International Journal of Clinical Endocrinology and Metabolism
Protective Effect of Alkaloids from Amaranthus Viridis Linn. Against Hydrogen Peroxide Induced Oxidative Damage in Human Erythrocytes (RBC)
Vadivukkarasi Sasikumar*, Arunambiga Subramaniam, Anila Aneesh and Ganapathy Saravanan
Cite this as
Sasikumar V, Subramaniam A, Aneesh A, Saravanan G (2015) Protective Effect of Alkaloids from Amaranthus Viridis Linn. Against Hydrogen Peroxide Induced Oxidative Damage in Human Erythrocytes (RBC). Int J Clin Endocrinol Metab 1(1):049-053. DOI: 10.17352/ijcem.000011Plants are rich in antioxidants which play an essential role in disease prevention. Amaranthus Viridis Linn. Is a common medicinal plant, spread throughout the world is used in traditional ayurvedic medicine. The present study was aimed to examine the protective effect of partially purified alkaloids (PPA) from Amaranthus viridis against hydrogen peroxide induced oxidative damage in human erythrocytes in vitro conditons. The experiments are performed in groups (Control, H22 induced and H22 + 25, 50 mg conc of alkaloids). The enzymic antioxidants like CAT, SOD, GPx, GST and non enzymic antioxidants like vitamin C, vitamin E and GSH levels and LPO were evaluated to determine the protective effect of alkaloids. The alkaloids prevent the decline of antioxidant status which in turn decreases LPO levels by preventing MDA formation in erythrocytes. The results confirm the protective effect of alkaloids against free radical induced oxidative damage in human erythrocytes.
Abbreviations
CAT: Catalase; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; GST: Glutathione-S-Transferase; GSH: Glutathione; LPO: Lipid Peroxidation; MDA: Malondialdehyde; H22: Hydrogen Peroxide; D.H2O: Distilled Water; Conc.: Concentrated; ROS: Reactive Oxygen Sspecies; RBC: Red Blood Cells ; NADH: Nicotinamide Adenine Dinucleotide;CDNB: 1-Chloro-2,4-Dinitro Benzene; TCA: Trichloro Acetic Acid; EDTA: Ethylene Diamine Tetra Acetate; DTNB: 3,3‘-Dithio-Bis(6- Nitrobenzoic Acid); PBS: Phosphate Buffered Saline; Alk: Alkaloid; Hb: Hemoglobin;
Introduction
Free radicals are formed during the normal metabolic process in the biological system. Free radicals are highly reactive due to the presence of unpaired electron(s) [1]. Reactive oxygen species (ROS) are a type of free radicals that are produced during normal cell metabolism, both in animals and plants. The ROS including H22, superoxide and the hydroxyl radical are some of the metabolic intermediates in human body [2]. Excess of ROS leads to oxidative stress, resulting in oxidative DNA damage which is implicated in the pathogenesis of numerous disorders [3].
A.viridis (Amaranthaceae) is spread throughout the world, growing under a wide range of climatic conditions. They produce useful feed and food products [4]. A.viridis L. (Amaranthaceae) has also been used in Indian traditional system. It is also a very good source of vitamins including vitamin A, B6, and C, riboflavin and foliate, minerals like calcium, iron, zinc, magnesium [5]. The A.viridis leaf extracts protects the heart by preserving the integrity of membrane and by maintaining the activities of marker enzymes in the serum and heart of isoproterenol induced cardio toxic rats which may be due to antilipoperoxidative and antioxidant effects [6].
Alkaloids are plant derived pharmacologically active, basic compounds from amino acids that contain one or more heterocyclic nitrogen atoms. Plant derived alkaloids are suggested to be natural antioxidants associated with prevention of various diseases and pathological conditions including malaria, diabetics, cancer, cardiac dysfunction etc. and also used as local anesthetics and pain relievers [7]. Newly extracted alkaloids from medicinal plants are used as therapeutic agents [8]. Many alkaloids are used as anti-arrhythmic, anticholinergic, anti-tumor, vasodilating, antihypertensive, cough medicine, anesthetic, antiprotozoal, antidiabetic, antihyperlipidemic and antioxidant agents [6].
Erythrocytes are mammalian cells that are responsible for transporting oxygen and other substances in blood throughout the body. These erythrocytes are easy to extract and they lack nucleus and hence they can be effectively used for studying the effect of various pharmacologically active substances. They are highly susceptible to oxidative damage as a result of high polyunsaturated fatty acid (PUFA) content of their membranes and the high cellular concentrations of oxygen and hemoglobin (Hb), a potentially powerful promoter of oxidative processes [9].
The present study reveals the protective effect of isolated alkaloids from A. viridis leaves against H22induced oxidative damage in RBC.
Materials and Methods
Chemicals used
PBS (pH 7), hydrogen peroxide, phosphate buffer, ammonium hydroxide, potassium dichromate, glacial acetic acid, sodium phosphate buffer, absolute ethanol, chloroform, n-butanol, phenazine methosulphate, nitrobluetetrazolium, ADH, CDNB, reduced glutathione, sodium phosphate buffer, sodium azide, TCA, EDTA, sodium citrate, DTNB, oxalic acid, sulphuric acid, 2,4-dinitro phenyl hydrazine, thiourea, ascorbic acid, disodium hydrogen phosphate, petroleum ether, 2,2-dipyridyl, ferric chloride were purchased from Sigma-Aldrich or Merck. All the chemicals used were of analytical grade.
Preparation of A.viridis leaf extract
The fresh plants of A.viridis were collected from local market in Erode, Tamil Nadu, India and was authenticated by Dr. R. Jagadesan, Horticulture, Horticultural College and Research Institute for Women, Trichy. The fresh leaves of A.viridis were collected and washed with tap water, followed by distilled water and shade dried. The dried leaves were manually ground to a fine powder and stored at 4°C until future use.
Isolation of alkaloids
5g of A.viridis powder was weighed in a 250ml conical flask and 50ml of 20% acetic acid in ethanol was added and covered to stand for 4hrs.This was filtered and the extract was concentrated using water bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added drop wise until the precipitation was complete. The whole solution was allowed to settle and the precipitate was collected by filtration and amount of alkaloid yield was weighed.
Purification of alkaloids
The filtered alkaloid was purified using the method proposed by Shi et al., 2002. 5g of silica gel (laboratory grade) were activated in hot air oven at 110˚C for 1 hr. The glass wool was fixed at the bottom of the column. The activated silica was added to the column, in small portion with gentle tapping after each addition, in order to ensure the uniform packing. The small quantity of solvent was allowed to remain at the top of the column (about 4 cm). Crude alkaloid residue (0.1g) was loaded to the top the column and eluted with the methanol solvent system. The presence of alkaloids in the extracted fractions were verified by various tests like Draggendroff’s test, Wagner’s test, Hager’s test and Mayer’s Test.
Preparation of erythrocytes suspensions [10]
Fresh blood samples from healthy volunteers (10–15ml) were collected and centrifuged at 3000 rpm for 15 minutes, plasma and puffy coats were removed. Red cells were washed with PBS (pH 7.00) for three times and erythrocytes were lysed with ice-cold distilled water.
Experimental design
Erythrocyte suspensions obtained from healthy donor were divided into four groups as Group I- Control [Erythrocyte suspension (750μl), PBS (1000μl) and D.H2O (250μl)], GroupII-H22 [Erythrocyte suspension (750μl), 10mM H22 (50μl), PBS (950μl) and D.H2O (250μl)], Group III- Alk (25 mg) [Erythrocyte suspension (750μl), 10mM H22 (50μl), alkaloids (500μl) (25mg alkaloids in 500μl PBS) and PBS (950μl)] and Group IV- Alk (50 mg) [Erythrocyte suspension (750μl), 10mM H22 (50μl), alkaloids (500μl) (50mg alkaloids in 500μl PBS) and PBS (950μl)]. These experimental groups were incubated at 37°C for 1 hour. Following the incubation, enzymic and non-enzymic antioxidant activity were determined.
Determination of antioxidant status
Measurement of Lipid peroxidation [11]: The lipid peroxidation was measured by the amount of formation of Malondialdehyde (MDA), the end product of lipid peroxidation. The MDA content was determined by the thiobarbituric acid (TBA) reaction.1.0ml of sample, 1.5 ml of 0.67% TBA and 1.0ml of water was added. A pink colour developed is read at 535nm. A series of standards (2-10 nmoles) was treated in a similar manner along with a reagent blank. The results were expressed for nmoles of MDA formed/mg protein.
Measurement of catalase activity [12]: Catalase causes rapid decomposition of hydrogen peroxide to water. Dichromate in acetic acid was converted to perchloric acid and then to chromic acetate when heated in presence of H22. The chromic acetate thus produced is measured spectrophotometrically at 610nm. To 0.9ml of phosphate buffer, 0.1ml of sample and 0.4ml of H22 was added. At 0sec and after 60sec 2.0ml of dichromate-acetic acid mixture was added. The tubes were kept in boiling water bath for 10 min and the colour formed as a result of chromic acetate formation was read at 620nm. Standards in the range of 1.2-6.0 µmol were taken and processed as test and blank containing reagent alone. The activity of catalase was expressed for erythrocytes as µmol of H22 decomposed/minutes/g of Hb.
Measurement of superoxide scavenging activity [13]: The determination of SOD is based on the inhibition of the formation of NADH-phenazine methosulphate and nitroblue tetrazolium formazon. 0.5ml sample was diluted to 1.0ml with water followed by addition of 2.5ml of ethanol and 1.5ml chloroform (chilled reagents were added). This mixture was shaken for 90 minutes at 4°C and then centrifuged. The assay mixture contained 1.2ml of sodium pyrophosphate buffer, 0.1ml of phenazine methosulphate, 0.3ml of nitroblue tetrazolium and appropriately diluted enzyme preparation in a total volume of 3ml. The reaction was started by the addition of 0.2ml NADH. After incubation at 30C for 90 min, the reaction was stopped by the addition of 1ml glacial acetic acid. The reaction mixture was stirred vigorously and shaken with 4ml of n-butanol. The mixture was allowed to stand for 10 min, centrifuged and n-butanol layer was separated. The colour density of the chromogen in n-butanol was measured in a Spectrophotometer at 520nm. A series of standard treated in a similar way to determine the enzyme activity. The enzyme concentration required to inhibit the chromogen produced by 50% in one minute under standard conditions was defined as one unit. The specific activity of the enzyme is expressed as unit/minutes/g of Hb.
Measurement of Glutathione-S-transferase activity [14]: Glutathione-S-transferase catalyses the reaction of 1-chloro 2,4 dinitrobenzene (CDNB) with the sulphydryl group of glutathione. To 1.0ml of buffer, 0.1ml of sample, 1.7ml of water, 0.1ml of CDNB were added and incubated at 37°C for 5 min. After incubation, 0.1ml of reduced glutathione was added. The increase in optical density of the enzyme was measured against blank at 340nm.The enzyme activity was calculated in terms of µmoles of CDNB conjugate formed/minutes/g of Hb.
Estimation of Ascorbic acid [15]: For the estimation of ascorbic acid, 1.0ml sample was taken and made up the volume to 3.0ml with distilled water. Added 1.0ml of dinitro phenyl hydrazine reagent followed by 1 to 2 drops of thiourea into each tube. A blank was done along with standard solution. Mixed the contents and incubated at 37°C for 3 h. After incubation the tubes were kept in the ice bath. Dissolved the orange red osazone crystals formed by adding 7.0ml of 80% sulphuric acid drop wise while the tubes were still in the water bath. The tubes in the ice bath were removed and allowed to stand for 30 min at room temperature and measured the absorbance at 540 nm. The result was expressed as μg/g of Hb.
Estimation of Glutathione [16]: This method was based on the development of yellow colour when 5, 5’Dithio-bis (2-nitrobenzoic acid) (DTNB) was added to compounds containing sulphydryl groups. 0.5ml of homogenate was pipetted out and precipitated with 2.0ml of 5% TCA.2.0ml of supernatant was taken after centrifugation and 1.0ml of Ellman’s reagent and 4.0ml of 0.3 M disodium hydrogen phosphate were added. The yellow colour developed was read in Spectrophotometer at 412nm. A series of standards (20–100µg) was treated in a similar manner along with a blank containing 1.0ml of buffer. The results were expressed in μ mol/g of Hb.
Statistical analysis
The data were expressed as mean ± S.D Statistical differences at p<0.05 between the groups were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test using SPSS 15.0 software.
Results and Discussion
Alkaloid yield
The amount of alkaloids obtained from A.viridis is found to be 100 mg per 5g of leaves.
Test for alkaloids
The results for qualitative analysis of alkaloids using Draggendorf’s test, Wagner’s test, Hayer’s test, and Mayer’s test proved the presence of alkaloids. The test results were presented in Table 1.
Antihaemolytic activity of alkaloids in H22 induced oxidative damage in erythrocytes
Under normal conditions, the continuous production of free radicals is compensated by the powerful action of protective enzymes like superoxide dismutase, catalase and gluthathione peroxidase that are believed as major antioxidant enzymes present in the human body that protect against the oxygen toxicity [17]. As a result, a lipid peroxidation (LPO) process occurs. Therefore, antioxidant enzyme activities and lipid peroxidation levels are accepted as important parameters in the evaluation of oxidative stress in aerobic organisms. The current study was carried out with the main aim of evaluating the antioxidant status of the alkaloids that are isolated from the leaves of Amaranthus viridis. The partially purified alkaloids from the leaves proved to protect the erythrocytes from the oxidative stress caused by H22. The results of the present study, i.e., the effect of various antioxidants on H22 induced oxidative damage on erythrocytes was depicted in Figures: 1-3.
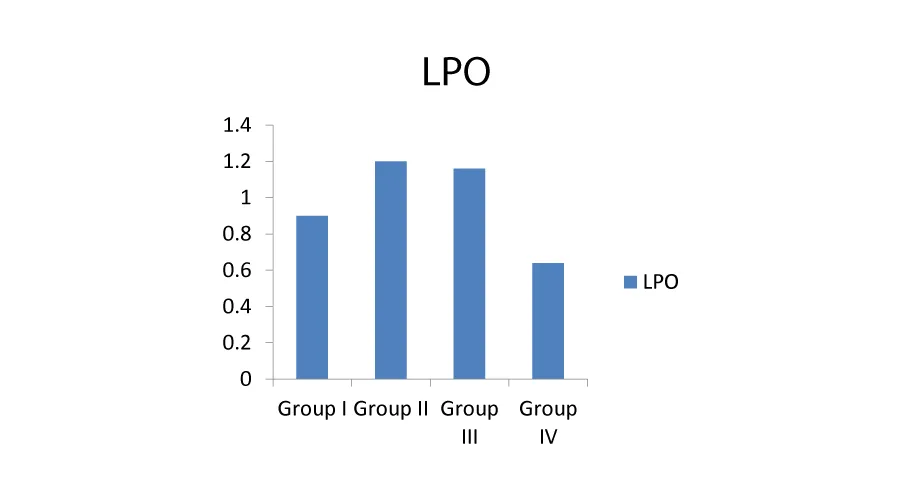
Effects of alkaloids on H22 induced lipid peroxidation: MDA is formed in high amounts during the lipid peroxidation process and its quantity reveals the extent of cell damage by peroxidation. MDA is a highly reactive, bifunctional molecule that can effectively cross link with the membrane phospholipids and proteins of the erythrocytes, thus impairing the membrane related functions ultimately leading to diminished survival of the erythrocytes [18]. The production of MDA was high in group II where the erythrocyte cells are treated with H22 alone (Table 2). Treatment with alkaloids prevented H22 induced MDA production and this inhibition was strongly dose dependent. The values in group IV are reversed and nearer to the control which indicates that alkaloids inhibits the membrane lipid peroxidation triggered by the injurious oxygen radicals generated from hydrogen peroxide (Figure 1).
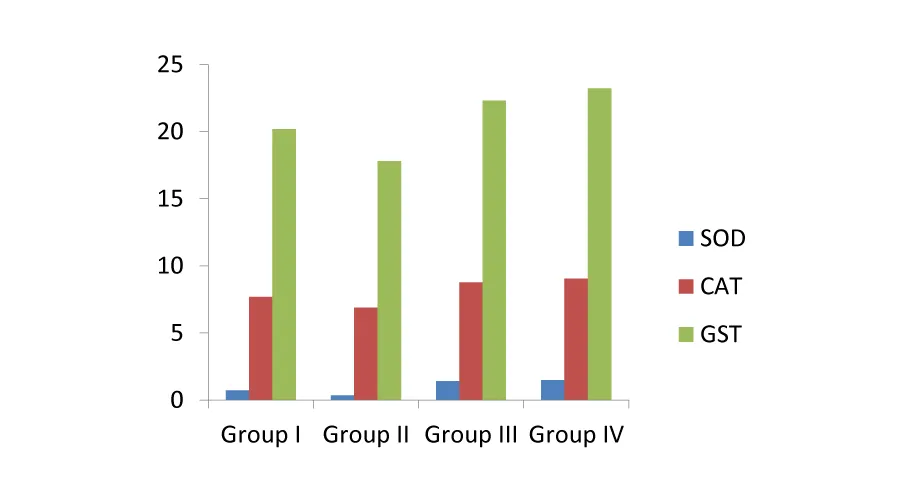
Effects of alkaloids on CAT, SOD and GST activity: Enzymic antioxidants, such as catalase, superoxide dismutase and glutathione S-transferase, play important roles in the scavenging of reactive oxygen species, such as superoxide radical, hydrogen peroxide, lipid hydroperoxides, and so forth. An aerobic organism can cope with the metabolic production of ROS under normal conditions via its antioxidant defense system; however, overproduction of ROS causes a dangerous process called “oxidative stress” [19].The antioxidant effect of CAT, SOD and GST on erythrocyte suspensions were presented in Table 3. The effect of all these three antioxidants, viz., CAT, SOD and GST was lowered in group II where H22 induced oxidative damage was high. The group IV samples with high concentration of the purified alkaloid showed more protective effect against oxidative damage caused by H22. The activity of the enzymic antioxidants in presence of the alkaloids was high and it is dose dependent, higher the alkaloid then there is higher free radical scavenging activity (Figure 2).
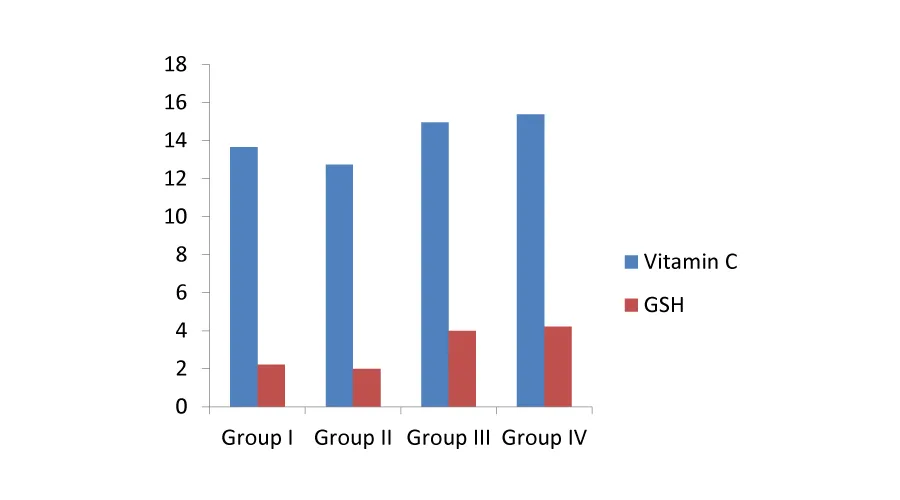
Effects of alkaloids on Ascorbic acid and Glutathione: Vitamin C and Glutathione are some of the non-enzymic antioxidants of animals which help those organisms to combat cellular damage due to oxidative stress. Vitamin C may accumulate at high concentration in photosynthetic tissues, in which it is intimately in the regulation of photosynthesis and protect the chloroplasts against damage caused by ROS such as O2, H22, hydroxyl radicals (OH) and singlet oxygen [20]. The effect of non-enzymic antioxidants on the erythrocyte suspension was presented in Table 4. Vitamin C is proved to have higher free radical scavenging activity in presence of the partially purified alkaloid from A.viridis. The protective effect of those non-enzymic antioxidants was preserved even in presence of H22 and their activity was nearly normal to that of the control sample (Figure 3). Glutathione is also a non-enzymic antioxidant which also possesses higher free radical scavenging activity and its antioxidant effect was found to be preserved at higher rates in presence of increased amount of the alkaloid from the leaf extract.
Conclusion
The antihaemolytic activity of the alkaloids from A.viridis was confirmed from this study. The results showed alkaloids present in the plant have good antihaemolytic activity. As a conclusion from the present study the alkaloids from A.viridis were found to have more protective effects on the antioxidant systems against H22 induced oxidative damage on erythrocytes. These alkaloids can effectively be used as a natural antioxidant for the treatment and prevention of lipid peroxidation related diseases.
- Abheri Das Sarma, Anisur Rahaman Mallick, Ghosh AK (2010) Free Radicals and their role in different clinical conditions: An Overview. Int J Pharm Sci Res 3: 185-192.
- Fang NA, Araiz, C, Bornet A, Delbosc S, Cristol, et al. (2005) Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fedrats. J. Agric. Food Chem 53: 151–157.
- Sudha G, Sangeetha Priya M, Indhu Shree R, Vadivukkarasi S (2011) In vitro free radical scavenging activity of raw Pepion fruit (Solanummuricatumaiton). Int J Curr Pharm Res. Res 3: 137-140
- Dini Somer T, Meiselman HJ (1993) Disorders of blood viscosity. Ann Med 25: 31–9.
- Kadashnikov C, Miller NJ, Paganga G (2008) Antioxidant properties of phenolic compounds. Trend. Plant Sci 2: 154–159.
- Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S (2013) Cardioprotective activity of Amaranthusviridis Linn: Effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infaracted rats. Int J Cardiol 165: 494 – 498.
- Cordell N, Tofani I, Maki K, Kojima K, Kojima Y, et al. (2001) Mechanical assessment of effects of grape seed proanthocyanidins extract on tibial bone diaphysis in rats. J Musculoskelet Neuronal Interact 5: 162–169.
- Edger A, Inoue S, Umegaki K (2002) Grape seed extract prevents H2O2 induced chromosomal damage in human lymphoblastoid cells. Biol Pharm Bull 27: 1459–1461.
- Clemens MR, Ruess M, Bursa Z, Waller HD (1987) The relationship between lipid composition of red blood cells and their susceptibility to lipid peroxidation. Free Radic Res Commun 3: 265–71.
- Wu YJ, Li WG, Zhang ZM, Tian X (1997) Antioxidative activity of 4-oxy and 4-hydroxy nitroxides in tissues and RBC from rats. Acta Pharmacol Sin 18: 150–154.
- Li, H.S. Principles and techniques of plant physiological biochemical experiment. Beijing: Higher Education Press. 2000. pp.260–261, 261–263.
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47: 389 – 394.
- Kakkar P. Vishwanathan PNA (1984) modified Spectrophotometric assay of Superoxide dismutase. Ind J Biochem Biophys 21: 130 – 132.
- Habig WH, Jakoby WB (1981) Assays for the differentiation of Glutathione S-transferases. Methods Enzymol 77: 398–405.
- Sadasivam S, Manickam A (1997) Estimation of dehydro ascorbic acid. Biochemical Methods. New Age International Publishers, New Delhi. pp:184-186.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70-77.
- Abraham S, Soundararajan CC, Vivekanandhan S, Behari M (2005) Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res 121: 111–115.
- Ault JG, Lawrence DA (2003) Glutathione distribution in normal and oxidatively stressed cells. Exp Cell Res 285: 9–14.
- Rajasekaran K (2005) Seizure-induced oxidative stress in rat brain regions: blockade by nNOS inhibition. Pharmacol Biochem Behav 80: 263-272.
- Müller R, Owen CA, Xue ZT, Welander M, Stummann BM (2002) Characterization of two CTR-like protein kinases in Rosa hybrida and their expression during flower senescence and in response to ethylene. J Exp Bot 53: 1223-1225.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
