International Journal of Clinical Endocrinology and Metabolism
Dipeptidyl Peptidase IV Inhibitory Activity of Berberine and Mangiferin: An In Silico Approach
Ipseeta Ray Mohanty1*, Selvaa Kumar2 and Rajesh Suman1
2School of Biotechnology and Bioinformatics, DY Patil University, Navi Mumbai, India
Cite this as
Mohanty IR, Kumar S, Rajesh Suman (2017) Dipeptidyl Peptidase IV Inhibitory Activity of Berberine and Mangiferin: An In Silico Approach. Int J Clin Endocrinol Metab 3(1): 018-022. DOI: 10.17352/ijcem.000024Background: Dipeptidyl peptidase-IV (DDP-IV) Inhibitors may represent single anti-diabetic drugs, the multiple actions of which may translate into demonstrable therapeutic benefits in diabetes. The marketed synthetic DPP-IV Inhibitors are expensive drugs and have been reported to cause unacceptable adverse effects such as pancreatitis, angioedema, thyroid and pancreatic cancers. In this scenario, research to identify DPP-IV Inhibitors from alternative sources is desirable.
Aims and Objective: The study was designed to elucidate the DPP-IV Inhibitory activity of Berberine and Mangiferin, determine the binding sites and affinity of Berberine and Mangiferin for DPP- IV enzyme using in silico studies and compare it to synthetic DPP-IV Inhibitor: Vildagliptin.
Material and Methods: The crystal structure of human DPP IV (PDB Id: 2QT9) was downloaded from Protein Databank. Berberine and Mangiferin were computationally designed and screened through in silico docking studies against crystal structure of DPP-IV. Computational in silico studies were used to identify the sites as well as amino acid residues on DPP-IV enzyme to which these natural DPP-IV Inhibitors binds.
Results: The DPP-IV Inhibitory activity of Mangiferin was found to be comparable to synthetic marketed DPP-IV Inhibitors; Vildagliptin and Sitagliptin. Like Vildagliptin, Berberine and Mangiferin bind within the active site pocket (the 1st largest pocket) of DPP-IV enzyme whereas, Sitagliptin prefers to bind in the second largest pocket of DPP-IV enzyme. Berberine prefers to bind to the active site pocket of DPP-IV enzyme. Berberine binds very close to Glu205 and Glu206. As delineated using in silico binding energy results, Mangiferin, possess superior DPP-IV inhibitory activity as compared to Sitagliptin and Vildagliptin.
Conclusion: In silico studies demonstrates that Berberine and Mangiferin possess significant DPP-IV Inhibitory activity comparable to marketed synthetic DPP-IV Inhibitors.
Introduction
Recently, one of the additions to this anti-diabetic armament of drugs is dipeptidyl peptidase-IV Inhibitors (DPP-IV) [1], DPP-IV Inhibitors increase incretin levels: Glucagon-like peptide-1 (GLP-1) and Glucagon inhibitory peptide (GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels [2,3]. A recent study has shown beneficial effects of DPP-IV Inhibitor on metabolic parameters in type 2 diabetic patients [4], DPP-IV Inhibitors have a large number of beneficial effects in the subgroup of metabolic syndrome with diabetes. Apart from glycemic control, they are known to lower blood pressure, are weight neutral, improve dyslipidemia, reduce inflammatory markers, diminish oxidative stress, improve endothelial function and reduce platelet aggregation in patients with type II diabetes [5], Therefore, it is plausible to explore the therapeutic potential of DPP-IV Inhibitors in experimental diabetes with metabolic syndrome.
As a drug class, DPP-IV Inhibitors are widely used clinically because of their low risk of hypoglycemia, once daily dosing, pharmacological effects such as weight neutral and preservation of beta cell mass [6], However, in spite of their beneficial effects, they have limitations: high cost of therapy and unacceptable adverse effects such as pancreatitis, pancreatic cancer, angioedema and thyroid cancer. In this scenario, it would be desirable to identify novel DPP-IV Inhibitors from alternative sources, that share the beneficial effects of the available synthetic DPP-IV Inhibitors and at the same time are cost effective. In this light, the DPP-IV Inhibitors obtained from natural source may be an alternative that needs to be explored, considering the rich biodiversity India is bestowed with. With these point in view, the present study was designed.
Asian countries such as India and China are already known for their contributions toward the usage of plant medicine in preventing and treating various diseases [7], Mangiferin, a major phytochemical in Mangifera indica has been reported to possess DPP-IV inhibitory activity. It belongs to family Anacardiaceae Mangiferin. Being a glucosylxanthone, it possesses strong antioxidant, anti-lipid peroxidation, immunomodulation, anti-diabetic cardiotonic, hypotensive, wound healing, anti-hyperlipidemic, anti-atherogenic and anti-degenerative properties [8], Mangiferin showed significant antihyperlipidemic and antiatherogenic activities as evidenced by significant alteration of lipid profile level and diminution of atherogenic index in diabetic rats [9], Although several benefits of Mangiferin have been reported by multiple pathways, there is no experimental evidence presently available in literature with regard to its DPP-IV inhibitory activity.
Berberine, an isoquinoline alkaloids originally isolated from the root of Berberis aristata belongs to family Berberidaceae. It has shown a wide array of pharmacological activities including antimicrobial, antitumor, anti-inflammation and antidiabetic activity [10], in 1988, the hypoglycemic effect of Berberine was found when Berberine was used to treat diarrhoea in diabetic patients in China. Since then, Berberine has been used as an anti-hyperglycemic agent also in a recent single-blind clinical observation, the study showed that Berberine supplementation was beneficial in correcting lipid metabolism disorders and reducing cardiovascular risk factors. Berberine has been reported in the several literatures to have beneficial effects in human type II diabetes. Experimentally, Berberine was found to inhibit human recombinant DPP-IV in vitro. The findings suggest that DPP-IV inhibition is, at least, one of the mechanisms that explain the anti-hyperglycemic activity of Berberine [11].
The DPP-IV Inhibitory activities of Berberine and Mangiferin were reported from the laboratory [12]. The results of in vitro and in vivo studies in the experimental model of diabetes co-existing with metabolic syndrome confirmed the DPP-IV Inhibitory activity of Berberine and Mangiferin. Serum DPP-IV levels were measured and was found to directly correlate to the antidiabetic efficacy of Berberine and Mangiferin in experimental rats [13], to further elucidate the binding sites and affinity of Mangiferin and Berberine for DPP-IV enzyme in silico docking studies were designed. Computational in silico studies were used to confirm the DPP-IV Inhibitory activity of Berberine and Mangiferin and identify the sites as well as amino acid residues on DPP-IV enzyme to which these natural DPP-IV Inhibitors binds.
Materials and Methods
The crystal structure of human DPP IV (PDB Id: 2QT9) [14], was downloaded from Protein Databank [15], which has a resolution of 2.1 Å. This was considered as a receptor for docking studies. The ligand selected includes sitagliptin, vildagliptin, mangiferin and berberine. All of them were downloaded from Pubchem Database [16]. The later was obtained from Chemfaces (www.chemfaces.com). All these structures were retrieved in SDF file format which was further converted into 3D format using Frog v2.14 (FRee On line druG conformation generation) [17]. In the Frog online server, the input was maintained as ‘1D to 2D’ and the input drug description was ‘SDF’ file. In the calculation parameters the output format was maintained as ‘PDB’. The minimize option was opted as ‘yes’. In the produce option we selected ‘single’. The total number of conformers generated was kept as 100. While rest of the options were left as default. The active site of DPPIV was retrieved through literature search and also predicted based on CASTp online server to identify pockets [18]. Now the receptor and the 3D generated ligands were considered for docking using Hex software 8.0.0 [19], it is an interactive molecular graphics program for calculating and displaying feasible docking modes which uses spherical polar Fourier (SPF) correlations to accelerate the calculations. During docking, in the docking parameter settings, we opted for ‘shape and electro’ for correlation type. Sampling method was ‘range angles’. Post processing was OPLS minimization. The rest of the options were left default. Here the 2QT9 was loaded along with their inhibitor 4-aryl cyclohexylalanine in complex state. Now this was considered as the reference pose for the docking of DPPIV of Homo sapiens against all the available chemical compounds. Top ten docked poses were downloaded and considered for further analysis using Swiss pdb viewer and CHIMERA software [20].
Results
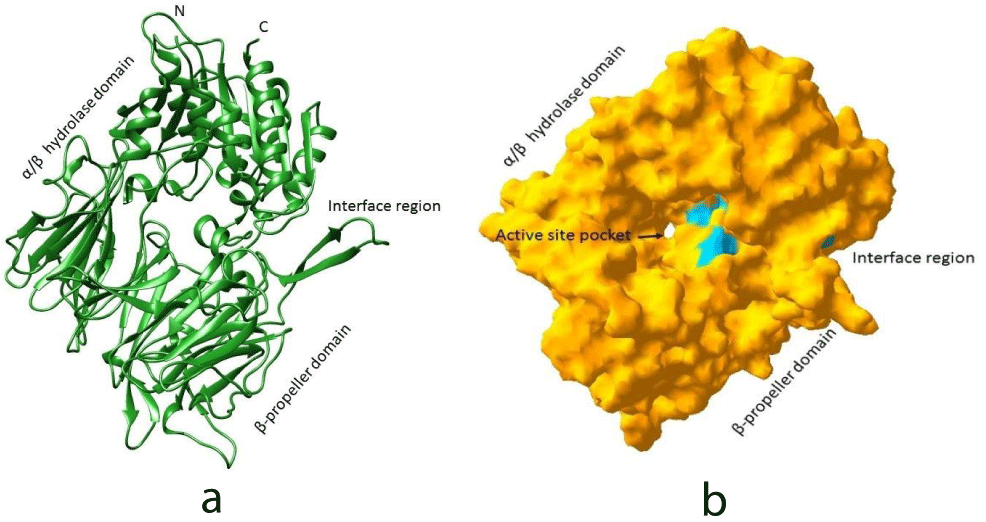
In this study, the crystal structure of DPP IV was considered as receptor which was docked against 14 chemical compounds. The available chemicals in 2D format were converted into 3D format and further minimized using Frog2 software. All these optimized chemical compounds were considered for docking against DPPIV based on the reference structure of DPPIV against 4-aryl cyclohexylalanine in complex state (2QT9). Basically, DPPIV is active in homo dimer form. The active site is a deep cleft in DPPIV which can be accessed via the opening of the propeller domain or through side opening formed at the interface of the β-propeller and hydrolase domains. Furthermore, the β propeller is a funnel shaped tunnel which extends to the active site (Figure 1a). All these chemical compounds were docked in the active site pocket (Figure 1b). In addition to this, the active sites were also predicted using CASTp software. This online server listed 198 pockets with area and volume. The first predicted pocket was the largest pocket with an area of 6086.4 Å2 and a volume of 16471 Å3. The second largest pocket has an area of 574.3 Å2 with a volume of 1171.8 Å3. Third largest pocket is observed proximal to the first largest pocket with an area 274.4Å2 and a volume of 191.5 Å3 (Figure 2).
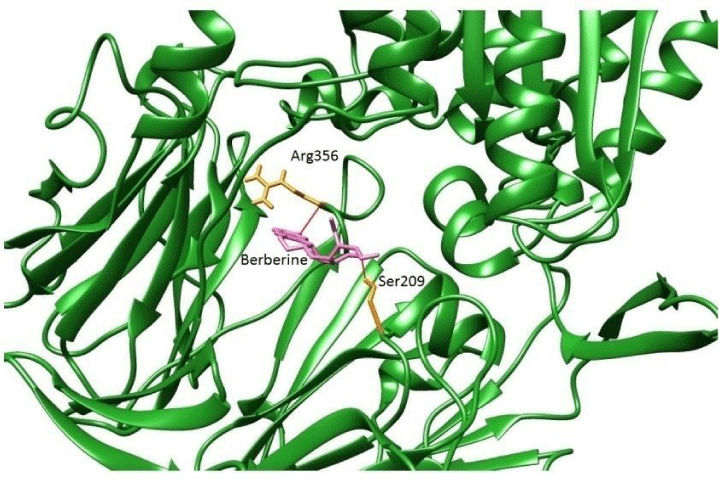
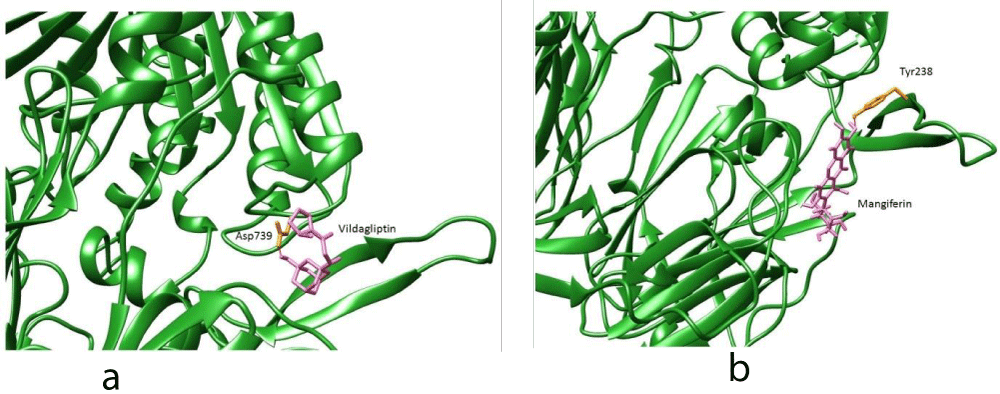
All these listed compounds were docked against the reference docked pose of 4-aryl cyclohexylalanine inhibitor binding site from 2QT9 crystal structure. Berberine prefers the active site pocket of DPP-IV enzyme. Of these, in particular, Berberine binds very close to Glu205 and Glu206 (Figure 3). Compounds like Vildagliptin and Mangiferin prefers to bind near the interface region of the DPPIV as their biological active forms are homodimer (Figure 4a,b). Sitagliptin binds near the α/β hydrolase domain (Figure 5). It was observed that few of compounds preferred to bind within the active pocket, whereas others prefer to interact with interface region, β propeller and α/β hydrolase domains. However, as per CASTp prediction, in addition to the active site, the region of interface and the α/β hydrolase and β propeller domain combo is also considered as part of the largest active site pocket. Berberine and Vildagliptin prefers to bind within the active site pocket (the 1st largest pocket). Mangiferin prefers to bind in the second largest pocket and Sitagliptin prefers to bind with the third largest pocket.
Discussion
Several anti-diabetic drugs are available for the treatment of type 2 diabetes mellitus. A relatively new class of oral hypoglycemic agent, DPP-IV Inhibitors has recently been introduced. DPP-IV Inhibitors increase incretin levels {GLP-1 (glucose dependent insulinotropic polypeptide) and GIP (glucagon-like peptide-1)}, which in turn increases insulin secretion and decreases blood glucose levels. DPP-IV Inhibitors work by enhancing the sensitivity of β cells to glucose, and have also been shown to improve markers of β cell function. Since the introduction of DPP-IV Inhibitors in 2006, they are flourishing as monotherapy and also used in combination with commonly prescribed anti-diabetic agents [22]. However, in spite of potential multiple beneficial effects of DPP-IV Inhibitors, they do have certain limitations: high cost of therapy and unacceptable adverse effects. The marketed synthetic DPP-IV Inhibitors are expensive drugs when indicated for long term therapy for a chronic disease like diabetes and are also reported to cause unacceptable adverse effects like pancreatitis, upper respiratory infections, anaphylactic reactions and pancreatic cancer (411). In this scenario, research to explore DPP-IV Inhibitors from alternate sources is of paramount importance. The present study was developed to identify DPP-IV Inhibitors from indigenous sources which may work in concert with body’s own defense mechanism, have fewer adverse effects and would be more affordable.
Computational in silico studies were used to confirm the DPP-IV Inhibitory activity of Berberine and Mangiferin and identify the sites as well as amino acid residues on DPP-IV enzyme to which these natural DPP-IV Inhibitors binds. The active principles Berberine and Mangiferin were computationally designed and screened through in silico docking studies against crystal structure of DPP-IV. The in silico methods have been demonstrated to be useful in predicting the potential of proteins as precursors of peptides in various bioactivities, such as DPP-IV. Such in silico computational methodologies have been widely used for virtual ligand and target based screening and profiling to predict biological activity. They can also be used to explore the target structures for possible active sites, generate candidate molecules, check for their drug generated likeness, dock these molecules with the target, rank them based on variations on the structures according to their binding affinities and further optimize the molecules to improve binding characteristics [23].
The crystal structure of DPP-IV shows that the enzyme is a serine protease that specifically cleaves N-terminal dipeptides from polypeptides with Pro and Ala at the penultimate position. In DPP-IV, each monomer consists of an N-terminal β-propeller domain (Lys56-Asn497) and a C-terminal catalytic domain (Gln508-Pro766, together with segment Leu45-Val55). Catalytic domain and propeller domain together embrace anegg-shaped cavity of approximate dimensions 40Å X 20Å X 20Å, which harbours the active centre [23].
The crystal structure of DPP-IV was considered as receptor which was docked against the Berberine, Mangiferin, Sitagliptin and Vildagliptin. All these optimized chemical compounds were considered for docking against DPP-IV based on the reference structure of DPP-IV against 4-aryl cyclohexylalanine in complexstate (2QT9). Basically, DPPIV is active in homo dimer form. The active site is a deep cleft in DPPIV which can be accessed via the opening of the propeller domain or through side opening formed at the interface of the β-propeller and hydrolase domains. Furthermore, the β propeller is a funnel shaped tunnel which extends to the active site. The propeller domain gets well packed against the hydrolase domain, and the catalytic triad (S630, H740, and D708) which is at the interface of the two domains [23]. The active site cavity is well guarded by residues like W659, Y631, Y547, P550, W629, Y752, F357, Y666, E205, E206, Y662, N710, R125, V656, S630 and V711 [24]. All these chemical compounds were docked in the active site pocket. In addition to this, the active sites were also predicted using CASTp software. This online server listed 198 pockets with area and volume. The first predicted pocket was the largest pocket with an area of 6086.4 Å2 and a volume of 16471 Å3. The second largest pocket has an area of 574.3 Å2 with a volume of 1171.8 Å3. Third largest pocket is observed proximal to the first largest pocket with an area 274.4Å2and a volume of 191.5 Å3.
Berberine and Mangiferin showed significant inhibition of DPP-IV enzyme. Berberine binds to the active site pocket, very close to Arg 356 and ser 209 of DPP-IV receptor. Mangiferin binds near the interface region near Tyr 238 of the DPP-IV receptor. Vildagliptin preferred to bind to Asp739 amino acid near the interface region of the DPP-IV as their biological active forms are homodimers and Sitagliptin binds near the α/β hydrolase domain. It was observed that few of theligands preferred to bind within the active pocket, whereas others prefer to interact with interface region, β propeller and α/β hydrolase domains. However, as per CAST prediction, in addition to the active site, the region of interface and the α/β hydrolase and β propeller domain combo are also considered as part of the largest active site pocket. The synthetic DPP IV inhibitors: Sitagliptin binds to amino acids: Glu452 and Vildagliptin to Asp739. Berberine and Mangiferin bind to the amino acid residues: Ser 209, Arg356 and Tyr 238. The binding energy is inversely proportional to the DPP-IV inhibitory activity. Based on the binding energy results, it was found all the ligands (Berberine, Mangiferin and Vildagliptin) studied had superior DPP-IV binding affinity as compared to Sitagliptin.
Previous in vitro and in vivo experimental studies from the laboratory demonstrated the DPP-IV Inhibitory activities of Berberine and Mangiferin. Results demonstrated that DPP-IV inhibition is one of the mechanisms attributing to the therapeutic efficacy of Berberine and Mangiferin in experimental diabetes with metabolic syndrome [12]. In the present study the DPP-IV inhibitory activity of Berberine and Mangiferin has been delineated using in silico docking studies against crystal structure of DPP-IV and compared to the synthetic DPP-IV Inhibitors. In silico results emphasize the potential of developing Berberine and Mangiferin as natural alternative to synthetic DPP-IV Inhibitors (Table 1).
Conclusion
Vildagliptin, Mangiferin prefers to bind near the interface region of the DPP-IV as their biological active forms are homodimer. Berberine prefers to bind to the active site pocket of DPP-IV enzyme. Berberine binds very close to Glu205 and Glu206. Sitagliptin binds near the α/β hydrolase domain of DPP-IV enzyme. Berberine, Mangiferin and Vildagliptin prefer to bind within the active site pocket (the 1st largest pocket) of DPP-IV enzyme whereas Sitagliptin prefers to bind in the second largest pocket of DPP-IV enzyme.
The study is funded by Indian Council of Medical Research, Government of India, vide grants received, no. 58/2/2014-BMS.
- Holst JJ (2002) Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes. Metab Res Rev 18: 430-441. Link: https://goo.gl/CkoU4i
- Ahrên B, Larsson H, Holst JJ (1997) Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin dependent diabetes mellitus. J Clin Endocrinol Metab 82: 473-478. Link: https://goo.gl/QaUB7h
- Mentlein R, Gallwitz B, Schmidt WE (1993) Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829-835. Link: https://goo.gl/jcQ6me
- Apaijai N, Chinda K, Palee S, Chattipakorn S, Chattipakorn N (2014) Combined Vildagliptin and Metformin Exert Better Cardioprotection than Monotherapy against Ischemia-Reperfusion Injury in Obese-Insulin Resistant Rats 9: 1-13. Link: https://goo.gl/rVuAFm
- Matteucci E, Giampietro O (2011) Dipeptidyl peptidase-4 inhibition: linking chemical properties to clinical safety. Curr Med Chem 18: 4753-4760. Link: https://goo.gl/v3bsq6
- Bharti SK, Sharma NK, Kumar A, Krishnan S, Gupta AK, et al. (2012) Dipeptidyl peptidase IV inhibitory activity of seed extracts of Castanospermum austral and molecular docking of their alkaloids. Topclass J Herb Med 1: 1-7. Link: https://goo.gl/RcgE7N
- Si-Yuan Pan, Gerhard Litscher, Si-Hua Gao, Shu-Feng Zhou, Zhi-Ling Yu, et al. (2014) Historical Perspective of Traditional Indigenous Medical Practices: The Current Renaissance and Conservation of Herbal Resources. Evidence-based complimentary alternative medicine 2014: 525340. Link: https://goo.gl/UBnhrq
- Pavan KM, Suman K, Mangiferin A (2015) Potential Natural Molecule for Management of Metabolic syndrome Int J Pharmacy Pharmaceutical Sci 7: 9-13. Link: https://goo.gl/v5yvh5
- Raihan H, Mirza, Nan Chi, Yuling chi (2013) Therapeutic potential of the Natural product Mangiferin in metabolic syndrome. J Nutri Therap 2: 74-79. Link: https://goo.gl/2DEiMY
- Zhang H, Wei J, Xue R, Wu JD, Zhao W, et al. (2010) Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression Metabolism 59: 285-292. Link: https://goo.gl/qPyWbk
- Gallwitz B (2011) GLP-1 agonists and dipeptidyl-peptidase IV inhibitors. Handbook of Experimental Pharmacology 203: 53-74. Link: https://goo.gl/hXz32z
- Suman RK, Mohanty IR, Maheshwari U, Borde MK, Deshmukh YA (2016) Natural dipeptidyl peptidase-IV inhibitor Mangiferin mitigates diabetes- and metabolic syndrome-induced changes in experimental rats .Diabetes, metabolic syndrome and Obesity: target and therapy 9: 261-272. Link: https://goo.gl/7xxRTv
- Suman RK, Manjusha Borde K, Ray I, Maheshwari U, Deshmukh YA (2016) Myocardial Salvaging effects of Berberine in experimental diabetes co-existing with myocardial infraction. Journal of Clinical and Diagnostic Research 10: FF13-FF18. Link: https://goo.gl/EubxTg
- Kaelin DE, Smenton AL, Eiermann GJ, He H, Leiting B, et al. (2007) 4-Arylcyclohexylalanine analogs as potent, selective, and orally active inhibitors of dipeptidyl peptidase IV. Bioorg Med Chem Lett 17: 5806-5811. Link: https://goo.gl/Yx7XRt
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, et al. (2000) The Protein Data Bank Nucleic Acids Research 28: 235-242. Link: https://goo.gl/KJMEdy
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, et al (2016) PubChem Substance and Compound databases. Nucleic Acids Res 44: 1202-1213. Link: https://goo.gl/asByZm
- Bohme Leite T, Gomes D, Miteva MA, Chomilier J, Villoutreix BO, et al. (2007) Frog a FRee Online druG 3D conformation generator Nucleic Acids Res 35: W568-W572. Link: https://goo.gl/EAzpmf
- Joe Dundas, Zheng Ouyang, Jeffery Tseng, Andrew Binkowski, YaronTurpaz, et al. (2006) CASTp computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucl Acids Res 34: W116-W118. Link: https://goo.gl/JsQxd1
- Ritchie D, Kozakov D, Vajda S (2008) Accelerating and focusing protein-protein docking correlations using multi-dimensional rotational FFT generating functions. Bioinformatics 24: 1865-1873. Link: https://goo.gl/xoxpQv
- Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss PdbViewer: An environment for comparative protein modeling. Electrophoresis 18: 2714-2723. Link: https://goo.gl/cF2gK7
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25: 1605-1612. Link: https://goo.gl/inDGnJ
- Kshirsagar AD, Aggarwal AS, Harle UN, Deshpande AD (2011) DPP-IV Inhibitors: successes, failure and future prospects. Diabetes Metab Syndr 105-112. Link: https://goo.gl/xnaiYw
- Aertgeerts K, Ye S, Tennant MG, Kraus ML, Rogers J, et al. (2004) Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation.Protein Sci 13: 412-421. Link: https://goo.gl/2LRcrX
- Neer EJ, Smith TF (1996) G protein heterodimers: New structures propelnew questions. Cell 84: 175-178. Link: https://goo.gl/BDPPSv
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
