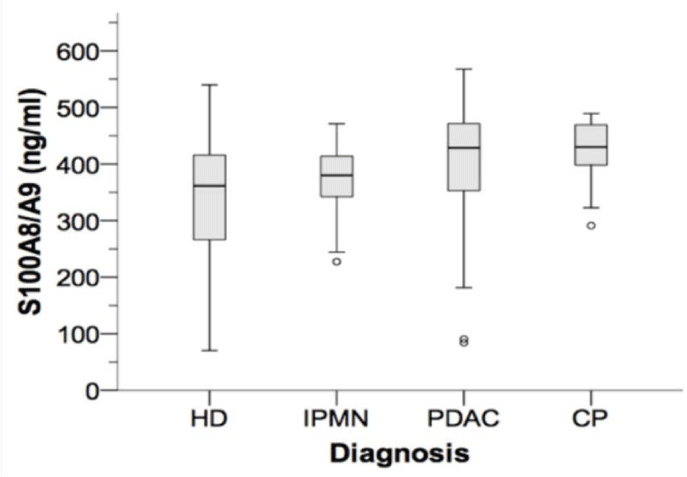
In 184 individual serum probes, we analyzed and quantified S100A8/A9 protein levels using ELISA. S100A8/A9 data were fairly normally distributed. Mean values for serum levels of S100A8/A9 were 402.02 (1st and 3rd quartile: 351.55 and 471.69, respectively) ng/mL in PDAC patients, 369.00 (342.23 and 414.16) ng/mL in IPMN patients, 426.51 (397.91 and 470.10) ng/mL in CP patients and 343.32 (266.09 and 417.76) in healthy donors, respectively (Table 1, Figure 1).
Interestingly, we found significantly increased S100A8/A9 protein serum levels in PDAC and CP patients, compared to IPMN patients and HD (p < 0.01; P=0.026 for PDAC vs. IPMN; table 3).
No difference was detected in both PDAC and CP patient serum levels. Also, there was no significant difference between HD and IPMN S100A8/A9 serum levels (Table 1,3).
Additionally, we investigated a possible relation between serum S100A8/A9 protein levels and histological grading, TNM-staging, survival time, serum CA 19-9 levels, leukocytes and CrP as well as patients’ age and gender.
We found that leukocytes and CrP serum levels correlate with S100A8/A9 protein serum levels on a statistically significant level (p < 0.01, data not shown).
However, no statistically significant difference was found between well (G1-G2) and poorly (G3) differentiated cancers as well as between T3 and T4 staging and lymph node status.
Statistical analyses and correlation of S100A8/A9 protein levels with survival time, CA 19-9, patients’ age or gender revealed no correlation at all.
To determine whether S100A8/A9 protein levels might be suitable as a possible stratification marker for pancreatic cancer, we chose a panel of four serum markers (Table 4).
A multivariate multinomial logistic regression model was developed using leukocytes, ln(CrP + 1), ln(CA19-9 + 1) and S100A8/A9 serum levels as covariates combined with the variables gender and age.
The final multivariate model after removal of redundant variables yielded 74.8% correctly classified diagnoses. In comparison, a univariate model based on ln(CA19-9 + 1) alone yielded 61.3% correctly classified cases (Table 4). Nagelkerke’s pseudo-R2 indicated that only the multivariate model fits the data relatively well.
Discussion
The current study reports for the first time serum protein levels of S100A8/A9 in PDAC and IPMN patients. Members of the S100 protein family are frequently up regulated in various types of autoimmune diseases such as rheumatoid arthritis, psoriasis or inflammatory bowel disease, but also in numerous cancer types [5,9,10,33].
A plethora of various proteomic profiling studies on potentially new serum markers for PDAC diagnosis were performed within the last years [41-46]. Remarkably, various studies already identified the pro-inflammatory S100A8/A9 protein as overexpressed in PDAC tumor tissue [34-37]. Moreover, it was previously shown that human pancreatic cancer cell lines Capan1, Panc-1, MiaPaCa2, and BxPC3 express high levels of S100A8/A9 [47].
In order to evaluate the diagnostic value of S100A8/A9 in pancreatic tumors and as an easy to obtain resource in clinical practice, patient serum samples were analysed. Elevated protein levels in PDAC sera as well as in sera obtained from patients diagnosed solely with CP were detected. While there was no significant difference in S100A8/A9 between PDAC and CP, S100A8/A9 levels were elevated in PDAC, compared to IPMN patients and HD.
Chen et al. previously found an overexpression of S100A8/A9 protein levels in pancreatic main duct fluid and reported that its concentration correlates with median survival [34]. In the present study S100A8/A9 serum level was not associated with outcome. Statistically significant correlations existed between S100A8/A9 protein levels and CrP as well as leukocyte levels, however, this is not surprising since S100A8/A9 protein is known to be an inflammatory-linked protein.
In colorectal cancer, Kim et al. identified S100A8/A9 protein plasma levels to be more specific and sensitive compared to the established tumor marker carcinoembryonic antigen CEA [26]. Also, in prostate cancer, Hermani et al. reported serum levels of S100A9 to be more sensitive than PSA when discriminating between prostate cancer and benign prostate hyperplasia [31]. Likewise, S100A8 and S100A9 serum levels were recently identified as potential biomarkers for renal cell cancer early-detection [48].
Our multivariate multinomial logistic regression model (containing the biomarkers leukocytes, CrP, CA 19-9 and S100A8/A9 protein levels, gender and age), performed better in estimating different histologic subtypes of pancreatic lesions than any univariate model, yielding about 75% correctly estimated diagnoses. In contrast, the CA 19-9-based model correctly classified only about 61% of the diagnoses.
The S100A8/A9 protein has been identified to be a potent chemoattractant for myeloid-derived suppressor cells (MDSC) [49-53]. While MDSC seem to be the main source of S100A8/A9 protein in cancer patients, it remains unclear to which degree malignant cells express the S100A8/A9 protein. Immunohistochemical studies of pancreatic cancer tissue identified the S100A8/A9 protein as exclusively expressed in myeloid cells infiltrating the tumor-stroma [36], although several pancreatic cancer cell lines are already known to express S100A8/A9 [47,54,55].
In conclusion, our study identified increased S100A8/A9 expression levels in patients suffering from PDAC, CP and IPMN. Also, the correlation of S100A8/A9 protein serum levels with commonly available clinical data revealed that it could help stratification to distinguish patients with malignant and/ or inflammatory disease from normal and non-malignant pathological conditions. The combined quantification of S100A8/A9 and CA-19-9 serum levels in patients points to a higher sensitivity for diagnosis. The latter, regarding the crux of late pancreatic cancer diagnosis, might be another important and necessary step towards early cancer detection.
- Sedaghat F, Notopoulos A (2008) S100 protein family and its application in clinical practice. Hippokratia 12: 198-204. Link: https://goo.gl/GzYp1P
- Moore BW (1965) A soluble protein characteristic of the nervous system. Biochemical and biophysical research communications 19: 739-744. Link: https://goo.gl/eDZ2Fo
- Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS (2006) Calcium-dependent and -independent interactions of the S100 protein family. The Biochemical journal 396: 201-214. Link: https://goo.gl/MMYiDf
- Bhardwaj RS, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, et al. (1992) The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. European journal of immunology 22: 1891-1897. Link: https://goo.gl/Wh7ea5
- Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clinica chimica acta; international journal of clinical chemistry 344: 37-51. Link: https://goo.gl/o6Mx6j
- Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N (1991) Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. The Journal of biological chemistry 266: 7706-7713. Link: https://goo.gl/nsVFwL
- Kerkhoff C, Klempt M, Sorg C (1998) Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochimica et biophysica acta 1448: 200-211. Link: https://goo.gl/bhgUzo
- Broome AM, Ryan D, Eckert RL (2003) S100 protein subcellular localization during epidermal differentiation and psoriasis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 51: 675-685. Link: https://goo.gl/3Vc2WA
- Gebhardt C, Nemeth J, Angel P, Hess J (2006) S100A8 and S100A9 in inflammation and cancer. Biochemical pharmacology 72: 1622-1631. Link: https://goo.gl/9qPB5j
- Nacken W, Roth J, Sorg C Kerkhoff C (2003) S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microscopy research and technique 60: 569-580. Link: https://goo.gl/VyJyVx
- Rachana Shah D, Chenyi Xue, Hanrui Zhang, Sony Tuteja, Mingyao Li, et al. (2017) Expression of Calgranulin Genes S100A8, S100A9 and S100A12 Is Modulated by n-3 PUFA during Inflammation in Adipose Tissue and Mononuclear Cells. Link: https://goo.gl/m2Jfu3
- Marie-Pierre Manitz, Basil Horst, Stephan Seeliger, Anke Strey, Boris Skryabin V, et al. (2003) Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol 23: 1034-1043. Link: https://goo.gl/6iBWcL
- Eue I, Pietz B, Storck J, Klempt M, Sorg C (2000) Transendothelial migration of 27E10+ human monocytes. International immunology 12: 1593-1604. Link: https://goo.gl/rb4s7y
- Kerkhoff C, Klempt M, Kaever V, Sorg C (1999) The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. The Journal of biological chemistry 274: 32672-32679. Link: https://goo.gl/nz6Jnn
- Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, et al. (2005) The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J 19: 467-469. Link: https://goo.gl/GGTd15
- Doussiere J, Bouzidi F, Vignais PV (2001) A phenylarsine oxide-binding protein of neutrophil cytosol, which belongs to the S100 family, potentiates NADPH oxidase activation. Biochemical and biophysical research communications 285: 1317-1320. Link: https://goo.gl/7PGvkn
- Goyette J, Geczy CL (2011) Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino acids 41: 821-842. Link: https://goo.gl/kXnJMd
- Farkas G Jr, Tiszlavicz Z, Takács T, Szabolcs A, Somogyvári F, et al. (2014) Analysis of plasma levels and polymorphisms of S100A8/9 and S100A12 in patients with acute pancreatitis. Pancreas 43: 485-487. Link: https://goo.gl/XaztxH
- Felix K, Gaida, MM (2016) Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. Int J Biol Sci 12: 302-313. Link: https://goo.gl/gnwgRb
- Stefania Moz, Daniela Basso, Dania Bozzato, Paola Galozzi, Filippo Navaglia, et al. (2016) SMAD4 loss enables EGF, TGFbeta1 and S100A8/A9 induced activation of critical pathways to invasion in human pancreatic adenocarcinoma cells. Oncotarget 7: 69927-69944. Link: https://goo.gl/fgz2s4
- Jordan KR, Kapoor P, Spongberg E, Tobin RP, Gao D, et al. (2017) Immunosuppressive myeloid-derived suppressor cells are increased in splenocytes from cancer patients. Cancer Immunol Immunother CII 66: 503-513. Link: https://goo.gl/SdvsQX
- Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, et al. (2013) Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 190: 794-804. Link: https://goo.gl/nbvVWK
- El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida CW, et al. (2002) Gastric cancers overexpress S100A calcium-binding proteins. Cancer research 62: 6823-6826. Link: https://goo.gl/M7Kjs1
- Ott HW, Lindner H, Sarg B, Mueller-Holzner E, Abendstein B, et al. (2003) Calgranulins in cystic fluid and serum from patients with ovarian carcinomas. Cancer research 63: 7507-7514. Link: https://goo.gl/VZ4f1v
- Stulík J, Osterreicher J, Koupilová K, Knízek, Macela A, et al. (1999) The analysis of S100A9 and S100A8 expression in matched sets of macroscopically normal colon mucosa and colorectal carcinoma: the S100A9 and S100A8 positive cells underlie and invade tumor mass. Electrophoresis 20: 1047-1054. Link: https://goo.gl/13VVjT
- Kim HJ, Kang HJ, Lee H, Lee ST, Yu MH, et al. (2009) Identification of S100A8 and S100A9 as serological markers for colorectal cancer. Journal of proteome research 8: 1368-1379. Link: https://goo.gl/VzCYuT
- Liang Duan, Rui Wu, Liwei Ye, Haiyan Wang, Xia Yang, et al. (2013) S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/beta-catenin pathway. PloS one 8: e62092. Link: https://goo.gl/Mgn66g
- Ito Y, Arai K, Nozawa R, Yoshida H, Hirokawa M, et al. (2009) S100A8 and S100A9 expression is a crucial factor for dedifferentiation in thyroid carcinoma. Anticancer research 29: 4157-4161. Link: https://goo.gl/UBcLLy
- Minami S, Sato Y, Matsumoto T, Kageyama T, Kawashima Y, et al. (2010) Proteomic study of sera from patients with bladder cancer: usefulness of S100A8 and S100A9 proteins. Cancer Genomics Proteomics 7: 181-189. Link: https://goo.gl/yNEA1G
- Arai K, Yamada T, Nozawa R (2000) Immunohistochemical investigation of migration inhibitory factor-related protein (MRP)-14 expression in hepatocellular carcinoma. Medical oncology 17: 183-188. Link: https://goo.gl/F9mHbi
- Alexander Hermani, Jochen Hess, Barbara De Servi, Senad Medunjanin, Rainer Grobholz, et al. (2005) Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 11: 5146-5152. Link: https://goo.gl/mg4iDF
- Arai K, Takano S, Teratani T, Ito Y, Yamada T, et al. (2008) S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Current cancer drug targets 8: 243-252. Link: https://goo.gl/Qzovq2
- Shizhen Zhang, Zhen Wang, Weiwei Liu, Rui Lei, Jinlan Shan, et al. Distinct prognostic values of S100 mRNA expression in breast cancer. Sci Rep 7: 39786. Link: https://goo.gl/a622Kk
- Chen KT, Kim PD, Jones KA, Devarajan K, Patel BB, et al. (2014) Potential prognostic biomarkers of pancreatic cancer. Pancreas 43: 22-27. Link: https://goo.gl/tDRKuD
- Chen R, Pan S, Brentnall TA, Aebersold R (2005) Proteomic profiling of pancreatic cancer for biomarker discovery. Molecular & cellular proteomics: MCP 4: 523-533. Link: https://goo.gl/bKnv19
- Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, et al. (2007) The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells. Proteomics 7: 1929-1940. Link: https://goo.gl/tWJcLa
- Shen J, Person MD, Zhu J, Abbruzzese JL, Li D (2004) Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer research 64: 9018-9026. Link: https://goo.gl/zLqkxk
- Eileen Ke, Bhavinkumar B, Patel MD, Tiffany Liu, Xin-Ming Li, et al. (2009) Proteomic analyses of pancreatic cyst fluids. Pancreas 38: e33-SS42. Link: https://goo.gl/SQgrWK
- Nicola Waddell, Marina Pajic, Ann-Marie Patch, David Chang K, Karin Kassahn S, et al. (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518: 495-501. Link: https://goo.gl/znvn7N
- Kleinbaum DG, Klein M (2010) Logistic regression, a self-learning text. 3rd edition (Springer, New York, Heidelberg, London, Dordrecht. Link: https://goo.gl/tYxV3y
- Zhang Y, Yang J, Li H, Wu Y, Zhang H, et al. (2015) Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med 8: 11683-11691. Link: https://goo.gl/qRTm5g
- Ye HL, Li DD, Lin Q, Zhou Y, Zhou QB et al. (2015) Low RASSF6 expression in pancreatic ductal adenocarcinoma is associated with poor survival. World J Gastroenterol 21: 6621-6630. Link: https://goo.gl/4952JS
- Agrawal S (2017) Potential prognostic biomarkers in pancreatic juice of resectable pancreatic ductal adenocarcinoma. World J Clin Oncol 8: 255-260. Link: https://goo.gl/jMc7B5
- Slotwinski R, Slotwinska SM (2016) Diagnostic value of selected markers and apoptotic pathways for pancreatic cancer. Cent Eur J Immunol 41: 392-403. Link: https://goo.gl/9xHbdB
- Saraswat M, Joenväärä S, Seppänen H, Mustonen H, Haglund C, et al. (2017) Comparative proteomic profiling of the serum differentiates pancreatic cancer from chronic pancreatitis. Cancer Med 6: 1738-1751. Link: https://goo.gl/YbQF9D
- Kuo KK, Kuo CJ, Chiu CY, Liang SS, Huang CH, et al. (2016) Quantitative Proteomic Analysis of Differentially Expressed Protein Profiles Involved in Pancreatic Ductal Adenocarcinoma. Pancreas 45: 71-83. Link: https://goo.gl/QjwGB5
- Basso D, Bozzato D, Padoan A, Moz S, Zambon CF et al. (2014) Inflammation and pancreatic cancer: molecular and functional interactions between S100A8, S100A9, NT-S100A8 and TGFbeta1. Cell communication and signaling: CCS 12: 20. Link: https://goo.gl/aHrEhw
- Limin zhang, Haowen jiang, Gang xu, Hui wen, Bin gu, et al. (2015) Proteins S100A8 and S100A9 are potential biomarkers for renal cell carcinoma in the early stages: Results from a proteomic study integrated with bioinformatics analysis. Molecular medicine reports 11: 4093-4100. Link: https://goo.gl/DmBHGJ
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, et al. (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. Journal of immunology 181: 4666-4675. Link: https://goo.gl/Ra9fUD
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj SP, et al. (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. The Journal of experimental medicine 205: 2235-2249. Link: https://goo.gl/ivPnX2
- Newton RA, Hogg N (1998) The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. Journal of immunology 160: 1427-1435. Link: https://goo.gl/EBGjvS
- Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, et al. (2012) Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer immunology, immunotherapy: CII 61: 1373-1385. Link: https://goo.gl/W358CS
- Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, et al. (2015) Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol 21: 2807-2815. Link: https://goo.gl/VVQH2S
- Basso D, Greco E, Padoan A, Fogar P, Scorzeto M, et al. (2011) Altered intracellular calcium fluxes in pancreatic cancer induced diabetes mellitus: Relevance of the S100A8 N-terminal peptide (NT-S100A8). Journal of cellular physiology 226: 456-468. Link: https://goo.gl/FxGRJE
- Fanjul M, Renaud W, Merten M, Guy-Crotte O, Hollande E, et al. (1995) Presence of MRP8 and MRP14 in pancreatic cell lines: differential expression and localization in CFPAC-1 cells. The American journal of physiology 268: C1241-1251. Link: https://goo.gl/2U7VRs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
