Global Journal of Obesity, Diabetes and Metabolic Syndrome
Sinapic Acid Regulates Glucose Homeostasis by Modulating the Activities of Carbohydrate Metabolizing Enzymes in High Fat Diet Fed-Low Dose STZ Induced Experimental Type 2 Diabetes in Rats
Ramesh Nithya, Vellai Roshana Devi, Rajendran Selvam and Sorimuthu Pillai Subramanian*
Cite this as
Ramesh N, Devi VR, Rajendran S, Subramanian SP (2017) Sinapic Acid Regulates Glucose Homeostasis by Modulating the Activities of Carbohydrate Metabolizing Enzymes in High Fat Diet Fed-Low Dose STZ Induced Experimental Type 2 Diabetes in Rats. Glob J Obes Diabetes Metab Syndr 4(2): 054-061. DOI: 10.17352/2455-8583.000024Diabetes Mellitus is a chronic metabolic disorder arises due to absolute lack of insulin secretion (T1DM) or its action or both (T2DM). Alterations in glucose metabolism in DM are frequently accompanied by impairment in the activities of enzymes that regulate carbohydrate metabolism. Liver is a vital organ that acts as primary site of endogenous glucose production through gluconeogenesis or glycogenolysis. The enzymes that control glucose metabolism in the liver tissue are considered as potential targets for the maintenance of normal glycemic control in diabetic individuals. Search for new drugs with more efficacies and without side effects preferably from plant origin continues. Sinapic acid is one such phytochemical which lacks scientific validation for its folklore use. It is a naturally occurring carboxylic acid belongs to phenylpropanoid family. It is widely distributed in the various sources such as rye, mustard, berries and vegetables In the present study it was aimed to systematically study the efficacy of sinapic acid (25mg/kg.b.w./rat for 30 days) in the regulation of glucose homeostasis modulating the activities of carbohydrate metabolizing enzymes in hepatic tissues of high fat diet fed-low dose STZ induced experimental type 2 diabetes in rats. The altered activities of carbohydrate metabolizing enzymes such as glucokinase, pyruvate kinase, glucose-6-phosphatase, fructose-1,6-bisphosphatase, glucose-6-phosphate dehydrogenase, lactate dehydrogenase in hepatic tissues of diabetic rats were significantly reverted to near normalcy upon oral treatment with sinapic acid. In addition, oral administration of sinapic acid to experimental diabetic groups of rats showed significant reduction in the levels of fasting blood glucose and glycosylated hemoglobin and increased level of plasma insulin and hemoglobin. Thus, the present data demonstrated that the oral administration of sinapic acid to diabetic rats regulates glucose homeostasis by regulating the activities of carbohydrate metabolizing enzymes.
Introduction
Diabetes Mellitus (DM) is a chronic metabolic disorder arises due to absolute lack of insulin secretion (T1DM) from the β-cells of pancreas or its action or both (T2DM) [1]. It is characterized by a persistent elevation in both fasting as well as postprandial blood glucose levels. The prevalence of DM is predicted to rise 642 million by the year 2025 and T2DM accounts for more than 90% of the total diabetic population. Several drugs such as biguanides, sulphonylureas, meglitinides, thiazolidiones, α-glucosidase inhibitors, bile acid sequestrants, SGLT2 inhibitors, GLP-1 receptor agonists and insulin with different mode of action are currently used either as monotherapy or combinatorial strategy for the treatment of DM [2]. However, these drugs elicit undesirable side effects and develop resistance after prolonged use. Thus, the maintenance of normoglycemia remains a major task in the treatment of patients with diabetes mellitus and hence the search for new drugs with more efficacies and without side effects preferably from plant origin continues.
Liver is a one of the major storage organ for glucose reserve in the human body and thus plays a pivotal role in the regulation of blood glucose homeostasis. Several studies have provided evidence that the impairment of hepatic glucose regulation plays an important role in the development of primary and secondary complications of diabetes mellitus and diminution of hepatic glucose production has certainly been considered as a successful strategy for the treatment of diabetes. It has been reported that the chronic hyperglycemia in diabetes decreases the activities of enzymes in glycolytic and pentose pathways while increases the activity of gluconeogenesis and glycogenolytic pathways [3].
Phytochemicals are ecologically derived plant secondary metabolites which protect them against environmental stress such as UV radiation, pollution, high temperature, extreme cold, drought, flood, tissue damage and microbial attacks [4]. Interestingly, these secondary metabolites are known to play a pivotal role in alleviating the primary and secondary complications of dreadful human diseases such as cancer, diabetes and atherosclerosis. More than 60% of the currently available allopathic drugs are originally identified from traditional medicinal plants. The medicinal value of plants lies in some chemical substances that elicit a definite physiological action on the human body. The WHO recommends phytotherapy in its health programs and suggested basic procedures for scientific validation of phytoingredients for their beneficial as well as pharmacological properties.
Sinapic acid (3, 5-dimethoxy 4-hydroxy cinnamic acid) is one such phytochemical which lacks scientific validation for its folklore use. It is a naturally occurring carboxylic acid belongs to phenylpropanoid family. It is widely distributed in the plant kingdom and is obtained from various sources such as mustard, rye, berries and vegetables [5]. Sinapic acid is known to possess antioxidant [6], anti-inflammatory [7], anxiolytic [8], peroxynitrite scavenging [9], neuroprotective [10] and antihyperglycemic properties [11]. Serum albumin has been reported to be responsible for the transport of sinapic acid in blood due to its ability to bind with serum albumin through hydrophobic interaction and hydrogen-bonding. Maximum plasma-sinapic acid level has been described as 40nM with a bioavailability of 3% of the total phenolics present in the non-processed cereal meal. Moreover, the small intestine was reported as the major site for absorption of orally administered sinapic acid through active Na+ gradient-driven transport [12]. Recently, we have reported the antidiabetic and antioxidant properties of sinapic acid in experimental type 2 diabetes in rats [13,14].
Among the various animal models to study the toxicity and pharmacological screening of newly developed therapeutical agents, high fat diet fed-low dose STZ induced experimental type 2 diabetes in rats is widely accepted as it closely resembles the clinical features of human type 2 diabetes in terms of insulin resistance coupled with insufficient insulin secretion. Therefore, in the present study it has been employed as an animal model to systematically study the efficacy of sinapic acid in the regulation of glucose homeostasis.
Material and Methods
Chemicals
Sinapic acid and Streptozotocin were purchased from Sigma-aldrich, St.Louis, USA. Ultra-sensitive ELISA kit for rat insulin was purchased from Crystal Chem Inc., Life Technologies, and India. All other reagents used in the present study were of analytical grade.
Experimental animals
Male albino rats of Wistar strains (160–180 g) were procured from Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Chennai. Rats were housed randomly in spacious polypropylene cages lined with husk under controlled environment (12:12 ± 1 h light/dark cycle; temperature 22°C ± 3°C; relative humidity 55% ± 10%). Before the initiation of the experiments, all animals were acclimatized to standard husbandry conditions for one week to overcome stress. Animals were fed with commercial pellet rat chow (Hindustan Lever Ltd., Bangalore, India), and allowed to have free access to water ad libitum. All the experiments were designed and conducted in strict accordance with the current ethical norms approved by Ministry of Social Justice and Empowerment, Government of India and Institutional Animal Ethical Committee guidelines.
High Fat Diet fed streptozotocin induced diabetes
The rats were divided into two dietary regimens by feeding either normal or high fat diet (HFD) for the initial period of two weeks [15]. The ingredients and chemical composition of the HFD was followed as before reported. After two weeks of dietary manipulation, the groups of rats fed with HFD were injected intraperitoneally with a low dose of STZ (35 mg/kg b.w) dissolved in 0.1M cold citrate buffer, pH 4.5). One week after STZ injection, the rats were screened for blood glucose level. Rats having fasting blood glucose (FBG) >250mg/dl that exhibited random hyperglycemia and glycosuria were selected for the experiment. The rats were allowed to continue to feed on the respective diets until the end of the experiments.
Experimental Protocol
The rats were divided into four groups each group comprising six rats.
➢ Group 1 : Control rats
➢ Group 2 : Diabetic rats (HFD-low dose STZ (35 mg/kg b.w.)
➢ Group 3 : Diabetic rats treated with sinapic acid (25 mg/kg b.w.)
➢ Group 4 : Diabetic rats treated with metformin (50 mg/kg b.w.)
Sinapic acid was dissolved in 0.2% dimethyl sulfoxide and administrated to rats orally using an intragastric tube daily for a period of 30 days.
Oral glucose tolerance test
Overnight fasted rats of all groups were subjected to oral glucose tolerance test on the last week of the experimental period. The blood glucose levels were monitored at 0, 30, 60, 90 and 120 min using One Touch glucometer (Life scan, Johnson and Johnson Company) after oral administration of 2 g/kg b.w. glucose as aqueous solution [16].
Sample collection
After 30 days of treatment, the animals were fasted overnight and sacrificed by cervical decapitation. Blood was collected with and without anticoagulants for the separation of plasma and serum. The liver and kidney were carefully removed, weighed and washed in ice-cold saline. The liver, kidney and muscle tissues were sliced into pieces and homogenized in an appropriate buffer (pH 7.0). The homogenates were centrifuged at 3000 rpm for 10 min at 0oC in cold centrifuge. The supernatant was separated and used for various biochemical estimations.
Studies on the biochemical parameters
Fasting blood glucose, percent hemoglobin, glycosylated hemoglobin by colorimetric assay, plasma protein, blood urea, uric acid and serum creatinine levels were estimated [17-23]. Plasma insulin level was assayed using an ELISA kit (Linco Research, St Charles, MO, USA) for rat insulin assay. The presence of urine sugar was detected using urine strips (Bayers, Diastix). The activities of pathological marker enzymes such as Aspartate transaminase (AST), Alanine transaminase (ALT) and Alkaline phosphatase (ALP) in serum were assayed [24,25].
Assay on insulin resistance
The insulin resistance developed in the experimental animals was evaluated by a homeostasis model of insulin resistance (HOMA-IR). The HOMA-IR was calculated by the method of Mathews et al. [26], as follows.
Carbohydrate metabolizing enzymes
The liver homogenate was centrifuged at 10,000 rpm to remove the debris and the supernatant was used as enzyme source for the assays of hexokinase [27], pyruvate kinase [28], glucose-6-phosphate dehydrogenase [29], glucose-6-phosphatase [30], fructose-1, 6- bisphosphatase [31], glycogen synthase [32], glycogen phosphorylase [33] and lactate dehydrogenase [34]. Another portion of wet liver tissue was used for the estimation of glycogen [35], content in control and experimental groups of rats.
Statistical analysis
The results were expressed as mean ± SD of six rats per group and statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS (version 16) program followed by LSD. Values were considered statistically significant when p < 0.05.
Results and Discussion
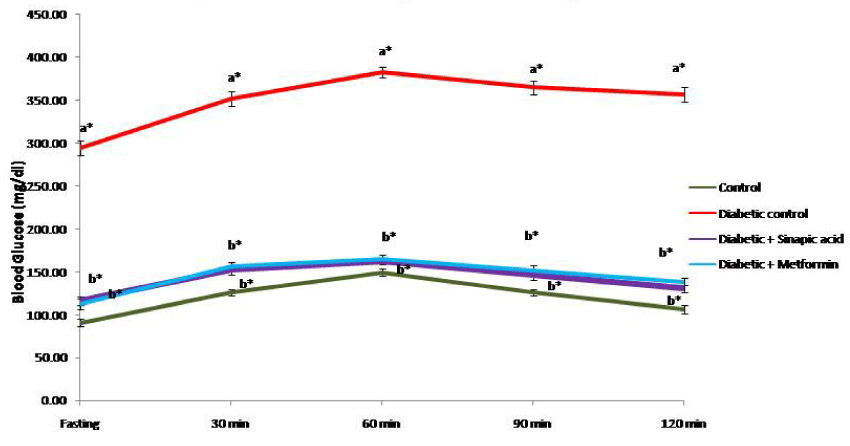
Based on the data obtained through toxicity and dosage fixation studies, the optimum dose for sinapic acid was fixed as 25mg/kg.b.w./rat/day for 30 days. The effect of oral administration of sinapic acid on oral glucose tolerance test (OGTT) in control and experimental groups of rats is presented as Figure 1. In control group of rats, the blood glucose level attained the maximum peak at 60 min after an oral glucose load and it was progressively reverted back to physiological level at 120 min indicating the maintenance of normal glucose homeostasis. On the other side, the blood glucose levels in type 2 experimental diabetic rats reached the maximum peak at 60 min and remained unsubsidized over the next 60 min. Oral treatment with sinapic acid as well as metformin resulted in a significant decrease in both fasting 30 as well as 60 min compared with untreated diabetic rats suggesting the efficacy of sinapic acid in the maintenance of blood glucose homeostasis during oral glucose load which in turn may be due to its insulin stimulatory and/or insulin mimetic properties.
Epidemiological studies and clinical trials strongly support that chronic hyperglycemia is the principal cause of diabetic complications and an effective glucose control is the key factor in preventing the metabolic disorders. The OGTT signifies the body’s ability to use glucose, the main source of energy. It is a test of immense value instead of using fasting blood glucose concentration alone to facilitate the diagnosis of diabetes. This test can also be used to diagnose pre-diabetes and diabetes. The glucose lowering effect of sinapic acid was comparable with metformin treated group of rats indicating the significant antidiabetic properties of sinapic acid.
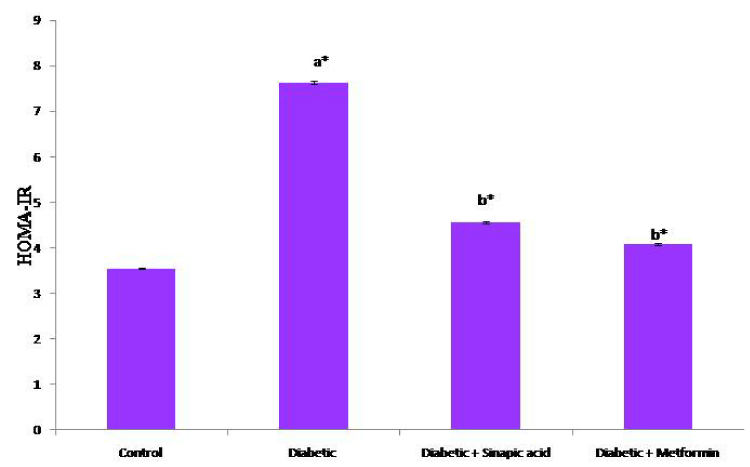
Table 1 depicts the effect of oral administration of sinapic acid on the levels of fasting blood glucose, plasma insulin and glycosylated hemoglobin in HFD fed- low dose STZ induced experimental type 2 diabetic rats. The levels of fasting blood glucose and glycosylated hemoglobin were significantly increased with a concomitant decrease in the levels of plasma insulin and hemoglobin. Treatment with sinapic acid significantly decreased the altered levels of fasting blood glucose and glycosylated hemoglobin in diabetic groups of rats. The plasma insulin and hemoglobin levels were improved to target range upon oral administration with sinapic acid for a period of 30 days. Accordingly, the altered value of HOMA-IR (Figure 2) was reverted back to reference range in diabetic rats treated with sinapic acid.
The FBG level is an established predictor of diabetes mellitus. It is a widely accepted biochemical marker used in the diagnosis and prognosis during the management of diabetes mellitus in both clinical and experimental settings. Insulin regulates the activities of various enzymes involved in carbohydrate metabolism in its target organs including liver to maintain glucose homeostasis. Incessant exposure to elevated glucose levels is known to have deleterious effects on insulin secretion and is correlated with the onset of peripheral insulin resistance. Increased level of plasma insulin was observed in experimental type 2 diabetic rats indicated that sinapic acid stimulates insulin secretion from the remnant β-cells or regenerated β-cells. The increased level of HbA1c is directly proportional to the decreased level of total hemoglobin in diabetic rats. HbA1c is used as a most reliable marker and standard diagnosis practices for estimating the degree of protein glycation during diabetes mellitus [36]. Protein glycation is a non-enzymatic reaction between excess glucose present in the blood and free amino groups on hemoglobin. Measurement of HbA1c level provides information of long-term glycemic status and to correlate with various complications related to DM. Experimental diabetic rats orally treated with sinapic acid significantly decreased the HbA1c level probably due to the glucoregulatory mechanisms which reveal the decreased protein glycation condensation reactions.
HOMA-IR is the most convenient method for assessment of insulin resistance [37]. Significant elevation in HOMA-IR values in diabetic group of rats indicating the development of insulin resistance. Upon oral administration of sinapic acid, HOMA-IR values were significantly decreased to desirable range which shows the efficacy of sinapic acid in the maintenance of glucose homeostasis. In epidemiological studies, the mathematical modeling of insulin resistance is often preferred due to its simplicity and cost effective although the gold standard is the clamp model.
The levels of plasma protein, blood urea, serum uric acid and serum creatinine in control and experimental groups of rats are presented in table 2. The level of total protein was found to be decreased in experimental type 2 diabetic rats. The levels of blood urea, serum uric acid and serum creatinine were elevated in HFD fed-STZ induced diabetic rats. These biochemical markers were reverted back to reference range upon the oral administration of sinapic acid for the period of 30 days.
Deficiency of insulin stimulates gluconeogenesis as an alternative route of glucose supply. This route is sustained by increased proteolysis which releases free glucogenic amino acids into the plasma that are deaminated in the liver with the consequence of increased urea in the blood. The observed decrease in the levels of protein with a simultaneous increase in blood urea in diabetic rats may be accredited to increased muscle proteolysis, reduced protein synthesis and hepatic gluconeogenic enzymes stimulating gluconeogenesis [38]. Creatinine is a metabolite of muscle creatine. The amount of creatinine is usually constant and hence the elevated levels indicate the impairment of renal function. Uric acid is the major metabolic product of purine metabolism and its elevated level in the serum signifies kidney impairment. After administration of sinapic acid to the diabetic rats the blood urea, uric acid and creatinine were decreased and the concomitant increase in the total protein. This indicates the beneficial effect of sinapic acid ameliorating diabetic associated renal complications.
Table 3 depicts the effect of sinapic acid on AST, ALT and ALP in the serum of control and experimental groups of rats. Significant elevation of these pathological marker enzymes were observed in diabetic rats. Oral administration with sinapic acid restores the elevated levels of these enzymes to physiological range. The aspartate transaminase and alanine transaminase are well-known cytosolic enzymes used as biomarkers to predict possible toxicity to the hepatic tissues [39]. Elevation of these transaminases was observed in experimental diabetic rats suggested damage to the liver cells as well [40]. Alkaline phosphatase is a liver marker enzyme often employed to assess the integrity of plasma membrane and endoplasmic reticulum [41]. The activities of ALT, AST and ALP were significantly elevated in HFD fed- low dose STZ induced diabetic group of rats. The increased activity of these cytoplasmic marker enzymes may be due to the cellular damage in the liver which is caused by STZ, used for the induction of experimental diabetes. Upon treatment with the sinapic acid to the diabetic rats the activity of these enzymes to desirable range indicating the non-toxic as well as tissue protective nature of the compound.
Liver plays a unique role in controlling carbohydrate metabolism by regulating glucose levels and it is achieved by a tightly regulated array of enzymes. This process is controlled by glucoregulatory mediators among which insulin plays a key role. In type 2 diabetes, alterations in hepatic glucose metabolism like an increased post-absorptive glucose production together with diminished glucose uptake following carbohydrate ingestion occur, implying insulin resistance as a central pathological principle [42]. Maintenance of glucose homeostasis as well as insulin resistance is a prerequisite to develop new therapeutic approaches in diabetes.
The activities of hexokinase, pyruvate kinase and lactate dehydrogenase in liver tissues of control and experimental groups of rats were shown in table 4. The activities of hexokinase and pyruvate kinase were significantly decreased in hepatic tissues of diabetic rats. Sinapic acid modulates the enzymes activity in hepatic tissues of high fat diet fed- STZ induced diabetic rats. The activity of lactate dehydrogenase in diabetic rats was significantly elevated when compared to control rats. Altered activity of LDH in diabetic rats is reverted to reference range upon oral administration with sinapic acid complex as well as metformin.
Table 5 depicts the activities of glucose-6- phosphatase, fructose-1, 6-bisphosphatase and glucose-6-phosphate dehydrogenase in liver tissues of control and experimental group of rats. HFD-low dose STZ induced type 2 diabetic rats showed significantly increased activities of glucose-6-phosphatase and fructose-1, 6-bisphosphatase. The activity of Glucose-6-phosphate dehydrogenase was significantly decreased in diabetic rats. These altered activities were bringing back to target range by oral administration of sinapic acid. Metformin treated diabetic rats also exhibit similar results.
Table 6 shows the level of liver glycogen and the activities of glycogen synthase and glycogen phosphorylase in control and experimental groups of rats. Diabetic rats showed considerable decrease in the liver glycogen content and glycogen synthase activity and a concomitant increase in glycogen phosphorylase activity. Upon oral administration of sinapic acid as well as metformin to diabetic rats restored glycogen level in liver and the activities of glycogen synthase, glycogen phosphorylase was also brought back to physiological levels when compared to control rats.
Hexokinase is key enzyme for glycolysis which catalysis the glucose to glucose-6-phosphate [43]. Hexokinase is an important regulatory enzyme in the oxidation of glucose [44]. Being an insulin-dependent enzyme, the hepatic hexokinase activity of diabetic rats is almost entirely inhibited or inactivated due to the absence of insulin [45]. This impairment results in a marked reduction in the rate of glucose oxidation via glycolysis, which ultimately leads to hyperglycemia. In diabetic condition, the activity of this enzyme is inhibited or inactivated due to insulin resistance because it is an insulin-dependent enzyme. Reduced insulin level in HFD-low dose STZ induced type 2 diabetic rats leads to reduced activity of hexokinase which ultimately results in the onset of chronic hyperglycemia. Upon oral treatment with Sinapic acid, the diabetic rats showed significantly increased activity of hexokinase.
Pyruvate kinase (PK) is a ubiquitously expressed, rate controlling, terminal key glycolytic enzyme that catalyzes the irreversible phosphoryl group transfer from phosphoenol pyruvate to ADP, yielding pyruvate and ATP. Its altered activity during diabetic conditions could be expected to diminish the metabolism of glucose and ATP production. Hence, the observed decline in the activity of PK in the liver of experimental diabetic rats is responsible for the reduced glycolysis and amplified gluconeogenesis signifying that these two pathways are distorted in diabetes [46]. The treatment with sinapic acid to diabetic rats showed a notable increase in plasma insulin that induces a decrease in ATP, a known allosteric inhibitor of PK, thereby increases the PK activity to near normalcy.
Lactate dehydrogenase (LDH) is a cytosolic enzyme that catalyzes the conversion of pyruvate to lactate in anaerobic glycolysis, which is subsequently converted to glucose in gluconeogenic flux. In diabetic condition, an increased activity of LDH was observed due to the impairment in glucose-stimulated insulin secretion [47]. Thus, increased activity of LDH interferes with normal glucose metabolism and insulin secretion in the pancreatic β-cells and it may be directly responsible for insulin secretory defects in diabetes. Oral administration of sinapic acid as well as metformin treated diabetic groups of rats showed a significant reduction in the LDH activity, probably due to the regulation of NAD+/NADH ratio by the oxidation of glucose.
Glucose-6-phosphatase, a key enzyme in the homeostatic regulation of blood glucose concentration, is expressed mainly in the liver and kidney and is critical in providing glucose to other organs during diabetes, prolonged fasting or starvation [48]. It catalyzes the dephosphorylation of glucose-6-phosphate to free glucose as the terminal step in gluconeogenesis and glycogenolysis. Fructose-1, 6-bisphosphatase is a key regulatory enzyme of the hepatic gluconeogenesis and appeared as a target for efficient and safe glycemic control in diabetes [49]. Glucose-6-phosphate dehydrogenase (G6PDH), the first and rate limiting enzyme, catalyzes the oxidation of glucose-6-phosphate to 6-phospho gluconate and at the same time reducing NADP+ to generate NADPH in the pentose phosphate pathway (PPP) that supplies reducing energy to the cells. NADPH is essential to renew reduced glutathione and decreased NADPH production may facilitate oxidative stress [50]. Hyperglycemia can lead to decrease the activity of G6PDH. G6PDH deficiency could be a risk factor for the pathogenesis of diabetes.
The activities of glucose-6-phosphatase and fructose-1, 6-bisphosphatase were significantly increased in the liver of the diabetic rat possibly because of insulin deficiency. Administration of sinapic acid improved the reversal of high glucose-6-phosphatase and fructose-1, 6-bisphosphatase activities in high fat diet fed-low dose STZ induced experimental type 2 diabetic rats which in turn revealed its role in the regulation of gluconeogenesis. In the present study, the oral administration of sinapic acid considerably increased the activity of glucose-6-phosphate dehydrogenase indicating the regulatory role of sinapic acid in maintaining normoglycemia in experimental diabetes.
Glycogen is a branched polymer of glucose residues which is synthesized by the enzyme glycogen synthase [51]. Glycogen synthase and glycogen phosphorylase are the rate-limiting enzymes in glycogen metabolism. During diabetic conditions, the glycogen levels, glycogen synthase activity and responsiveness to insulin signaling are diminished and glycogen phosphorylase activity is significantly increased. The observed decrease in glycogen content and altered activities of glycogen synthase and phosphorylase in diabetic rats were significantly improved to expected range upon oral administration of sinapic acid. Thus it may be concluded that the oral administration of sinapic acid significantly modulates the glycogen metabolism in high fat diet fed-low dose STZ induced experimental type 2 diabetes in rats.
Conclusion
The present study has demonstrated that sinapic acid significantly improved the levels of fasting blood glucose and plasma insulin and thereby modulating the activities of carbohydrate metabolizing enzymes in the hepatic tissues of high fat diet fed-low dose STZ induced experimental type 2 diabetes in rats. The data obtained also evidenced that the efficacy of sinapic acid at a concentration of 25mg/kg.b.w was comparable with metformin which was administered relatively at a higher concentration (50 mg/kg.b.w.). Therefore, sinapic represents a potential target for the development of an alternative medicine in the treatment and management of diabetes and its secondary complications.
- American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32: S62–S67. Link: https://goo.gl/HkS59n
- Clemmensen C, Müller TD, Finan B, Tschöp MH, Marchi RD (2016) Current and Emerging Treatment Options in Diabetes Care. Handb Exp Pharmacol 233: 437-459. Link : https://goo.gl/W3raay
- Peter M, Thulé (2012) Mechanisms of current therapies for diabetes mellitus type 2. Adv Physiol Educ 36: 275–283. Link: https://goo.gl/xo5Z2y
- Gibson EL, Wardel J, Watts CJ (1998) Fruit and Vegetable Consumption, Nutritional Knowledge and Beliefs in Mothers and Children. Appetite 31: 205-228. Link: https://goo.gl/fes7Ym
- Niciforovic N, Abramovi H (2014) Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity, Comprehensive Reviews in Food Science and Food Safety 13. Link: https://goo.gl/z6xfUC
- Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H (2002) Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem 50: 2161–2168. Link: https://goo.gl/oXEjBC
- Yun KJ, Koh DJ, Kim SH, Park SJ, Ryu JH, et al. (2008) Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J Agric Food Chem 56: 10265–10272. Link : https://goo.gl/CW7ka8
- Yoon BH, Jung JW, Lee JJ, Cho YW, Jang CG, et al. (2007) Anxiolytic-like effects of sinapic acid in mice. Life Sci 81: 234–240. Link: https://goo.gl/dZ7Bfq
- Zou Y, Kim AR, Kim JE, Choi JS, Chung HY (2002) Peroxynitrite scavenging activity of sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J Agric Food Chem 50: 5884–5890. Link: https://goo.gl/jFXZeA
- Kim DH, Yoon BH, Jung WY, Kim JM, Park SJ, et al. (2010) Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology 59: 20-30. Link: https://goo.gl/EL4ZVW
- Kanchana G, Shyni WJ, Rajadurai M, Periasamy R (2011) Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. Global Journal of Pharmacology 5: 33–39. Link: https://goo.gl/wH95TR
- Ader P, Grenacher B, Langguth P, Scharrer E, Wolffram S (1996) Cinnamate uptake by rat small intestine: transport kinetics and transepithelial transfer. Experimental Physiology 81: 943–955. Link: https://goo.gl/g9vrF6
- Nithya R, Subramanian S (2015) Sinapic Acid, a Naturally Occurring Carboxylic Acid Derivative Ameliorates Hyperglycemia in High Fat Diet-Low Dose STZ Induced Experimental Diabetic Rats. International Journal of Scientific Engineering and Technology Research 4: 5746-5750. Link: https://goo.gl/XXYLuh
- Nithya R, Subramanian S (2017) Antioxidant properties of sinapic acid: in vitro and in vivo approach. Asian journal of pharmaceutical and clinical research 10: 255-262. Link: https://goo.gl/AzeiGR
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52: 313-320. Link: https://goo.gl/8b3c1z
- Conn JW (1940) Interpretation of the glucose tolerance test: necessity of standard preparatory diet. Am J Med Sci 199: 555–564. Link: https://goo.gl/9Jy33j
- Sasaki T, Masty S, Sonae A (1972) Effect of acetic acid concentration on the colour reaction in the Otoluidine boric acid method for blood glucose estimation. Rinshbo Kagaku 1: 346-353. Link: https://goo.gl/VwhtuQ
- Drabkin DL, Austin JH (1932) Spectrophotometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J Biol Chem 98: 719–733. Link: https://goo.gl/BQ14QW
- Nayak SS, Pattabiraman TN (1981) A new colorimetric method for the estimation of glycosylated hemoglobin. Clin Chim Acta 109: 267-274. Link: https://goo.gl/6XWLum
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. Link: https://goo.gl/mmwxZ8
- Natelson S, Scott ML, Beffa C (1951) A rapid method for the estimation of urea in biologic fluids. Am J Clin Pathol 21: 275-281.Link: https://goo.gl/9y8u9K
- Shang S, Harton M, Tamayo MH, Shanley C, Palanisamy GS, et al. (2011) Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 91: 378-385. Link: https://goo.gl/UUo6eR
- Brod J, Sirota JH (1948) The renal clearance of endogenous "creatinine" IN MAN. J Clin Invest 27: 645-654. Link: https://goo.gl/EbNaTa
- King J (1965a) The transaminases: alanine and aspartate transaminases, In: Practical Clinical Enzymology (Ed.) Van D. Nostrand Co., London, 1965a 363-395.
- King J (1965b) The hydrolases-acid and alkaline phosphatases, In: Practical clinical enzymology. (Ed.) Van D. Nostrand Co., London, 199-208. Link: https://goo.gl/rsyxfq
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419. Link: https://goo.gl/W4yRCP
- Panserat S, Capilla E, Gutierrez J, Frappart PO, Vachot C, et al. (2001) Glucokinase is highly induced and glucose-6-phosphatase poorly repressed in liver of rainbow trout (Oncorhynchus mykiss) by a single meal with glucose. Comp Biochem Physiol B Biochem Mol Biol 128: 275-283. Link: https://goo.gl/kdrGJ8
- Pogson CI, Denton RM (1967) Effect of alloxan diabetes, starvation and refeeding on glycolytic kinase activities in rat epididymal adipose tissue. Nature 216: 156-157. Link: https://goo.gl/7X3LBQ
- Ells HA, Kirkman HN (1961) A colorimetric method for assay of erythrocytic glucose-6-phosphate dehydrogenase. Proc Soc Exp Biol Med 106: 607-609. Link: https://goo.gl/KaNNcs
- Koide H, Oda T (1959) Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin Chim Acta 4: 554-561. Link: https://goo.gl/LwK7iP
- Gancedo JM, Gancedo C (1971) Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non-fermenting yeasts. Arch Mikrobiol 76: 132-138. Link: https://goo.gl/8ZoHYF
- Leloir LH, Goldemberg SH (1962) Glycogen synthetase from rat liver: (Glucose)n + (UDPG)?(Glucose)n+1 +UDP, In: Colowick SP, Kalpan NO (Eds.), Methods in Enzymology. Academic Press, New York 145–147. Link: https://goo.gl/bS1jUd
- Cornblath M, Randle PJ, Parmeggiani A, Morgan HE (1963) Regulation of glycogenolysis in muscle. Effects of glucagon and anoxia on lactate production, glycogen content, and phosphorylase activity in the perfused isolated rat heart. J Biol Chem 238: 1592-1597. Link: https://goo.gl/ZDhy7u
- King J (1959) A routine method for the estimation of lactic dehydrogenase activity. J Med Lab Technol. 16: 265-272. Link: https://goo.gl/qEMduX
- Morales MA, Jabbagy AJ, Terenizi HR (1973) Mutation effecting accumulation of glycogen. Neurospora Newsletter 20: 24-25. Link: https://goo.gl/LYbxx8
- Goldstein DE, Little RR, Wiedmeyer HM, England JD, McKenzie EM (1986) Glycated hemoglobin: methodologies and clinical applications. Clin Chem 32: B64–70. Link: https://goo.gl/3U162y
- Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365: 1415-1428. Link: https://goo.gl/837Rnq
- Sunmonu TO, Afolayan AJ (2013) Evaluation of Antidiabetic Activity and Associated Toxicity of Artemisia afra Aqueous Extract in Wistar Rats. Evid Based Complement Alternat Med 2013: 929074. Link: https://goo.gl/FndjR3
- Liu Z, Que S, Xu J, Peng T (2014) Alanine aminotransferase-old biomarker and new concept: a review. Int J Med Sci 11: 925-935. Link: https://goo.gl/wSzvQp
- Olaoluwa T, Osilesi O, Adebawo OO, Onajobi FD, Oyedemi SO et al. (2015) Alkaline Phosphatase (ALP), Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Activities in Selected Tissues of Rats Fed on Processed Atlantic Horse Mackerel (Trachurus trachurus). Advances in Bioscience and Biotechnology 6: 139-152. Link: https://goo.gl/yedFR9
- Akanji MA (1993) Effect of chronic consumption of metabisulphite on the integrity of the rat kidney cellular system. Toxicology 81: 173–179. Link: https://goo.gl/TRXgzq
- Raddatz D, Ramadori G (2007) Carbohydrate metabolism and the liver: actual aspects from physiology and disease. Z Gastroenterol 45: 51-62. Link: https://goo.gl/t2WyFG
- Laakso M, Malkki M, Deeb SS(1995) Amino acid substituents in hexokinase II among patients with NIDDM. Diabetes44:330‐334. Link: https://goo.gl/bYVebm
- O‘Doherty RM, Lehman DL, Telemaque‐Potts S, Newgard CB (1999) Metabolic impactof glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 48: 2022‐2027. Link: https://goo.gl/Lg7Sey
- Gupta D, Raju J, Prakash J, Baquer NZ (1999) Change in the lipid profile, lipogenic and related enzymes in the livers of experimental diabetic rats: effect of insulin and vanadate. Diabetes Res Clin Pract 46: 1‐7. Link : https://goo.gl/VDPhmy
- Gupta, Vibhor, Bamezai, Rameshwar NK (2010) "Human pyruvate kinase M2: A multifunctional protein". Protein Science 19: 2031–2044. Link: https://goo.gl/PrTdyz
- Ainscow EK, Zhao C, Rutter GA (2000) Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes 49: 1149-1155. Link: https://goo.gl/szfzQh
- Nordlie RC, Foster JD, Lange AJ (1999) Regulation of glucose production by the liver. Annu Rev Nutr 19: 379-406. Link: https://goo.gl/Njiwef
- Aoki K, Saito T, Satoh S, Mukasa K, Kaneshiro M, et al. (1999) Dehydroepiandrosterone suppresses the elevated hepatic glucose-6-phosphatase and fructose-1,6-bisphosphatase activities in C57BL/Ksj-db/db mice: comparison with troglitazone. Diabetes 48: 1579-1585. Link: https://goo.gl/ZeUy66
- Jain M, Cui L, Brenner DA, Wang B, Handy DE, et al. (2004) Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation 109: 898-903. Link: https://goo.gl/LWsT68
- Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, et al. (2005) Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem 280: 17260-17265. Link: https://goo.gl/EHdae1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
