Global Journal of Obesity, Diabetes and Metabolic Syndrome
Decrease of microglia and fatty liver in obese mice by germinated Sang-Yod rice
Nuntika Wangpradit1, Supattra Prom-in1, Jaya Kumar2, Kien Hui Chua2 and Jasadee Kaewsrichan1*
2Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, 56000 Kuala Lumpur, Malaysia
Cite this as
Wangpradit N, Prom-in S, Kumar J, Chua KH, Kaewsrichan J (2020) Decrease of microglia and fatty liver in obese mice by germinated Sang-Yod rice. Glob J Obes Diabetes Metab Syndr 7(2): 036-041. DOI: 10.17352/2455-8583.000046Our previous study indicated that learning and cognition of obese mice were enhanced by germinated Sang-Yod rice intervention. We recently discover that inferior effects of high fat diet on lipid metabolisms and functions of two vital organs, including the liver and the brain are attenuated by germinated Sang-Yod rice. Thirty-two male C57BL/6J mice are divided into 4 groups (n = 8), and distinctly assigned to receive a different diet for 12 weeks, including normal diet (CONTROL group), high fat diet (HFD group), high fat diet plus 20 mg simvastatin/kg/day (POSITIVE group), and high fat diet plus 0.5% germinated Sang-Yod rice/kg/day (HFD-GR group). Biochemical and histological assessments are performed using blood and tissue samples, respectively. The levels of blood glucose, triglycerides, and high-density lipoproteins for all groups of mice are not different. Increases of low-density lipoprotein and total cholesterol concentrations and the albumin/globulin ratio are found for mice fed with high fat diet. The activities of alanine transaminase and aspartate transaminase enzymes are not different among these groups of mice. Fat degeneration, cytoplasmic vacuoles, and lipid droplets in the livers of mice treated with simvastatin and germinated Sang-Yod rice are reduced. Lastly, cells of the prefrontal cortex of these treated mice are morphologically normal with an absence of microglia. In contrast, microglia is simply observed in this brain area of mice in HFD group. Limitation of high fat in foods should be attended to maintain healthy life.
Abbreviations
HFD: High Fat Diet; GABA: γ-Aminobutyric Acid; LDL: Low-Density Lipoprotein; NOS: Nitric Oxide Synthase; HDL: High-Density Lipoprotein; TC: Total Cholesterol; A: Albumin; G: Globulin; TP: Total Protein; ALT: Alanine Transaminase; AST: Aspartate Transaminase ; ALP: Alkaline Phosphatase; H&E: Hematoxylin and Eosin
Introduction
Increased production of γ-aminobutyric acid (GABA) during germination of brown rice has been notified [1]. GABA content ranging between 10 and 14 mg/100 g germinated Sang-Yod rice was quantified (unpublished data). The potential of this germinated rice on cognitive improvement of obesity-induced mice has been reported recently [2]. So far, GABA is known to play major roles in the central nervous system, such as for modulation of synaptic transmission and for promotion of neuronal development and relaxation [1]. To date, GABA is found to exert non-neuronal effects in a variety of peripheral organs and has been used as anti-hypertensive, anti-diabetic, anti-cancer, and hepato-reno-protective agents according to the existing anti-inflammatory and anti-oxidant activities [3]. Concerning the brain, it is made up of different cell types. But the most common brain cells are neurons and non-neuron cells called glia. Previous studies have indicated that inflammation of glial cells is linked with many degenerative diseases such as Parkinson’s, Alzheimer’s, and multiple sclerosis [4]. Emerging information has illustrated that overnutrition and obesity can be inducers of malfunctioning glia, resulting in impaired learning and memory [5]. Simvastatin is typically prescribed to hyperlipidemic patients for lowering cholesterol production and increasing low-density lipoprotein (LDL) removal owning to inhibition of HMG-CoA reductase enzyme [6]. Nowadays, it is dispensed as a neuroprotective drug based on potentials in modulation of nitric oxide synthase (NOS), improvement of immune response, and decrease of oxidative stress and inflammation [7]. At present, foods rich in GABA have been recommended as daily intakes for controlling high profiles of LDL and triglycerides in blood circulation [8]. In this project, we continue to elucidate other benefits of germinated Sang-Yod rice focusing to the brain and the liver of obese mice about which levels of blood glucose, proteins and lipids, as well as histopathology of the liver and the brain were explored.
Materials and methods
Preparation of germinated sang-yod rice
Sang-Yod is a red rice variety from southern Thailand. The rice was dehulled within 4 months of harvest. The obtained brown rice with perfect germ was washed 3-4 times with tap water and soaked for 12 h in the dark at room temperature. Next, the rice was taken out, wrapped with cheesecloth, and maintained in moist condition with good ventilation for 18 h. The germinated rice obtained was dried at 45 °C for 8 h in a hot air oven and ground into fine powder by using multi-function disintegrator (WF-20B). The rice powder was kept at 4°C until use.
Animals
Thirty-two male C57BL/6J mice with age of 6 weeks and weight around 22±3 grams were purchased from Monash University, Kuala Lumpur, Malaysia. They were housed 4 per cage in a room at 22±2OC and a 12-hours light-dark cycle with free accessibility to food and water for 2 weeks. After that mice were divided into 4 groups depending on a designed diet. Four types of diets were prepared for feeding mice, such as normal diet (CONTROL group), high fat diet (HFD group), high fat diet plus 20 mg simvastatin/kg/day (POSITIVE group), and high fat diet plus 0.5% germinated Sang-Yod rice/kg/day (HFD-GR group). The former two diets were acquired from Altromin Spezialfutter GmbH & Co., KG, Germany, and compositions therein were shown in Table 1. The latter two diets were obtained by mixing high fat diet with either simvastatin or germinated Sang-Yod rice and separately pelleting in a mold to mimic the previous form of high fat diet. A group of mice was provided with a corresponding diet for 12 weeks. The mice body weight was recorded weekly. Animal study was carried out regarding to ethical guidelines for laboratory animal at Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Mice were killed by using an overdose of pentobarbital (80mg/kg) with a volume of 0.1 ml per 10 g of body weight in 1 ml syringe 23-gauge, 5/8-inch needle and cardiac puncturing. The liver and the brain were carefully taken out and kept in suitable containers at -80OC until use. Blood sample was collected in a tube without anticoagulants. Serum was obtained by centrifugation of the blood at 2,000 rcf for 15 min.
Measurement of blood glucose, serum proteins and lipids, and liver enzymes
Accu-Chek kit was used for measuring blood glucose in regard to protocols as recommended by Roche Diagnostic Co., Ltd. Serum lipids, such as triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and total cholesterol (TC); serum proteins, such as albumin (A), globulin (G) and total protein (TP); and liver enzymes, such as alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP), were assayed by Pathlab, Petaling Jaya, Selangor, Kualalumper, Malaysia.
Histological analysis
Samples of the liver were cut into a 5-mm thick slice, fixed in 10% neutral buffered formalin overnight, and paraffin-embedded. An embedded tissue was sectioned at a 3-µm thickness by using Leica RM2235 manual rotary microtome. A section was expanded on a slide at -20OC for 1 h and stained with hematoxylin and eosin (H&E) (Sigma-Aldrich, St. Louis, MO, USA) by using standard protocols. For Oil red O staining, a section with a thickness range of 5-10 μm was prepared by using cryo-section method. Then, it was air-dried for 30 min at room temperature, fixed in 10% iced-cold formalin for 2 h, and air-dried again. After washing with dH2O and left for completely drying, the section was stained with Oil red O by using standard procedures. Brain samples were cut into two halves. One-half samples were fixed in 10% neutral buffered formalin for 4 h, and other halves were stored in suitable containers at -80OC until use. The fixed ones were dehydrated with serial diluted ethanol, embedded in paraffin blocks, and coronally cut at a thickness range of 5-10 μm by using standard cryo-section method. These specimens were then stained by H&E. Tissue samples on slices were visualized by 3 independent scientists using a bright field fluorescence microscope (Nikon, DS-Fi2-U3 model, Faculty of Dentistry, PSU).
Statistical analysis
Data were expressed as mean ± SEM (n=8). Individual group difference between the means was evaluated by one-way analysis of variance (ANOVA), followed by Tukey’s multiple range tests using SPSS 19.0 statistical software packages. Differences were statistically significant at p < 0.05.
Results
Body weight
Changes of the weekly body weight in response to a different diet were recorded and summarized in Table 2. Increase of the body weight of mice in CONTROL, HFD, and HFD-GR groups was not significantly different. But that belonging to POSITIVE and HFD mice was statistically enhanced since the 2nd week of the trial. At the end, mice of all groups showed significant weight gain. There was difference in time period required for adapting to a distinct diet during which the body weight was not changed, i.e., 3 weeks for CONTROL mice, 4 weeks for HFD mice, 7 weeks for HFD-GR mice, and 8 weeks for POSITIVE mice.
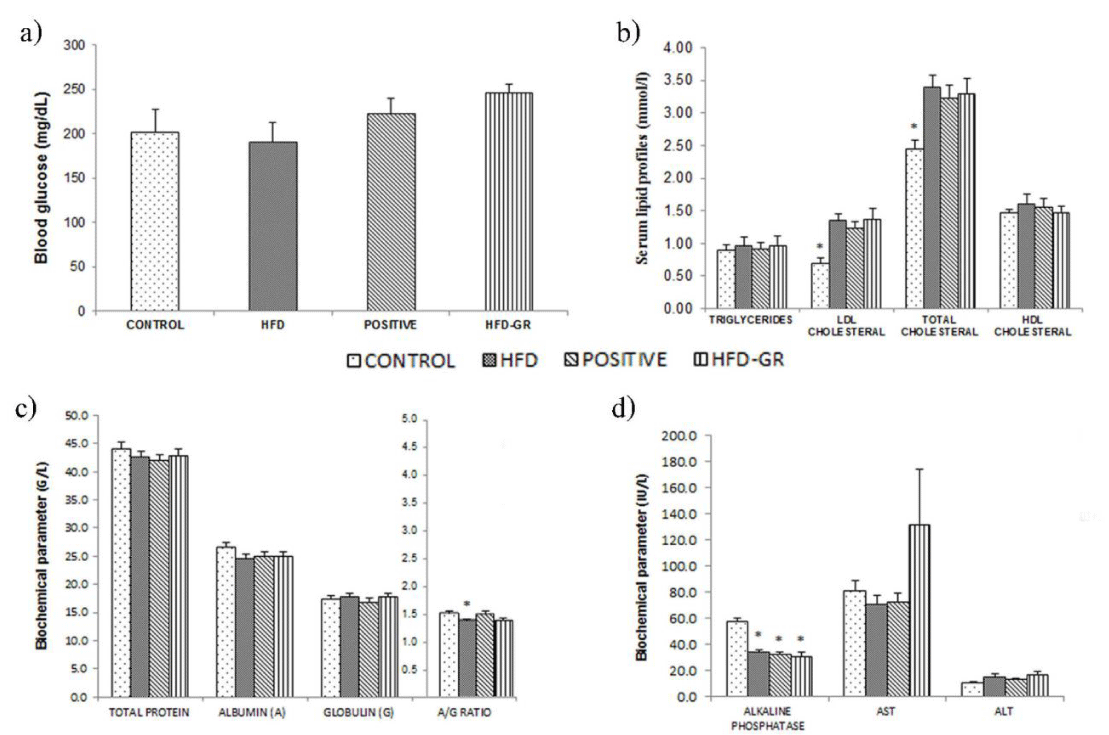
Levels of blood glucose
Glucose concentrations in blood samples were analyzed by using Accu-Chek kit and results were shown in Figure 1a. The glucose levels of mice in CONTROL, HFD, POSITIVE and HFD-GR groups were not significantly different. In respect to the diets that differed in either fat contents or supplements, pathological conditions concerning insulin resistance and glucose intolerance were not implicated to occur.
Serum lipid profiles
Serum lipids including TG, LDL, TC, and HDL were measured after 12 weeks of the feeding programs. Results of Figure 1b indicated that mice of all groups presented comparative TG and HDL levels. Instead, all of the mice being fed with high fat diet, e.g., mice of HFD, POSITIVE or HFD-GR groups, showed higher LDL and TC profiles in compared to that of CONTROL mice. Therefore, dyslipidemia was apparent as a result of feeding mice with high fat diet for 12 weeks.
Measurements of serum proteins and liver enzymes
The profiles of serum proteins, such as total protein (TP), albumin (A) and globulin (G) were determined after 12 weeks of animal feeding. Data were shown in Figure 1c, indicating insignificant difference of these protein fractions among 4 groups of mice respective to a distinct diet. But the A/G ratio of HFD mice was statistically decreased in comparison with other remaining groups of mice (p < 0.05). Furthermore, activities of liver enzymes in serum specimens, including ALT, AST and ALP were measured. Results in Figure 1d showed that there were insignificant differences in the ALT and AST activities for all groups of mice. In contrast, the ALP activity of CONTROL mice was significantly greater than that of HFD, POSITIVE, and HFD-GR mice. Based on the data concerning serum protein levels and liver enzyme activities, hepatic injury and hepatic sinusoidal obstruction could not be certainly assumed by high fat diet intake.
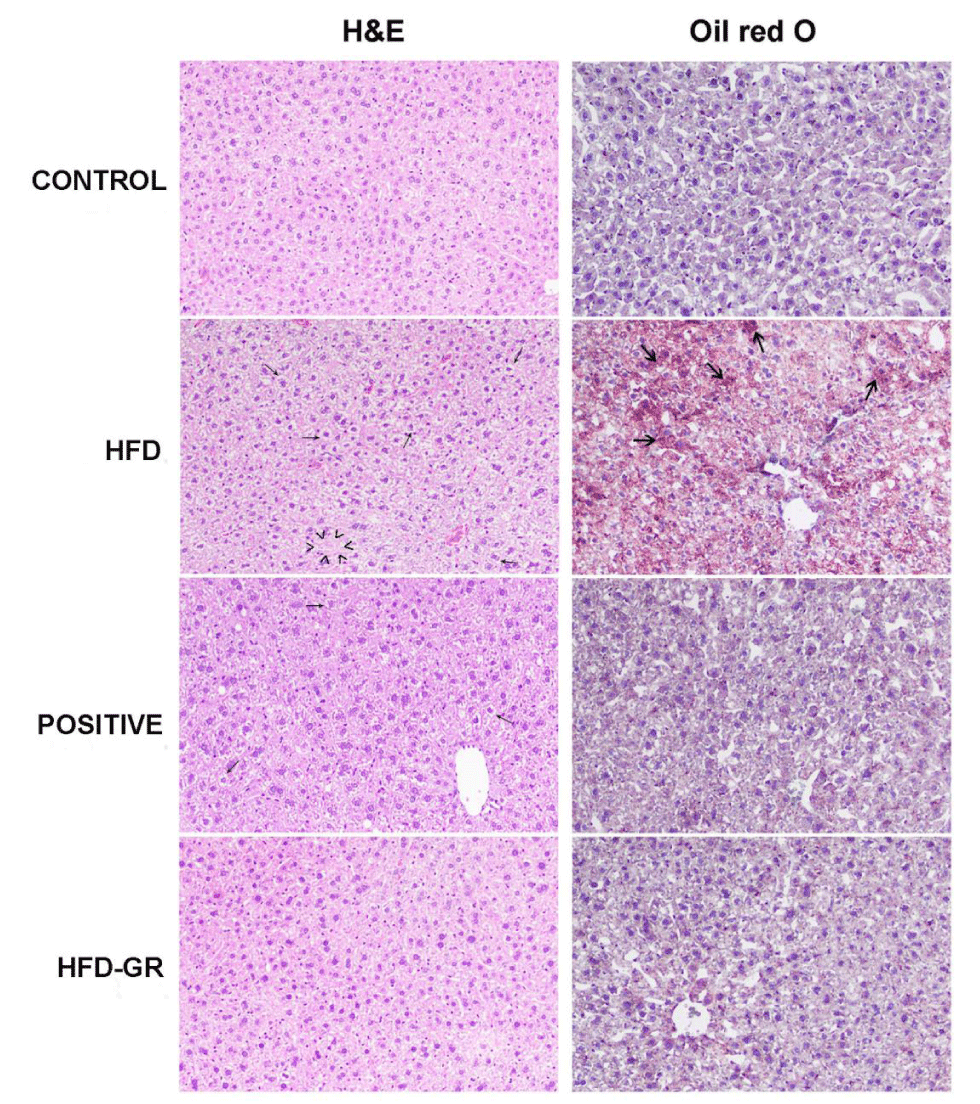
Histological study of the liver
Liver samples from 4 groups of mice fed with different diets for 12 weeks were histologically determined. Representative results of liver tissues stained by H&E and Oil Red O were shown in Figure 2. Signs of liver abnormalities for CONTROL mice were not apparent. In contrast, fat degeneration and cytoplasmic vacuoles were clearly notified by liver tissues of HFD mice. These mice were obese and left untreated. Decrease of fat degeneration was evident for mice treated with simvastatin (POSITIVE mice). Interestingly, a huge reduction of hepatic steatosis was demonstrated for mice having germinated Sang-Yod rice as an intervention (HFD-GR mice). Concerning Oil Red O stain, there was little difference in pathological signs for the livers of POSITIVE and CONTROL mice. But deposition of lipid droplets in the livers of HFD mice was considerable in compared to that of CONTROL mice. The extent of lipid accumulation was remarkedly decreased when treating HFD mice with simvastatin (POSITIVE mice). Such decrease was much greater when HFD mice were supplemented with germinated Sang-Yod rice (HFD-GR mice). In addition, cell components corresponding to tissue inflammation were not detected for all specimens. Taken together, diets containing high percentages of fats could be somehow as inducers of fatty liver.
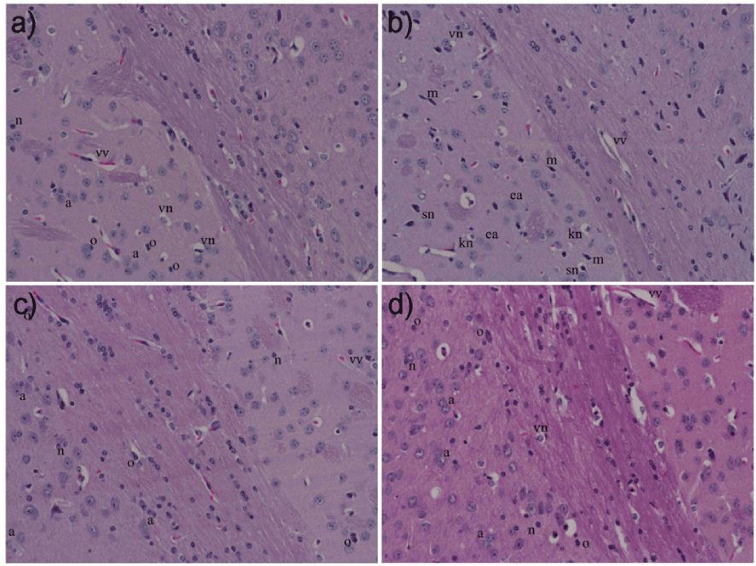
Histopathology of the brain
Representative photomicrographs of the prefrontal cortex stained by H&E were shown in Figure 3. Morphologies of neurons and non-neuronal cells, such as astrocytes and oligodendrocytes for mice in CONTROL, POSITIVE and HFD-GR groups were apparent to be normal with an absence of microglial cells. But there were pathological changes of brain cells in the prefrontal cortex of HFD mice, indicating as shrunken nucleus, karyorrhectic or eosinophilic neurons. In addition, numerous microglia were noticeably detected. In accordance, this brain part was susceptible to toxic factors produced by fat metabolism or induced to be produced by obesity.
Discussion
Obesity is a global health concern, characterized by excess fat accumulation in adipose and non-adipose tissues. It is also considered as a chronic low-grade inflammatory disorder because there is an extreme production of pro-inflammatory molecules in obese people [9]. The liver is most susceptible to these cytokines by causing liver damage [10]. Moreover, insulin resistance and type 2 diabetes are prone to develop in people who are obese. Blood glucose levels and glucose utilization are thus deviated by these conditions [11]. Loss of cognition or impaired learning and memory in relation to obesity has been reported by several clinical and preclinical researches [2,12]. Therefore, we continued to clarify if using germinated Sang-Yod rice as a supplement would be sufficient for improving liver- and brain-health of obese mice induced by high fat diet.
According to Figure 1a, blood glucose levels as compared among 4 groups of mice, i.e., CONTROL, HFD, POSITIVE, and HFD-GR groups, were not significantly different. Instead, gaining of the body weight for mice in HFD, POSITIVE and HFD-GR groups was reported (Table 2). It seemed that diets containing high percentage of fats being used were not competent for inducing insulin resistance and type 2 diabetes to develop in this mouse strain. In agreement with previous reports, mice of B6 strain are more susceptible than those of C57BL/6J strain when to mimic metabolic defects in human. The former mice become obese easily with several inferior conditions such as hyperinsulinemia, hyperglycemia, and hypertension after feeding with high fat diets [13]. However, there is some controversy to this regard recently [14]. In Figure 1b, hyperlipidemia was possibly developed after feeding mice with the high fat diet for 12 weeks. With this pathophysiological condition, increased levels of TG, LDL, and TC in serums were demonstrated. Treatments by using simvastatin or germinated Sang-Yod rice were not effective for decreasing these lipids to normal levels. The liver is responsible for the synthesis of albumins (A) and globulins (G). In normal individuals, serum albumin concentrations are determined to be higher than that of globulins and usually normal even in cases with liver diseases. In consequence, the A/G ratio is investigated when to interpret liver function test, giving a normal A/G ratio of slightly over 1 [15]. In Figure 1c, the A/G ratio of mice fed with the high fat diet (HFD mice) was comparatively decreased (p < 0.05), suggesting imperfect liver function. Another liver marker to be considered was total protein (TP), which is the sum concentration of albumins and globulins in serum. Difference in the TP levels was not found for all groups of mice tested (Figure 1c), implicating no existence of liver damage in these mice. As results of liver function tests based on TP levels and the A/G ratio were inconsistent, it would be necessary to consider enzymes majorly produced by the liver, including AST, ALT, and ALP. Again, hepatic integrity can be predicted from ALT and AST activities in blood circulation [16]. Increased enzyme activities are used for estimation of hepatic injury, although AST is less sensitive and specific than ALT. Moreover, production of ALP is increased when there is intrahepatic or extrahepatic obstruction [17]. From Figure 1d, there was slight elevation of ALT activity in mice fed with high fat diet, i.e., less than 1¼ of ALT level of CONTROL mice. All of the mice fed with high fat diet, including HFD, POSITICE, and HFD-GR mice, showed significantly decreasing ALP activity in compared to CONTROL mice. Therefore, obstruction and lesion-occupying liver were unlikely to happen by high fat diet feeding [18]. It was interesting to note that less accumulation of lipid droplets in the livers of mice supplemented with germinated Sang-Yod rice was found (Figure 2, right column), suggesting hepatoprotective consequence of this rice [19]. Accordingly, GABA and other compounds present in germinated Sang-Yod rice might exhibit antioxidant and anti-inflammatory activities and take parts for the recovery of damaged hepatocytes to healthier cells, in agreement with previous studies [20]. Identification of mechanisms underlining these benefits is being carried out.
Previous literatures have demonstrated that hyperlipidemia can induce neurovascular damage and blood brain barrier dysfunction owning to inflammatory cytokines as increasingly secreted by adipocytes [21, 22]. Nevertheless, increase of blood lipid profiles is not proposed for their increment in the brain because the blood brain barrier will resist brain cells to take up chemical species from blood circulation [23]. In this sense, it would be interesting to determine whether hyperlipidemia and fatty liver being induced to happen in the recent mice could make some changes in microstructures of cells in the prefrontal cortex. Indeed, cognition loss of these mice has been previously reported by ours [2]. The area of prefrontal cortex was attractive to consider because the residing cells will provide primary inputs to the hippocampus that responsible for learning ability and memory. A great number of neurons and non-neurons with normal morphologies was observed in HFD-GR mice (Figure 3). In consistence, these mice performed better on learning and cognitive memory in comparison with HFD mice as acquired by Morris Water Maze test [2]. Instead, fewer intact brain cells were seen in HFD mice with the prompt existence of microglia, suggesting to be in response to brain injury and/or inflammation. Certainly, peripheral insults of hyperlipidemic disorder and fatty liver affected microstructures of the prefrontal cortex and such harmful effects could be diminished by germinated Sang-Yod rice supplement. In agreement with these findings, brain cells in the prefrontal cortex of obese animals have been highly predisposed to inflammatory insults [24], and increased expression of microglia in brain regions associating cognition and memory has been reported in mice fed with high fat diet for 5 months [25]. Altogether, long-time feeding of high fat diets would be inferior on cognition by influencing cells of forebrain regions in response to inflammations occurring peripherally and bring about central inflammation beyond the hippocampus to affect other areas relating to cognition. Future work is necessary to ascertain possible mechanisms in reducing microglial migration under fatness by germinated Sang-Yod rice.
Conclusion
Long-term consumption of high-caloric foods shows negative outcomes to people by increasing an incidence of obesity, metabolic syndrome, and degenerative disorders. These conditions can be accelerated or decelerated depending on different intakes to be chosen. Accordingly, interventions focused on suitable nutrition and proficient life-style factors would be promising to improve caloric metabolisms and holistic health.
The authors wish to thank Department of Pharmaceutical Chemistry and Drug Delivery System Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University (PSU), Thailand; and Department of Physiology, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia, for their assistance in experimentation, data collection, and valuable advice.
The financial supports of this project were obtained from Thailand’s Education Hub for ASEAN Countries for Ph.D. Studies; The Overseas Thesis Research Scholarship of Graduate School at Prince of Songkla University (PHA6102034N); FF-2018-401; and FF-2018-401/1.
- Byun JI, Shin YY, Chung SE, Shin WC, et al. (2018) Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: A randomized, double-blind trial. Clin Neurol 14: 291-295. Link: https://bit.ly/31vsedq
- Ngo DH, Vo TS (2019) An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 24: 2678. Link: https://bit.ly/3ghPPSO
- Nordengen K, Kirsebom BE, Henjum K, Selnes P, Gísladóttir B, et al. (2019) Glial activation and inflammation along the Alzheimer’s disease continuum. Journal of neuroinflammation 16: 46. Link: https://bit.ly/2BS0k0o
- Koeppen J, Nguyen AQ, Nikolakopoulou AM, Garcia M, Hanna S, et al. (2018) Functional consequences of synapse remodeling following astrocyte-specific regulation of Ephrin-B1 in the adult hippocampus. J Neurosci 38: 5710-5726. Link: https://bit.ly/3ifqs66
- Isley WL, Miles JM, Patterson BW, Harris WS (2006) The effect of high-dose simvastatin on triglyceride-rich lipoprotein metabolism in patients with type 2 diabetes mellitus. J Lipid Res 47: 193-200. Link: https://bit.ly/2YM8PTG
- McFarland A, Davey A, Anoopkumar-Dukie S (2017) Statins reduce lipopolysaccharide-induced cytokine and inflammatory mediator release in an in vitro model of microglial-like cells. Mediators Inflamm 2017: 2582745. Link: https://bit.ly/2YLk9PX
- Yeap SK, Beh BK, Ali NM, Yusof HM, Ho WY, et al. (2014) In vivo antistress and antioxidant effects of fermented and germinated mung bean. Biomed Res Int 2014: 694842. Link: https://bit.ly/38giGnX
- Farhangi MA, Mesgari-Abbasi M, Hajiluian G, Nameni G, Shahabi P (2017) Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation 40: 1688-1697. Link: https://bit.ly/2YQCdbD
- Yaghchiyan M, Roshangar L, Farhangi MA, Mesgari-Abbasi M, Rafiei L, et al. (2019) Histologic, Metabolic, and Inflammatory Changes in the Liver of High-fat Diet-induced Obese Rats before and after Vitamin D Administration. Iran J Allergy Asthma Immunol 18: 402-411. Link: https://bit.ly/3dOVIFx
- Akter R, Nessa A, Husain MF, Wahed F, Khatun N, et al. (2017) Effect of Obesity on Fasting Blood Sugar. Mymensingh Med J 26: 7-11. Link: https://bit.ly/3ingcce
- Collins S, Martin TL, Surwit RS, Robidoux J (2004) Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81: 243-248. Link: https://bit.ly/3ghSyM2
- Avtanski D, Pavlov VA, Tracey KJ, Poretsky L (2019) Characterization of inflammation and insulin resistance in high‐fat diet‐induced male C57BL/6J mouse model of obesity. Animal Model Exp Med 2: 252-258. Link: https://bit.ly/2BqAUHm
- Deng Y, Pang Q, Miao RC, Chen W, Zhou YY, et al. (2016) Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther 9: 5317-5328. Link: https://bit.ly/3eOfGSn
- Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M (2016) Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 33: 62-70. Link: https://bit.ly/2NHv5rH
- Walker HK, Hall WD, Hurst JW (1990) Headache--Clinical Methods: The History, Physical, and Laboratory Examinations. Link: https://bit.ly/2YOKSLw
- Newsome PN, Cramb R, Davison SM, Dillon JF, FoulertonM, et al. (2018) Guidelines on the management of abnormal liver blood tests. Gut 67: 6-19. Link: https://bit.ly/2NLrDMM
- Mostafa M, Abdelkader A, Evans JJ, Hagen CE, Hartley CP, et al. (2020) Fatty Liver Disease: A Practical Approach. Archives of pathology & laboratory medicine 144: 62-70. Link: https://bit.ly/2YOs6nG
- Debnath T, Park SR, Kim DH, Jo JE, Lim OB (2013) Anti-oxidant and anti-inflammatory activities of Inonotus obliquus and germinated brown rice extracts. Molecules 18: 9293-9304. Link: https://bit.ly/2YNbNHr
- Van Dyken P, Lacoste B (2018) Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Frontiers in neuroscience 12: 930. Link: https://bit.ly/3eTOFNe
- De Assunção SNF, Boa Sorte NCA, Alves CDA, Mendes PSA, Alves CRB, et al. (2018) Inflammatory cytokines and non-alcoholic fatty liver disease (NAFLD) in obese children and adolescents. Nutr Hosp 35: 78-83. Link: https://bit.ly/3dQ6z2a
- Czuba E, Steliga A, Lietzau G, Kowiański P (2017) Cholesterol as a modifying agent of the neurovascular unit structure and function under physiological and pathological conditions. Metab Brain Dis 32: 935-948. Link: https://bit.ly/38fxvqO
- Lauridsen JK, Olesen RH, Vendelbo J, Hyde TM, Kleinman JE, et al. (2017) High BMI levels associate with reduced mRNA expression of IL10 and increased mRNA expression of iNOS (NOS2) in human frontal cortex. Transl Psychiatry 7: e1044-e1044. Link: https://bit.ly/2ZwBM5s
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, et al. (2014) Obesity in aging exacerbates blood–brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 69: 1212-1226. Link: https://bit.ly/2CYR4Ir
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
