International Journal of Clinical Endocrinology and Metabolism
Efficacies of Eruca sativa and Raphanus sativus Seeds’ Oils in Streptozotocin-Induced Diabetic Rats
Osama M Ahmed, Eman S Abdel-Reheim*, Mohammed B Ashour, Hanaa I Fahim and Hassnaa H Mohamed
Cite this as
Ahmed OM, Abdel-Reheim ES, Ashour MB, Fahim HI, Mohamed HH (2016) Efficacies of Eruca Sativa and Raphanus Sativus Seeds’ Oils in Streptozotocin-Induced Diabetic Rats. Int J Clin Endocrinol Metab 2(1): 034-043. DOI: 10.17352/ijcem.000020Objective: The study was planned to investigate the effect of Eruca sativa seeds’ oil (ESSO) and Raphanus sativus seeds’ oil (RSSO) on impaired glucose tolerance, lipid profile and oxidative stress in streptozotocin-induced diabetic albino rats. Also, the probable mechanisms of action were suggested.
Methods: Experimental diabetes mellitus was induced by a single dose of 45 mg/kg b. wt streptozotocin. The diabetic rats were then divided into three groups: the 1st one was given 1% caboxymethylecellulose (CMC) and was considered as diabetic control, the 2nd group was treated with 50 mg/kg b. wt ESSO dissolved in 5 ml CMC and the 3rd group was treated with 50 mg/kg b. wt. RSSO dissolved in 5 ml CMC by gastric intubation daily for 30 days. The diabetic control rats were compared with normal group.
Results: The obtained data revealed that both ESSO and RSSO treatments led to a significant amelioration of glucose tolerance, lipid profile, serum insulin and liver glycogen content and the activities of glucose-6-phosphatase, aspartate aminotransferase and alanine aminotransferase as well as the antioxidant defense system in diabetic treated rats. The histopathological examination of the pancreas revealed that both ESSO and RSSO increased the pancreatic islets’ size and the number of α- and β-cells within the islet.
Conclusion: the present results proved that Eruca sativa oil and Raphanus sativus oil improved glucose tolerance, serum insulin level and metabolic pathways in streptozotocin-induced diabetic rats. Raphanus sativus oil seemed to be more potent than Eruca sativa oil in improving serum insulin level and many aspects of carbohydrate metabolism and lipid profile in the diabetic rats. The antidiabetic effects may be mediated via enhancement of the insulin secretory response, peripheral glucose uptake and the antioxidant activity as well as attenuation of the intestinal glucose absorption.
Introduction
Diabetes mellitus is a chronic metabolic disease that results from either insulin deficiency (type 1 diabetes mellitus) or insulin resistance (type 2 diabetes mellitus) [1-3]. It is associated with long-term complications and its prevalence has been steadily increasing worldwide over the past few decades [3-5]. Experimental diabetes mellitus has been induced in laboratory animals by several methods. The most common method for creating diabetes in animals is injecting alloxan or streptozotocin (STZ). These materials inflate and ultimately degenerate the Langerhans islets beta cells [7]. STZ (N-nitro derivative of glucosamine) is a naturally occurring, broad spectrum antibiotic and cytotoxic chemical that is particularly toxic to the pancreatic, insulin producing beta cells in mammals [8]. STZ injection leads to the degeneration of the Langerhans islets beta cells [9], resulting in disturbances in carbohydrate, protein and lipid metabolism as a result of absolute or relative insufficiency of insulin secretion [10,11]. However, persistent and chronic hyperglycemia leads to insulin resistance [12].
Traditional plant medicines or herbal formulations might offer a natural key to unlock diabetic complications [13]. Flavonoids are a group of naturally occurring polyphenolic compounds primarily from fruits and vegetables [14]. Several small dietary intervention trials have shown that consumption of flavonoid-rich foods such as tea and onions was associated with a significant increase in plasma levels of flavonoids in diabetic patients [15]. Flavonoids may preserve β-cell function by reducing oxidative stress-induced tissue damage and therefore protect against the progression of insulin resistance to type 2 diabetes. However, the relation of dietary flavonoids to the development of type 2 diabetes is less well studied. A prospective study in Finland recently showed that the intakes of some specific types of flavonoids including quercetin and myricetin were inversely associated with risk of incident type 2 diabetes [16]. In terms of antioxidant compounds, rocket salad species are a good source of vitamins, like vitamin C, carotenoids, and polyphenols, which play a very important role among natural antioxidants [17]. Eruca sativa seeds (ESS) oil as antidiabetic adjuvant and antioxidative agent. Oral supplementation of ESS oil before or after alloxan treatment resulted in lower serum glucose levels, higher serum insulin values and an improved lipid profile as well as hepatic glycogen content and its regulating enzyme as compared with rats treated with alloxan alone [18]. Sulforaphane, the principal component of ESS oil, was found to be a potent inducer of phase II detoxication enzymes due to its antioxidant functions [19]. Raphanus sativus seeds (RSS) were found to contain alkaloid like coumarins, saponins, flavonoids and anthocyanins. They decrease uric acid level in the serum which related to circulating markers of inflammation and free radical reactions [20]. The anthocyanins are important group of dietary antioxidants that have many physiological functions. They protect living cells from oxidative damage resulting in the prevention of diseases [21]. Besides, RSS contain isothiocyanate that has antimicrobial activity, antimutagenic, anticarcinogenic and antiatherosclerosis activity [22].
Therefore, this study is designed to assess the anti-hyperglycemic and anti-hyperlipidemic effects of Eruca sativa seeds’ oil (ESSO) and Raphanus sativus seeds’ oil (RSSO) and to suggest their mechanisms of action in STZ-induced diabetic albino rats.
Material and Methods
Male albino rats weighting about 150 – 200 g were used as experimental animals in the present investigation. They were obtained from the animal house of National Research Institute, El-Giza, Egypt. They were kept under observation for about 15 days before the onset of the experiment to exclude any intercurrent infection. The chosen animals were housed in polypropylene cages with stainless steel covers at normal atmospheric temperature (25 ± 5ºC) as well as normal 12 hours light/dark cycle. Moreover, they were given access of water and supplied daily with standard diet of known composition. All animal procedures are in accordance with the guidelines and instructions of the Experimental Animal Committee of Faculty of Science, Beni-Suef University, Egypt. All efforts were done to reduce the number and suffering of animal.
Eruca sativa seeds oil (ESSO) and Raphanus sativus seeds oil (RSSO) was obtained from Captain Company (Cap – pharm) Elobour city, Cairo, Egypt.
Diabetes mellitus was experimentally induced in animals fasted for 16 hours by intraperitoneal injection of 45 mg/kg b. wt STZ (Sigma Company) dissolved in citrate buffer, PH 4.5 [23]. Ten days after STZ injection, rats were screened for blood glucose levels. The rats with serum glucose level ranging from 180-300 mg/dl after oral glucose loading (3g/kg b. w.) were considered as mild diabetic and included in the experiment.
The rats were divided into 3 groups of 10 rats, as follows: group I was considered as diabetic control and orally given 1% carboxymethylcellulose CMC (5 ml /kg b. wt); rats of group II were orally treated with ESS oil at a dose level (50 mg/kg b. wt) dissolved in 5ml CMC; and group III was orally treated with RSS oil at a dose level (50 mg/kg b. wt) dissolved in 5ml CMC. All treatments were given daily for 30 days by oral gavage. By the end of the experiment, animals were sacrificed and blood samples, visceral adipose tissue, pancreas and liver were obtained.
Body weight of experimental groups was recorded at the beginning and at the end of the treatment period. The % change of body weight gain of each group was calculated from the formula.
% change of body weight = (Final body weight – Initial body weight/Initial body weight) x 100
On the day before sacrifice, oral glucose tolerance test (OGTT) was performed in normal, diabetic control and diabetic rats treated with ESS oil and RSS oil. Blood samples were obtained from lateral tail vein of rats deprived of food overnight (10- 12 hours). Successive blood samples were then taken at 0, 60, 120 and 180 minutes following the administration of glucose solution (3 g/kg b. w.) by oral gavage. Blood samples were left to coagulate, centrifuged, and clear non-haemolysed serum was obtained for determination of glucose concentration according to the method of Trinder et al. [24], using reagent kit purchased from Spinreact Company (Spain).
Serum insulin was assayed in the Radioactive Isotopes Unit, Middle Eastern Regional Radioisotope Center (Dokki, Giza) by radioimmunoassay kits of DPC (Diagnostic Products Corporation, Los Angeles, USA) [coat-A-count] according to the methods of Marschner et al. [25].
After sacrifice and dissection, Liver glycogen content was determined according to the method of Seifter et al. [26]. Liver glucose-6-phosphatase was determined according to the method of Begum et al. [27]. The librated inorganic phosphate from glucose-6-phosphate was estimated according to the method of Munoz et al. [28], using reagent kits obtained from Biosystems Company (Spain). Serum alanine aminotranferase (ALT) activity and liver aspartate aminotranferase (AST) activity were determined according to the kinetic method of Schumann and Klauke [29] using reagent kits purchased from Human Diagnostics Chemical Company (Germany).
Serum total lipids [30], triglycerides [31], cholesterol [32], HDL-cholesterol [33], levels were determined. Serum LDL-cholesterol level was calculated from formula: LDL cholesterol = total cholesterol – triglycerides/5 – HDL-cholesterol). Serum vLDL-cholesterol concentration was calculated according to Nobert [34], formula (vLDL-cholesterol = triglycerides/5).
Liver lipid peroxidation, reduced glutathione and total thiols were also measured according to the methods of Preuss et al. [35], Beutler et al. [36] and Koster et al. [37], respectively. Humalyzer 2000 Chemical Analyzer (Germany) was used for spectrophotometric measurements.
An intestinal perfusion technique [38], using variable flow mini-pump (Control Company, Texas, USA) was adopted to study the effect of ESS oil and RSS oil on intestinal glucose absorptions in normal rats fasted for 24 h and anesthetized with intraperitoneal sodium thiopental solution (50 mg/kg b. wt). Perfusion rate was 20 mL/h to a total volume of 20 mL via a thermo-regulator set at 38oC through 20-cm intestinal segment in situ of the anesthetized rats. The perusing solution was composed of the following (in g/L): The perfusion solution was composed of the following (in g/L): 7.37 NaCl, 0.2 KCL, 0.065 NaH2PO4.2 H2O, 0.213 MgCl2.6 H2O, 1.02 CaCl2, 0.6 NaHCO3 and 1.0 glucose, at pH 7.5. The results were expressed as percentage glucose absorption calculated from the amount of glucose in solution before and after perfusion with 100,200 and 300 mg/ml of both ESS oil and RSS oil in the solution.
Histological sections of pancreas and staining with haematoxylin and eosin were performed in the histopathology department, National Cancer Institute, Cairo University, Cairo, Egypt.
The data were analyzed using the one-way analysis of variance (ANOVA) [39], followed by least significant difference (LSD) test to compare various groups with each other. Results were expressed as mean ± standard error (SE) and values of P>0.05 were considered non-significantly different, while those of P<0.05 and P<0.01 were considered significant and highly significant, respectively. F-probability expresses the general effect between groups. The means that were not significantly different are followed by the same superscript symbol(s).
Results
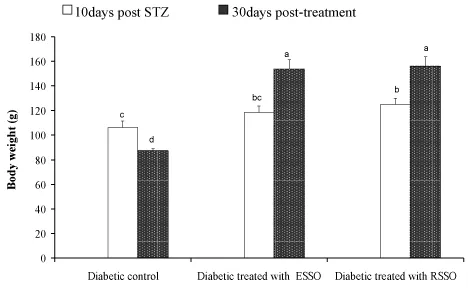
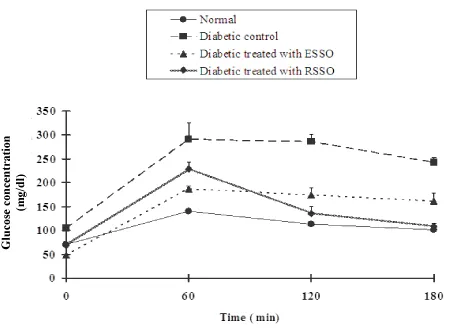
The data in Figure 1 showed ameliorative effect (P< 0.01) of ESSO and RSSO on the decrease in body weight of the diabetic rats (153.83±7.92 g and 156.17±7.70 g Vs 87.63±1.44 g, respectively). The normal rats had a fasting serum glucose level of 71.17±4.38 mg/dl and ESSO and RSSO treated group measures 48.17±1.11 and 68.75±5.25 mg/dl respectively that were much lower than that of the diabetic control one. The OGTT curves of normal, diabetic control and treated rats attained its maximal level after 60 minutes of glucose administration but the glucose tolerance of diabetic control rats was the most deteriorated one (Figure 2). While ESSO was more effective in decreasing the elevated serum glucose level at fasting and 60 minutes after oral glucose loading, the RSSO was more potent at 120 and 180 minutes.
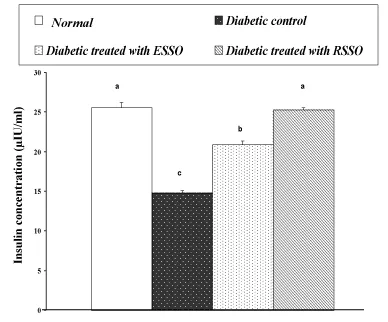
As indicated in Figure 3, serum insulin level exhibited a significant decrease in diabetic rats as compared to the normal level. Treatment of diabetic animals with ESSO and RSSO induced a profound amendment of these deteriorated changes of insulin concentration; RSSO seemed to be more effective. The changes in liver glycogen content and liver glucose-6-phosphatase as well as serum AST and ALT activities are indicated in Table 1. The lowered liver glycogen content in diabetic rats was significantly increased as a result of ESSO and RSSO administration. On the other hand, the elevated liver glucose-6-phosphatase activity was significantly improved after treatment of diabetic rats with ESSO and RSSO. The elevated gluconeogenic transaminase enzymes, AST and ALT activities in serum were significantly decreased in diabetic rats treated with ESSO and RSSO as compared to the diabetic control.
The effects of ESSO and RSSO on lipid profile of diabetic rats are presented in Table 2. Diabetic rats exhibited a highly significant increase (P<0.01; LSD) in serum total lipids, cholesterol, triglycerides, LDL- and vLDL-cholesterol levels as compared with the non-diabetic group. The treatment of diabetic rats with ESSO and RSSO significantly ameliorated these elevated values. On the other hand, HDL-cholesterol level was affected in an opposite manner, as it was decreased (P<0.01; LSD) in diabetic rats and markedly increased in response to treatments. As indicated in Table 3, the elevated atherogenic indices represented by the ratios of LDL and total cholesterol to HDL-cholesterol in diabetic rats were significantly alleviated (P<0.01; LSD) as a result of treatments with ESSO and RSSO. It was found that RSSO seemed to be more potent than ESSO in improving the lipid profile and atherogenic indices (Tables 2,3).
Oxidative stress parameters, liver lipid peroxidation and total thiols content & reduced glutathione content variations as the result of treatment of diabetic rats with ESSO and RSSO are presented in Table 4. The elevated liver lipid peroxidation and the lowered liver total thiols content were significantly alleviated in diabetic rats treated with ESSO and RSSO as compared to the diabetic control. However, while the ESSO was more potent in decreasing the elevated lipid peroxidation, RSSO was more effective in increasing the lowered total thiols and glutathione contents.
Data of the effects of ESSO and RSSO at varying concentrations on in situ intestinal glucose absorption at three different concentrations (5, 10 and 15 mg/ml) are given in Table 5. All concentrations of ESSO and RSSO produced a marked decrease in the intestinal glucose absorption. However, while the effect of RSSO was dose dependent and significant at the three tested concentrations, the effect of ESSO was significant only at 10 mg/ml. Additionally, RSSO seemed to be more effective than ESSO in reducing the intestinal glucose absorption.
Data recorded in Table 6 showed that the addition of ESSO as well as RSSO to the incubation media caused an increase of glucose uptake by rat diaphragm in vitro. Both agents produced a highly significant (LSD; P<0.01) increase in glucose uptake with increasing doses as composed with the blank. The effect of ESSO at low concentration (0.2 mg/ml) seemed to be more potent than higher one, while at high concentration, the RSSO appeared to be more effective.
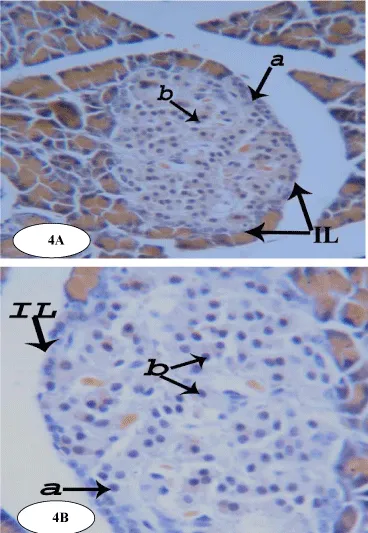
The pancreas is made up of an acinar exocrine component and a diffuse endocrine one, which constitutes the pancreatic islets or islets of Langerhans. The exocrine portion consists of lobules which in turn consist of pancreatic acini that are tightly packed together with very little connective tissue in between and their constituent acinar cells appear roughly triangular in outline (Figure 4).
In normal animals, the endocrine portion of pancreas, islets of Langerhans, is scattered throughout the pancreas as irregular, spheroidal masses of pale stained cells with rich vascular supply. In the islets, cells are arranged in irregular cords between which are distributed capillaries (Figure 4).
Modified aldehyde fuchsin method demonstrates three main types of cells, alpha (a), beta (b) and delta (d) cells. All are irregular, granular and polygonal cells with central spherical nuclei. Beta (b) cells are the most abundant cells found in the endocrine pancreas, occupying the core of the islets and contain numerous granules. Alpha (a) and delta (d) cells form the periphery of the islets. Delta cells are usually located adjacent to alpha cells and are somewhat larger in size (Figure 4).
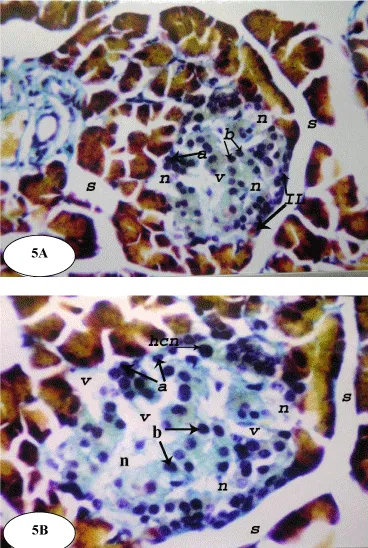
Following STZ administration, at a dose level of 45 mg/kg b. wt., subtle alterations in the pancreatic islet cells were observed (Figure 5) and the architecture of the normal islets was disrupted. The islets of the diabetic control rats showed vacuolation and necrosis. Many nuclei appear irregular and hyperchromatic and many cells showed extreme hydropic degenerations (Figure 5).
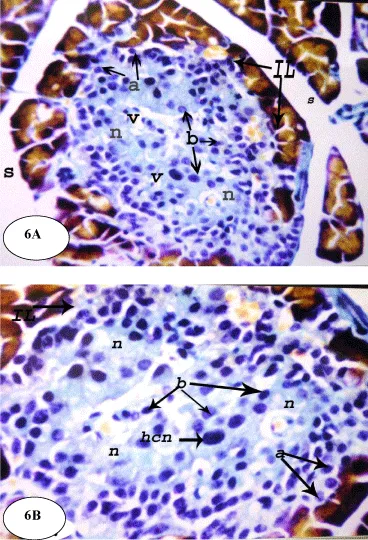
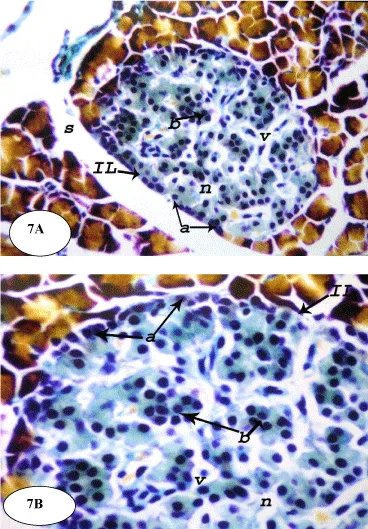
On the other hand, the treatment of the diabetic rats with ESSO and RSSO produced potential improvement of the islets architecture (Figures 6 and 7). At the end of the experiment, the islets regained their normal architecture with enlarged size and the number of alpha (a) and beta (b) cells was obviously increased. There are still few vacuolations (v) and necrotic areas (n).
Discussion
Study results revealed the treatment of diabetic rats with either ESSO or RSSO lead to significant amelioration of glucose tolerance. A decrease in elevated serum glucose levels is in agreement with the results of Sajeeth et al. [40], who revealed similar effect of ES in STZ-induced diabetic rats. ES might have exerted its effect by preventing the death of beta cells or may have helped in the recovery of partially destroyed beta cells [41]; this action may be due to its antioxidant properties [42]. The present results are also in accordance with the data of El-Missiry and El Gindy [18], who reported the effect of ESS oil on alloxan diabetic rats and are in concordance with those of Pari and Rajarajeswari [43], who evaluated the antidiabetic effect of coumarin, a rich secondary constituents of Eruca sativa [44], in STZ-nicotinamide-induced diabetic rats. Moreover, the antihyperglycemic effect of RSSO may also be attributed to the presence of substances other than flavonoids, i.e. glycosides, saponin and coumarin. Such compounds have been reported to be responsible for hypoglycemic action [45]. In comparison with normal control rats, the present study revealed a profound decrease in fasting insulin level of STZ diabetic rats. This finding agrees with Akhani et al. [46] and Modi et al. [47] and may be ascribed to the diabetogenic effect of STZ, which leads to marked degenerative changes in β-cells, as indicated in the present histological study. Serum insulin concentration was increased markedly as a result of treating diabetic rats with both ESSO and RSSO. ESSO by its ability to inhibit lipid peroxidation [18], prevents STZ induced oxidative stress and protects β-cells resulting in increased insulin secretion and decrease in elevated blood glucose levels. Also, RSSO increased insulin secretion and decreased in elevated blood glucose levels. This effect may be due to it contains anthocyanins that have antioxidant effect that prevent cell from oxidative damage as reported by Matsufuji et al. [21] and to the presence of coumarins and flavonoids which are probably responsible for recovery in the altered biochemical variables and amendment of pancreatic islets histological architecture and histology as reported by Kröncke et al. [48] and evidenced in the present investigation.
The decrease in liver glycogen observed in diabetic rats is in accordance with the finding of Ferrannini et al. [49] and Ferreira et al. [50] and supported the suggestion of increased glucose output during insulin deficiency. The increased hepatic glucose output in diabetes may be derived from glycogenolysis and/or gluconeogenesis as reported by Raju et al. [51]. Our results revealed an enormous depletion in hepatic glycogen content accompanied by profound elevation of glucose-6-phosphatase, as compared to that of normal control ones. These results are in accordance with those of Ahmed et al. [52] and Ahmed et al. [53], who found that STZ-induced diabetes reduced hepatic glycogen content and increased glucose-6-phosphatase activity in diabetic rats. This decrease in glycogen content of the hepatocytes in the liver could be due to the displacement of glycogen in the cytoplasm of hepatocytes as accumulation of lipid droplets [54]. The elevation of liver glycogen content in the present study after treatment with ESS and RSS oil may be due to their ability to improve insulin level.
With regards to the in situ-intestinal glucose absorption in the current study, it was found to be decreased in a dose dependent manner as a result of the presence of ESS RSSO in the perfusion solution. Moreover, RSSO was more effective in decreasing intestinal absorption of glucose than ESS oil. Thus, based on the results, it can be suggested that the reduction of intestinal glucose absorption might have a role in the mechanism of action to augment the hypoglycemic activity of the tasted materials.
In some other instances, the present study revealed that both RSSO and ESSO potentially increased peripheral glucose uptake in the rat diaphragm in vitro. Thus, these tested oils may also produce their anti-hyperglycemic effects by enhancing the peripheral glucose uptake in addition to their effects in decreasing the hepatic glucose output and intestinal glucose absorption.
In view of the lipid profile, the diabetic rats exhibited marked elevation of serum total lipids, triglycerides, total cholesterol, LDL-cholesterol and vLDL-cholesterol concentrations. These results are in agreement with the findings of El-Missiry and El Gindy [18], Rajagopal and Sasikala [55], Baxi et al. [56] and Lanjhiyana et al. [57], who recorded significant increased levels of these lipids in the alloxanized diabetic rats, and in agreement with Vergès [58], who revealed an increased triglycerides and LDL-cholesterol levels in type 1 diabetes. On the other hand, HDL-cholesterol exhibited a different behavioral pattern where it was detectably decreased in the diabetic rats. This agrees well with observation Modi et al. [47], Vergès [58] and Lanjhiyana et al. [57]. Additionally, Rajagopal and Sasikala [55] and Singh et al. [59] reported that diabetogenic agents significantly increase the cholesterol and triglycerides levels. The abnormally high concentration of serum lipids in DM is mainly due to an increase in the mobilization of free fatty acids from the peripheral fat depots, since insulin inhibits the hormone-sensitive lipase [60]. The marked hyperlipidemia that characterizes the diabetic state may, therefore, be regarded as a consequence of the uninhibited actions of lipolytic hormones on the fat depots. Excess of fatty acids in plasma produced by STZ promotes the liver conversion of some fatty acids to phospholipids and cholesterol [61].
In the present study, treatments of STZ-induced diabetic rats with ESS and RSS oils produced potential improvement of the altered serum lipid variables. These results are in agreement with El-Missiry and El Gindy [18], who revealed the amelioration of alloxan-induced diabetes mellitus and oxidative stress in rats by oil of ESS that improved the levels of serum lipid parameters as compared with the diabetic group. Also, the finding is in concurrence with Bhavsar et al. [62], who demonstrated the decreasing effect of saponins, one of RSS components, on triglycerides and total cholesterol levels and with Kwon et al. [63], who revealed that anthocyanins, one of RSS components, were also effective in improving the lipid profile. These chemical constituents significantly reduced the levels of serum triglyceride and cholesterol.
Our study showed that the aminotransferases (AST and ALT) activities were significantly increased in the liver of STZ-induced diabetic rats. This increase in aminotransferases levels may be due to the cellular damage in the liver caused by STZ-induced diabetes. This agrees with Zafar et al. [64], who revealed the altered liver morphology and enzymes in STZ-induced diabetic rats. In contrast to the present study, Okada et al. [65], reported that AST activity was lower than the amount of enzyme in diabetic rat tissues. It was suggested that this may be due to the inactivation of cytosolic AST in the diabetic rat tissues by a glycation reaction, accompanied by impairment in glucose utilization in STZ induced diabetes.
In the present study treatments with both ESS and RSS oil decrease the AST and ALT levels. These results are in agreement with those of Okamoto et al. [66], who reported the ability of coumarin, one of rich constituents of ES, to lower plasma ALT activity. Also, the effect of the tested plants may be due to their ability to prevent cell damage and recover the cellular damage in the liver.
In view of oxidative stress point of view, the present results indicated that STZ-induced diabetes produced marked elevation of liver lipid peroxidation and detec tablereduction of liver total thiols, glutathione content. These data are consistent with those of several authors. El-Missiry and El Gindy [18], Ahmed [67], Adewole et al. [68] and Moussa [69], reported that abnormalities of antioxidant defense systems have been demonstrated in both experimentally induced diabetes and in patient with diabetes mellitus. In the study of Ha et al. [70], it was shown that oxidative stress is one of the important mediators of vascular complications in diabetes including nephropathy. High glucose produces reactive oxygen species (ROS) as a result of glucose auto-oxidation, metabolism, and the development of advanced glycosylation end products.
Lipid peroxidation is one of the characteristic features of chronic diabetes. Oxidative damage induced by STZ resulted in the formation of highly reactive hydroxyl radical, which stimulates the lipid peroxidation that causes destruction and damage to the cell membrane [71]. Glutathione is an important biomolecule against chemically induced toxicity and can participate in the elimination of reactive intermediates by reduction of hydroperoxides in the presence of glutathione peroxidase. Glutathione also functions as free radical scavenger and in the repair of radical caused biological damage [72]. It also inhibits free radical mediated lipid peroxidation [73]. Decreased glutathione levels in diabetes have been considered to be an indicator of increased oxidative stress [74].
In the present study, the treatment of diabetic rats with ESSO and RSSO produced a significant decrease in the liver lipid peroxidation. This decrease could be attributed to the increase in glutathione peroxidases in rats treated with the herbal formulation since this enzyme has been shown to inactivate lipid peroxidation. The present results are in accordance with El-Missiry and El Gindy [18] who reported that ESS oil has a significant decrease in lipid peroxidation and increase glutathione content in alloxan-induced diabetic rats and in concordance with Sajeeth et al. [71], who reported the same effect of ESS. Additionally, RSS oil has the same effect which may be due to the presence of anthocyanins. The anthocyanins are important group of dietary antioxidants that have many physiological functions. They protect living cells from oxidative damage resulting in the prevention of diseases [21].
In conclusion, ESSO an RSSO have potent anti-hyperglycemic and antihyperlipidemic efficacies in STZ-induced diabetic rats. These effects may be due their ability to improve the islets architecture, insulin secretory response and antioxidant activity.
- WHO (World Health Organization) (2011) Diabetes, Fact sheet No 312. Link: https://goo.gl/3udgFH
- WHO (World Health Organization) (2016) Global Report on Diabetes. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland. Link: https://goo.gl/obwRAk
- American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28: S37-S42. Link: https://goo.gl/HqwjvU
- Tripathi BK, Srivastava AK (2006) Diabetes mellitus: Complications and therapeutics, Med Sci Monit 12: RA130-147. Link: https://goo.gl/IbzZjB
- Mealey BL, Ocampo GL (2007) Diabetes mellitus and periodontal disease, Periodontology 44: 127-153. Link: https://goo.gl/bnv3mv
- Standl E, Maxeiner S, Raptis S (2006) Once-daily insulin glargine administration in the morning compared to bedtime in combination with morning glimepiride in patients with type 2 diabetes: an assessment of treatment flexibility. Horm Metab Res 38: 172-177. Link: https://goo.gl/WLX5GX
- Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, et al. (2007) Induction of diabetes by streptozotocin in rats. IJCB 22: 60-64. Link: https://goo.gl/NAVdqQ
- Hayashi K, Kojima R, Ito M (2006) Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharmaceut Bull 29: 1110-1119. Link: https://goo.gl/YKrXq9
- Ikebukuro K, Adachi Y, Yamada Y, Fujimoto S, Seino Y, et al. (2002) Treatment of Streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone Marrow cells via portal vein in rats. Transplantation 73: 512-518. Link: https://goo.gl/yuqxOh
- Shukla R, Sharma SB, Puri D, Murthy PS (2000) Indigenous Drugs for Diabetes Mellitus. J K Science 2: 128-130. Link: https://goo.gl/qPv2nY
- Geiger PC, Han DH, Wright DC, Holloszy JO (2006) How muscle insulin sensitivity is regulated: testing of a hypothesis. Am J Physiol Endocrinol Metab 291: E1258-63. Link: https://goo.gl/MCsO3c
- Kawahito S, Kitahata H, Oshita S (2009) Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J Gastroenterol 15: 4137-4142. Link: https://goo.gl/GYTNAf
- Nammi S, Boini MK, Lodagala SD, Behara RB (2003) The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complementary and Alternative Medicine 3: 1472-6882. Link: https://goo.gl/1oPjdS
- Hollman PC, Katan MB (1999) Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol 37: 937-942. Link: https://goo.gl/1bJTJp
- Lean ME, Noroozi M, Kelly I, Burns J, Talwar D, et al. (1999) Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes 48: 176-181. Link: https://goo.gl/RNAHGY
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, et al. (2002) Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 76: 560-568. Link: https://goo.gl/KG8u1n
- Martinez-Sanchez A, Gil-Izquierdo A, Gil MI, Ferreres FA (2008) Comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J Agric Food Chem 56: 2330-2340. Link: https://goo.gl/VOadmd
- El-Missiry MA, El Gindy AM (2000) Amelioration of Alloxan Induced Diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann Nutr Metab 44: 97–100. Link: https://goo.gl/RXRau3
- Fahey JW, Talalay P (1999) Antioxidant functions of sulforaphane: A potent inducer of phase II detoxicating enzymes. Food Chem Toxicol., 37: 973–979. Link: https://goo.gl/FPzhuC
- Zaman RU (2004) Study of cardioprotective activity of Raphanus sativus L. in the rabbits. Pak J Biol Sci 7: 843-847. Link: https://goo.gl/v4PJgj
- Matsufuji H, Otsuki T, Takeda T, Chino M, Takeda M (2003) Identification of reaction products of Acylated Anthocyanins from red radish with peroxyl radicals J Agric Chem 51: 3157- 3161. Link: https://goo.gl/sRW4Bu
- Suh SJ, Moon SK, Kim CH (2006) Raphanus sativus and its isothiocyanates inhibit vascular smooth muscle cells proliferation and induce G1 cell cycle arrest. International Immunopharmacology 6: 854-861. Link: https://goo.gl/5MgJjL
- Dandu AM, Inamdar NM (2009) Evaluation of beneficial effects of antioxidant properties of aqueous leaf extract of Andrographis paniculata in STZ-induced diabetes. Pak J Pharm Sci 22: 49-52. Link: https://goo.gl/OjX8R4
- Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6: 24-27. Link: https://goo.gl/1VRPGI
- Marschner I, Bottermann P, Erhardt F, Linke R, Maier V, et al. (1974) Group experiments on the radio-immunological insulin determination. Horm Metab Res 6: 293-296. Link: https://goo.gl/EyWyDf
- Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with anthrone reagent. Arch Biochem 25: 191-200. Link: https://goo.gl/r9oFkg
- Begum N, Moses SG, Shanmugasundaram KR (1978) Serum enzymes in human and experimental diabetes mellitus. Indian J Med Res 68: 774-784. Link: https://goo.gl/3Wtav5
- Munoz MA, Balon M, Fernandez C (1983) Direct determination of inorganic phosphorus in serum with a single reagent. Clin Chem 29: 372-374. Link: https://goo.gl/Z22Ukx
- Scumann G, Klauke R (2003) New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 327: 69-79. Link: https://goo.gl/jOfmeg
- Frings CS, Frendley TW, Dunn RT, Queen CA (1972) Improved determination of serum total lipids by the sulpfo-phospho-vanillin reaction. Clin Chem 18: 673-674. Link: https://goo.gl/6niygy
- Fossati P, Prencip L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28: 2077-2080. Link: https://goo.gl/2a8wlU
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20: 470-475. Link: https://goo.gl/uNMB5Q
- Burnstein M, Selvenick HR, Morfin R (1970) Rapid method for isolation of lipoprotein from human serum with polyanions. J Lipid Res 11: 583-395. Link: https://goo.gl/sw7vuV
- Nobert WT (1995) Clinical guide to laboratory tests, 3rd edition. Philadelphia: WB Saunders Company Link: https://goo.gl/FyaoUW
- Preuss HG, Jarrel ST, Scheckenbach R, Lieberman S, Anderson RA (1998) Comparative effects of chromin, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr 17: 116-123. Link: https://goo.gl/lQqz8S
- Beutler E, Duron O, Kelly BM (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61: 882-888. Link: https://goo.gl/ebHhxe
- Koster JF, Biermond P, Swaak AJG (1986) Intracellular and extracellular sulphhydryl levels in rheumatoid arthritis. Ann Rheum Dis 45: 44-46. Link: https://goo.gl/V0bWRL
- Zarzuelo A, Risco S, Gamez MJ, Jimenez J, et al. (1990) Hypoglycemic action of Salvia lavandulifolia vahl.ssp. oxyodon: A contribution to studies on the mechanism of action . Life Science 47: 909-915. Link: https://goo.gl/32PqiU
- Roa M, Blane K, Zonneberg M (1985) One way analysis of variance. Version IA (C). PC-STAT, Program coded by University of Georgia, USA 1985. Link:
- Sajeeth CI, Manna PK, Manavalan R, Jolly CI (2010) Antidiabetic activity of a polyherbal formulation ESF/AY/500 in streptozotocin induced diabetic male albino rats: a research. International Journal of Drug Formulation & Research 1: 311-322. Link: https://goo.gl/WB9Wly
- Ahmeda I, Adeghatea E, Sharmaa AK, Pallota DJ, Singh J (1998) Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Research and Clinical Practice 40: 145-151. Link: https://goo.gl/SGhswN
- Chattopadhyay RR, Bandyopadhyay M (2005) Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and Streptozotocin induced diabetic rats. African Journal of Biomedical Research 8: 101-104. Link: https://goo.gl/4M6kSS
- Pari L, Rajarajeswari N (2009) Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem Biol Interact 181: 292-296. Link: https://goo.gl/ctnBXh
- Sadiq A, Hayat MQ, Mall SM (2014) Qualitative and Quantitative Determination of Secondary metabolites and Antioxidant Potential of Eruca sativa. Nat Prod Chem Res 2: 137. Link: https://goo.gl/UzDU6S
- Vessal M, Zal F, Vasi M (2003) Effects of Teucrium polium on oral glucose tolerance test, regeneration of pancreatic islets and activity of hepatic glucokinase in diabetic rats. Arch Iran Med 6: 35-39. Link: https://goo.gl/PoSiVV
- Akhani SP, Vishwakarma SL, Goyal RK (2004) Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol 56: 101-5. Link: https://goo.gl/C0jfwQ
- Modi KP, Vishwakarma SL, Goyal RK, Bhatt PA (2006) Beneficial effects of Coenzyme Q10 in Streptozotocin-Induced Type I Diabetic Rats. IJPT 5: 61-65. Link: https://goo.gl/wtzCfX
- Kröncke KD, Fehsel K, Sommer A, Rodriguez ML, Kolb-Bachofen V (1995) Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-nitroso-urea: Streptozotocin contributes to islet cell DNA damage. Biol Chem Hoppe-Seyler 376: 179-185. Link: https://goo.gl/5KeoXA
- Ferrannini E, Lanfranchi A, Rohner-Jeanrenaud F, Manfredini G, Van de Werve G (1990) Influence of long-term diabetes on liver glycogen metabolism in the rat. Metabolism 39: 1082-8. Link: https://goo.gl/wc2qCg
- Ferreira LD, Bräu L, Nikolovski S, Raja G, Palmer TN, et al. (2001) Effect of streptozotocin-induced diabetes on glycogen resynthesis in fasted rats post-high-intensity exercise. Am J Physiol Endocrinol Metab 280: E83-91. Link: https://goo.gl/WKIEFp
- Raju, J, Gupta D, Rao AR, Yadava PK, Baquer NZ (2001) Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reserving the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem 224: 45-51. Link: https://goo.gl/VNzEfG
- Ahmed OM, Abdel Moneim A, Abul Yazid I, Mahmoud AM (2010) Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetologia Croatica 39: 15-35. Link: https://goo.gl/Jz33xO
- Ahmed OM, Abdel-Hamid H, Bastawy M, Hasona NA (2006) Antihyperglycemic effects of Plantago ispoghula seeds aqueous extracts in diabetic and hypercholesterolemic rats. J Egypt Ger Soc Zool 51: 371-393. Link:
- Thulesen J, Orskov C, Holst JJ, Poulsen SS (1997) Short term insulin treatment prevents the diabetogenic action of streptozotocin in rats. Endocrinology 138: 62-8. Link: https://goo.gl/nzR6iJ
- Rajagopal K, Sasikala K (2008) Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats, Singapore Med J 49: 137-141. Link: https://goo.gl/AgcyiL
- Baxi DB, Singh PK, Doshi AA, Arya S, Mukherjee R, et al. (2010) Medicago Sativa leaf extract supplementation corrects diabetes induced dyslipidemia, oxidative stress and hepatic renal functions and exerts antihyperglycaemic action as effective as Metformin, Annals of Biological Research 1: 107-119. Link: https://goo.gl/AYpQBS
- Lanjhiyana S, Garabadu D, Ahirwar D, Bigoniya P, Rana AC, et al. (2011) Antihyperglycemic potential of Aloe vera gel in experimental animal model. Annals of Biological Research 2: 17-31. Link: https://goo.gl/fE7EYI
- Vergès B (2009) Lipid disorders in type 1 diabetes. Diabetes Metab 35: 353-60. Link: https://goo.gl/pAjdnm
- Singh AK, Singh J, George M, Joseph L (2010) Anti-Diabetic Effect Of Flacourtia Jangomas Extract In Alloxan-Induced Diabetic RatsPharmacologyonline2: 253-259. Link: https://goo.gl/tx4S1w
- Al-Shamaony L, Al-Khazraji SM, Twaij HA (1994) Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. J Ethnopharmacol 43: 167-71. Link: https://goo.gl/RCU1x8
- Bopanna KN, Kannan J, Sushma G, Balaraman R, Rathod SP (1997) Antidiabetic and anti-hyperlipaemic effects of neem seed kernel powder on alloxan diabetic rabbits. Indian J Pharmacol 29: 162-7. Link: https://goo.gl/BjYwNy
- Bhavsar SK, Singh S, Giri S, Jain MR, Santania DD (2009). Effect of saponins from Helicteres isora on lipid and glucose metabolism regulating genes expression, Journal of Ethnopharmacology 124: 426-433. Link: https://goo.gl/L3bxpK
- Kwon SH, Ahn IS, Kim SO, Kong CS, Chung HY, et al. (2007) Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J Med Food 10: 552-6. Link: https://goo.gl/2gG97m
- Zafar M, Naqvi S, Ahmed M, Kaimkhani Z (2009) Altered Liver Morphology and Enzymes in Streptozotocin Induced Diabetic Rats. Int J Morphol 27: 719-725. Link: https://goo.gl/rGCwkP
- Okada M, Murakami Y, Miyomoto E (1997) Glycation and inactivation of aspartate aminotransferase in diabetic rat tissues. J Nutr Sci Vitaminol 43: 463-9. Link: https://goo.gl/TzeJqQ
- Okamoto T, Kobayashi T, Yoshida S (2005) Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas. Curr Med Chem - Anti-Cancer Agents 5: 47-51. Link: https://goo.gl/wzbhLK
- Ahmed OM (2003) Evaluation of the hypoglycemic and antioxidant effects and the probable mechanism of action of chromium and selenium in streptozotocin-induced diabetic albino rats. J Egypt Ger Soc Zool 41: 163-193. Link:
- Adewole SO, Ezekiel AC, Ojewole AO (2007) Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Trad CAM 4: 64-74. Link: https://goo.gl/0L7mIp
- Moussa SA (2008) Oxidative stress in diabetes mellitus. Romanian J Biophys 18: 225–236. Link: https://goo.gl/dRHlZY
- Ha H, Kim C, Son Y, Chung MH, Kim KH (1994) DNA damage in the kidneys of diabetic rats exhibiting microalbuminuria. Free Radic Biol Med 16: 271-274. Link: https://goo.gl/kQObPn
- Sajeeth CI, Manna PK, Manavalan R, Jolly CI (2010) Phytochemical Investigation and Antioxidant Activity of a Polyherbal Formulation (ESF/AY/500) on Streptozotocin Induced Oxidative Stress in Rats, Der Pharma Chemica 2: 184-189. Link: https://goo.gl/imsm9K
- Meister A (1984) New aspects of glutathione biochemistry and transport, selective alteration of glutathione metabolism. Nutr Rev 42: 397- 410. Link: https://goo.gl/UK0pfC
- Meister A, Anderson ME (1983) Glutathione. Annual Rev Biochem 52: 711-760.
- Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complication. Br Med Bull 49: 642-652. Link: https://goo.gl/T9bFNc
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
