Global Journal of Obesity, Diabetes and Metabolic Syndrome
Study of Resting Energy Expenditure and Weight Changes during Pregnancy
D Jackemeyer1, Erica Forzani1,2 and Corrie Whisner3*
2School for Engineering of Matter, Transport, and Energy, Arizona State University, USA
3School of Nutrition and Health Promotion, Arizona State University, USA
Cite this as
Jackemeyer D, Forzani F, Whisner C (2017) Study of Resting Energy Expenditure and Weight Changes during Pregnancy. Glob J Obes Diabetes Metab Syndr 4(1): 016-023. DOI: 10.17352/2455-8583.000018In the present study, we have followed 4 pregnant women during pregnancy. The participants measured their resting energy expenditure (REE), weight, and activity, and recorded caloric intake. REE was measured with a mobile indirect calorimeter, BreezingTM on a weekly basis, and used to determine total energy expenditure (TEE) and daily caloric intake needs. The measured REE profiles indicated individual patterns in metabolic rate changes across pregnancy that could not be predicted by any known REE equation. The study outcomes suggest that the use of a mobile indirect calorimeter in conjunction with weight and physical activity measures allowed for the accurate estimate of caloric needs for pregnant women. The actual caloric intake (BreezingTM) was compared with the self-reported caloric intake and demonstrated to have non-significant differences in three of the four cases, and a significant difference in one of the four cases. In addition, the participants reported that knowledge gained from tracking health parameters positively affected weight gain during pregnancy and helped to gain within a healthy weight range. Furthermore, all the participants were able to fully recover their pre-pregnancy weight within the year following birth.
Introduction
Resting energy expenditure (REE), also known as resting metabolic rate (RMR) is the energy to support basic metabolic functions, and sustain the life of a person, as well as the gestation of a new life [1,2]. The human body transforms fat, carbohydrates, and proteins from food into energy, during which oxygen is exchanged for carbon dioxide [3]. The exchange of these gases can be detected by indirect calorimetry devices and used to calculate the energy (kcal/day) needed to support life [4,5]. In a pregnant woman, energy requirements for life and growth can be higher than in a non-pregnant state [6]. However, the energy needs during pregnancy can vary greatly by time and person and are unknown unless they are directly measured [7].
For the mother excessive gestational weight gain can result in elevated prenatal and post-partum risks. These include preeclampsia and gestational diabetes, and post-partum weight retention, which is associated with obesity and cardio-metabolic risk factors (e.g. heart disease and high blood pressure) later in life [8,9]. Preeclampsia and high blood pressure are difficult conditions to treat during pregnancy and can also have negative effects on the fetus [8,9].For the fetus, maternal excessive weight gain can lead to fetal macrosomia, a condition where the baby grows larger than normal in the uterus and makes delivery very difficult for the mother [8,9]. Additionally, the infant can incur injuries during the birthing process if their shoulders get trapped in the birth canal. Infants born with macrosomia also have an increased probability of developing childhood obesity and metabolic syndrome as adults. On the other hand, maternal excessive weight gain can also relate to in-utero growth restriction caused by inadequate nutrient transfer across the placenta [8,9]. Due to the above-mentioned consequences for the mother and the baby, healthy weight gain during pregnancy is key to preventing adverse maternal and fetal health risks [8,9].
In a recent study conducted on non-pregnant adults at the University of Arizona and Arizona State University [10], researchers found that metabolic equations can provide REE values with errors up to 1100 kcal/day from the true values of a participant’s resting energy expenditure. In addition, it has been shown that if someone uses energy expenditure based on calculated REE for weight maintenance, adhering to those inaccurate numbers can lead to weight gain [11]. For this reason, the present study aimed to: 1- measure REE and characterize longitudinal metabolic changes during pregnancy, and 2- compare measured REE values in pregnant women with calculated values corresponding to maternal weight, height, and age. In addition, the study also aimed to: 3- estimate the actual caloric intake in pregnant women from measured REE values and weight and 4- to correlate the measured caloric needs with the calorie intake values reported by the study participants. Furthermore, the study assessed the consequences of the knowledge of measured caloric needs in pregnant women via a survey taken after the study, and evaluation of post-partum weight recovery.
Material and Methods
Participants
Four adult women were tested as participants of the study. The study was approved by the Institutional Review Board of Arizona State University (IRB protocol #1012005855), and it was carried out from December 2014 to December 2016. All participants provided written informed consent prior to participation. Inclusion criteria for participants included being generally healthy and over the age of 18 years. Women were excluded if they had preexisting conditions including type 1 or 2 diabetes, high blood pressure, or intestinal or metabolic conditions that would affect energy metabolism.
Study design
All participants had baselines assessed for physical parameters before pregnancy. During pregnancy, they followed the standard-of-care as designated by their healthcare professionals (Obstetricians/Gynecologists). In addition, participants electronically measured and recorded REE, weight, activity, and caloric intake throughout the duration of the pregnancy. Data were uploaded and stored on iPhonesTM using BreezingTM, WithingsTM, and fitness and food tracker (FitbitTM, HealthTM, MyFitnessPalTM, EnquosTM) applications (apps) as detailed in Table 1. The study participants were trained in the use of the devices, and were allowed to communicate with a study researcher via e-mail throughout the study to resolve technical questions about the management of apps and trackers.
Each participant was given a mobile indirect calorimeter for self-assessment of REE at home. The calorimetry device was the BreezingTM Tracker (www.breezing.com), which uses indirect calorimetry to measure the exchange of oxygen and carbon dioxide from inhaled and exhaled breath. This method is recommended by the Academy of Nutrition and Dietetics, and American College of Sports Medicine [12] for weight management. The sensing principle of the mobile indirect calorimeter was validated against the Douglas Bag Method [13]. Following the recommendation of taking at least one measurement per week, the participants were provided with an REE-adjusted daily caloric intake recommendation through the app. Along this line, the Breezing app assessed the total energy expenditure (TEE) of each participant based on measured REE, lifestyle, structured exercise time, and intensity (metabolic equivalent, MET: intensity of exercise defined as “x” times REE); and provided a daily caloric intake goal defined as [3]: Calorie Intake Goal = TEE + caloric surplus, with: TEE = (lifestyle coefficient * REE) + Exercise = (lifestyle coefficient * REE) + ((Exercise hours / 24 hours) * MET * REE). For all participants in the study, the lifestyle was determined to be sedentary, and therefore a lifestyle coefficient equal to 1.179 (for females) was utilized [3]. Exercise was set mostly as light (MET = 2.35) due to the low intensity of exercise activities [14]. However, the time spent on structured exercise was negligible, rendering TEE with contributions from REE, and lifestyle.
On most of the occasions for each pregnancy, the participants measured their REE at home on a Saturday morning, and met REE measurement conditions, which included overnight fasting, a 12-hour period with no strenuous exercise, no-caffeine ingestion, and resting in a sitting position for 20 minutes prior to the measurement [15]. After the participants rested, they were instructed to relax and breathe normally during the measurement. REE values collected from the BreezingTM app were compared to caloric estimates provided by the following equations.
Harris-Benedict Eq. (female) [16,17]:
REE (kcal/day) = 655.1 + (9.563 × weight (kg)) + (1.850 × height (cm)) – (4.676 × age (years))
Mifflin-St Jeor Eq. (female) [18]:
REE (kcal/day) = (10 × weight (kg)) + (6.25 × height (cm)) - (5 × age (years)) - 161
More details on the apps used in the study
Resting Energy Expenditure and Caloric Intake Goal: As mentioned above, the BreezingTM app provided to the users the caloric intake goal after the measurements, and setting a target weight and weekly exercise target. All measurement, and goal/target information was exported from the app via e-mail in a csv file format, and e-mailed to the research scientist of the study at the given measurement time point.
Weight: The WithingsTM app was used to track weight via a Wi-Fi scale (Body analyzer from Withings, www.withings.com). Recorded weight results were exported to study staff using the user’s account dashboard.
Self-Reported Caloric Intake: The MyFitnessPalTM (www.myfitnesspal.com) or EnquosTM (www.enquos.com) apps were used for tracking dietary and caloric consumption. Participants were able to set the recommended caloric intake goal from the BreezingTM app as their daily nutrition goal in these partner apps. As foods consumed (breakfast, lunch, snacks, dinner) were entered into the apps, the remaining balance of calories for the day was displayed in the BreezingTM app. It is important to mention that no special diet was prescribed to the participants of the study. The apps were used as educational tools to learn about caloric needs during pregnancy and achieve adequate gestational weight gain. At the end of the study, all recorded results in the apps were exported with a Google Chrome extension, FoodFastFit, in the case of MyFitnessPal, or the use of the app’s analytics tool in the case of EnquosTM.
Step counting: Step counting measurements were performed using either the accelerometer from each participant’s iPhone, via WithingsTM or HealthTM apps; or a commercial step counter, FitbitTM zip (www.fitbit.com). Recorded results were exported using: 1- the user’s account dashboard from WithingsTM, or 2- QS access (https://quantifiedself.com/2014/10/qs-access-app-see-healthkit-data-table/) from the HealthTM app.
Data and statistical analysis
Averaged parameters were collected before or during the study at a certain period of time as mean ± standard deviation (SD). Weekly changes in weight for each participant were evaluated from the slopes on weight vs. week plots. Statistically significant differences in parameter changes were assessed by comparison via paired t-tests and defined as p < 0.05.
Results
Characteristic of the participants of the study
Table 2 shows a summary of baseline characteristics of the participants before pregnancy. All were generally healthy and without conditions with known adverse impacts on fetal growth and development.
Evaluation of health parameters during pregnancy
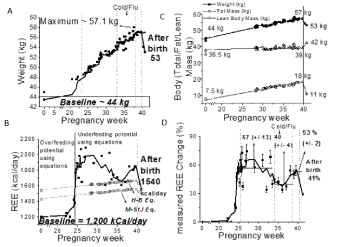
Regarding weight and REE, Figures 1A-C summarize changes in the weight, measured REE, and body composition of Participant-#1 across the pregnancy period. Figure 1B compares the measured REE (by BreezingTM) with estimates calculated using the Harris-Benedict [16,17], and Mifflin-St Jeor [18] equations. Figure 1D shows the percent change in REE as calculated from the pre-pregnancy REE value (1,200 kcal/day). In addition, Figures 2-4 show changes in weight (A) and measured REE (B) across the pregnancy period for Participants 2, 3, and 4.
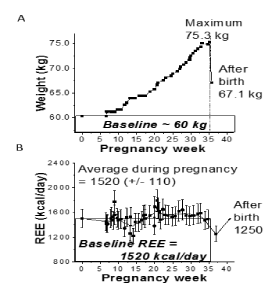
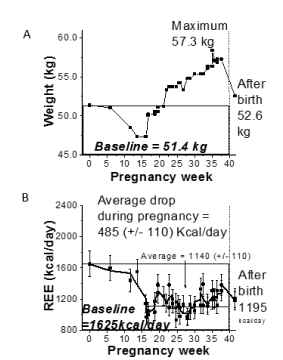
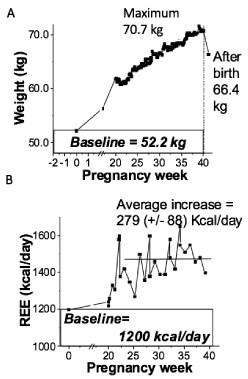
The four cases had distinctive weight and REE changes across pregnancy. For Participant-1 (Figure 1A,B), the weight increased 0.49±0.02 kg/week on average during the second and third trimesters, while the REE values increased during the second trimester and remained relatively stable through delivery, except with a small decrease due to a cold. Specifically, a sharp increase of ~ 690 kcal/day was observed after the second trimester of pregnancy which plateaued with an average of 1890 +/-150 kcal/day followed up by average values of 1680 +/-50 kcal/day and 1830 +/-30 kcal/day during and after the illness, respectively. Comparison of measured and estimated REE values were significantly different (p<0.05) for this participant. The weight for Participant-2 (Figure 2) increased by approximately 0.59±0.01 kg/week during the second and third trimesters, while the REE remained stable throughout pregnancy. REE for Participant-2 had an overall average of 1520±110 kcal/day during pregnancy in comparison to an average pre-pregnancy requirement of 1520 kcal/day. A weight decrease (Figure 3) was observed during early pregnancy for Participant-3 which was likely attributed to the participant experiencing nausea. Once the original weight was achieved again, a steady increase in weight was observed at 0.34±0.01 kg/week. In parallel, the REE values decreased from 1625 kcal/day to an average of 1140 +/- 110 kcal/day and remained low through late pregnancy, even when steady weight gain was occurring. For Participant-4 (Figure 4), weight increased steadily throughout pregnancy at a rate of 0.54±0.01 kg/week, and the REE values increased slightly from 1200 kcal/day in early pregnancy to 1480 kcal/day in later pregnancy. Occasional upward fluctuations occurred in the second and third trimesters.
Regarding the activity tracking, Table 3 shows a comparison of the average daily step counts and their standard deviation for the 4 study participants during the second and third trimesters of pregnancy. The table excluded the last 2 weeks of pregnancy due to observed decreased mobility or lack of step tracking quality. It is worth mentioning that for Participants 1, 3, and 4, total steps observed by phone accelerometers were less than 1,000 steps/day on some days, and those days were excluded from the count due to higher probability of lack of phone wearing compliance.
Table 4 shows a comparison of the mean self-reported intake of calories, protein, fat and carbohydrates for the 4 participants during the second and third trimesters of pregnancy. It is important to mention that with the exception of Participant-2 who reported her diet with almost 100% adherence during the full pregnancy, the remaining participant’s only fully tracked dietary intake for a few weeks of their pregnancies. More specifically, Participants 1, 3, and 4 tracked between 7 to 10 consecutive days in each of the trimesters, including second and third trimester with total daily calories consumed equal to or greater than the caloric intake goal.
Post-study evaluation of the participants
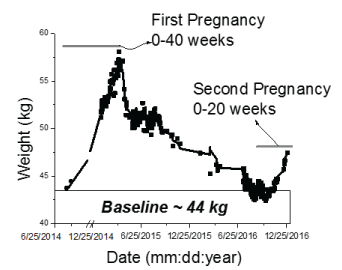
Table 5 shows the pre-pregnancy weight, the pregnancy maximum weight, the post-partum weight at 1 or 2 months after delivery, and the percentage of recovered weight compared with pre-pregnancy weight. Figure 5 shows the weight change of Participant-1, that got pregnant a second time 12 months after her first delivery.
Participants #1 through #4 reported average caloricintakes of 2130, 1860, 1870, and 2250, respectively. Since the study followed weight changes, activity and REE, an energy balance analysis was performed. Table 6 summarizes these findings, which were assessed as follows: 1- Total Energy Expenditure (TEE) estimated from average REE values for the second and/or third trimesters (depending on the case) and the activity level (sedentary) for each participant. 2- Given the weight gain experienced by Participants 1 through 4 which was approximately 0.49, 0.59, 0.34, and 0.54 kg/week, respectively; the surplus caloric intake supporting this weight gain was calculated and presented as caloric excess in kcal/day. 3- Surplus caloric intakes were added to each participant’s respective TEE value thereby rendering the estimated actual caloric intake. 4- The estimated actual caloric intake was compared to the self-reported caloric intake which rendered differences of approximately 570, 580, -150, and 90 kcal/day for Participants #1 through #4, respectively. Paired t-tests comparing the estimated actual caloric intake vs. the reported caloric intake were performed using the average values, SDs (calculated from a propagated error method) and total number of measurements. The tests indicated there were no significant differences between actual and reported caloric intakes for Participants #1, #3, and #4, while a significant difference between actual and reported caloric intake was found for Participant #2.
A survey was administered at the end of each pregnancy evaluating knowledge related to the following: 1- metabolism, 2- weight gain, and 3- caloric intake. In all cases, the participants indicated they increased their level of education and understanding of the three topics during the study. In particular, they reported that the use of the mobile indirect calorimeter and activity tracker improved their awareness of energy expenditure, and the use of the MyFitnessPalTM or EnquosTM app educated them about the caloric content and density of foods. Participants reported having an increasing awareness of caloric content of habitual food choices and interest in considering varied food choices while balancing quantity for proper weight gain.
Discussion
Data from this case analysis suggests that the use of mobile health tracking devices that include measures of indirect calorimetry to measure caloric needs may be beneficial for tracking and planning weight gain during pregnancy and achieving pre-pregnancy weights following delivery. Based on IOM guidelines for gestational weight gain all participants, with the exception of Participant-3, were expected to gain 25-35 lbs (11-16 kg); the pre-pregnancy BMI of Participant-3 corresponded with a recommended weight gain of 28-40 lbs (13-18 kg) [7]. Actual weight gain among the participants was 13.1, 15.0, 5.9 (from minimum) and 18.5 kg for Participants 1-4, respectively. This placed Participants 1 and 2 within expected ranges (Participant-4 was 2.5 kg over the upper bound) with Participant 3 gaining approximately 6.8 kg less than recommended.
Given that caloric intake needs are directly associated with REE [19], it was observed that if someone followed caloric recommendations based on equations, periods for risk of under- and over-feeding could occur as previously observed in adults participating in a weight loss intervention [11]. For Participant-1, data (Figure 1C) suggest that changes in body composition occurred during pregnancy. Given that published equations relate REE linearly to fat-free mass [20,21], the estimated REE values for this participant would have assumed a constant body composition throughout pregnancy (not shown). Our data suggest that REE equation estimates do not correlate well with pregnancy REE as measured by indirect calorimetry. Therefore, the simple math of: “the higher the Free Fat Mass (FFM), the higher the REE,” as assumed in many REE equations, is not valid during pregnancy.
Another interesting observation of this study was the macronutrient composition of the diets. Even though there were no specific instructions to follow a specific diet, all participants reported ingesting a relatively high level of protein within a range of 21-24% of kcal, and a ratio of carbohydrate: fat: protein between 3.0:1.0:1.1 and 5.0:1.0:1.6. Despite variation in dietary macronutrient content, all but one participant reported caloric intakes that did not differ from those estimated by the BreezingTM app. It is well-known that consistent diet tracking is difficult for participants. Despite inconsistent reporting by these participants, a minimum requirement of 7 consecutive days during a given season (period of the year) has been found to be representative of a person’s diet [22]. Therefore, we considered available dietary intake data to be relatively representative of these participants during pregnancy. Together, these data suggest that use of indirect calorimetry may be beneficial to pregnant women as they manage their increased needs for energy and monitor weight gain throughout gestation. This finding is promising but given the small sample size, further research is needed to evaluate whether this observation persists among larger and more diverse cohorts of pregnant women.
Overall, data from our 4 cases indicate that weight gain and REE changes are unique for each woman, and that there are likely complex factors that influence caloric needs during pregnancy. In fact, the unique patterns observed for changes in weight and REE suggest that REE estimates by equations are inaccurate, and the only way to assess the actual REE is to measure it.
When considering the health of both the mother and the child, it may be important to measure and track REE to adequately estimate caloric needs to avoid the risk of excessive weight gain during pregnancy. Along this line, it is very common to have general recommendations for weight gain during pregnancy that are backed by limited data. One example is the recommendation of 300 extra kcal each day [23]. Using the IOM weight gain guidelines [7] and the traditional assumption of ~ 1 lb. (~ 0.5kg) of weight gain/loss per 3,500 kcal we would expect the following with regard to excess daily caloric needs for adequate weight gain:
For a BMI < 18.5 kg/m2: a weight gain of 28-40 lbs (13-18 kg) is recommended, which accounts for an additional 350 - 500 kcal/day on average.
For a BMI range of 18.5-24.9 kg/m2: a weight gain of 25-35 lbs (11-16 kg) is recommended, which would require an additional 310 - 440 kcal/day on average.
For a BMI range of 25.0-29.9kg/m2: a weight gain of 15-25 lbs (7-11 kg) is recommended, which accounts for an average excess need of 190 - 310 kcal/day.
For a BMI >30.0 kg/m2: a weight gain of 11-20 lbs (5-9 kg) is recommended, which would require an additional intake of 140 - 250 kcal/day on average.
Activity levels among our participants remained low throughout pregnancy which correspond with previous findings that the majority of pregnant women in the US do not meet physical activity guidelines [24]. The average step trends were as follows: Participant-1 < Participant-4 < Participant-2 < Participant-3, with a variability higher than 35%. For the most active Participant (#3), the variability was the highest, meaning there were days with relatively high and others with relatively low step counts. No particular trends were observed in activity levels when comparing levels at the beginning of pregnancy with those at the end of pregnancy, with the exception of the few weeks preceding delivery when the participants’ movements were more restricted. This differs slightly from national reports indicating that American women reported greater moderate-to-vigorous physical activity in the first trimester when compared to the third trimester [25]. Overall, the average number of steps was relatively low for these cases such that the participants would be characterized as sedentary.
As mentioned before, REE can change greatly during pregnancy, and the way it changes can be different for each woman. Despite an increased need for calories, excessive gestational weight gain still affects many women, contributing to adverse maternal and child health outcomes [7-9,26]. In order to evaluate how effective the participants were at restoring weight after delivery of a baby, the weight of the study participants was analyzed 1-2 month after delivery. Table 4 illustrated that two of the four participants (#2 and #3) almost recovered their pre-pregnancy weight after 1-2 month, while the other two participants (#1 and #4) were at 40-50% of their beginning weight. With regard to Participants 1 and 4, both were able to recover the weight after the second post-partum month. As an example, Figure 5 showed weight recovery data for Participant 1, where a pre-pregnancy weight of approximately 45 kg was recovered by one year post-delivery. This case became pregnant again after recovering her pre-pregnancy weight and was successful at following a healthy trajectory in her second pregnancy with a similar rate of weight gain rate when compared to her first pregnancy. Weight loss following pregnancy can be difficult as recent data suggest that nearly 75% of women are heavier one year after birth compared to their pre-pregnancy weight [27]. Although postpartum weight retention has been found to be highest among African American women and those with less education, lower incomes and subsidized insurance [27], data are conflicting with regard to how the co-occurrence of multiple modifiable factors influence postpartum weight status [28].
While a review of 4 randomized controlled and 5 nonrandomized trials suggested that physical activity and dietary interventions could help minimize the risk of excessive gestational weight gain [29], other studies have shown limited effects [30,31]. In a recent publication, it was shown that real-time tracking of health parameters can improve outcomes related to weight management [10] but these effects have not been extensively studied in pregnant women. In the case of all four participants in our study, the successful recovery of weight seemed related to the educational component of tracking weight, metabolism, activity and caloric intake during pregnancy; however, other factors such as socioeconomic status, education level and lifestyle may have also had an impact. Further research is needed to assess the effects of indirect calorimetry, weight, diet and activity tracking in both obese and normal weight women from diverse socioeconomic backgrounds to evaluate the effectiveness of personal tracking on pregnancy health outcomes.
Conclusion
Four participants participated in a pilot study where the pre-pregnancy and pregnancy weight, resting energy expenditure, physical activity, and caloric intake were measured across gestation using mobile devices and smart phone apps. Phones had a calorie counter app, an activity tracker, and were linked to a resting energy expenditure (REE) mobile indirect calorimeter that connected to phones via Bluetooth. The weight was self-monitored with a Wi-Fi scale. In addition, the participants assessed their caloric intake needs based on the measured REE.
Personal weight tracking allowed the participants to have greater control overweight changes throughout the pregnancy, especially at the second and third trimesters when fetal growth is associated with greater maternal weight gain. In addition, the measurement of REE revealed unique metabolic profiles during the pregnancy among these participants, and further supported the inaccuracy and risks of using REE estimations from equations, as well as current clinical caloric consumption recommendations. In other words, it was concluded that careful tracking of REE is necessary to obtain accurate caloric intake goals during pregnancy.
The study also demonstrated that measuring weight, REE and lifestyle factors, via step counting, made it possible to estimate true caloric needs. We found that 3 of the 4 participants had true caloric intakes with no significant differences from self-reported caloric intakes, while one participant had a significant positive difference. In addition, at the end of the study, all participants indicated that they gained knowledge about energy balance concepts through the use of the mobile apps and tools, and understood the importance of weight management during pregnancy. Furthermore, analysis of post-pregnancy weight indicated that these pregnant women were able to recover their pre-pregnancy weight within a year or less post-delivery.
We would like to acknowledge the exceptional compliance of all 4 participants during the collection of data for this study as well as financial support from the Office of Knowledge Enterprise Development at Arizona State University, and the Flinn Foundation (Grant # 1904). We would also like to specially acknowledge Mr. Bill Read for his encouragement to pursue this study.
Author contributions
Corrie Whisner was responsible for study design, direction and support of study logistics and discussions. Erica Forzani was the lead on IRB logistics, data analysis, and discussion. David Jackemeyer was responsible for data collection.
- Leoluca Criscione, Marion Durr-Gross (2010) Vitasanas GmbH. Eating healthy and dying obese.
- Marion Nestle, Malden Nesheim (2012) Why Calories Count: from science to politics. University of California Press. Link: https://goo.gl/UkS9fT
- McArdle WD, Katch FI, Katch VL (2007) Exercise Physiology: Energy, Nutrition, & Human Performance. Lippincott Williams & Wilkins Link: https://goo.gl/YnBbpS
- Weir JBD (1949) New Methods For Calculating Metabolic Rate With Special Reference To Protein Metabolism. J Physiol 109: 1-9. Link: https://goo.gl/p4Ciec
- Weir JBD (1990) Nutrition Metabolism Classic - New Methods For Calculating Metabolic-Rate With Special Reference To Protein-Metabolism. Nutrition 6:213-221.
- Learn about: Pregnancy weight gain: https://www.babycenter.com/6_your-pregnancy-16-weeks_1105.bc (accessed on Jan 2017). Link: https://goo.gl/wXl3O2
- Institute of Medicine (US) (2009) Weight gain during pregnancy: reexamining the guidelines. Washington, DC. National Academies Press. National Academy of Sciences. Link: https://goo.gl/5kj07k
- Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, et al. (2009) A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol 201: 339. Link: https://goo.gl/vx19tT
- Gaillard R, Durmuş B, Hofman A, Mackenbach JP, Steegers EA, et al. (2013) Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 21: 1046-1055. Link: https://goo.gl/x24NJJ
- Stump C, Jackemeyer D, Abidov Y, Tao NJ, Forzani E, et al. (2016) Study of the effect of mobile indirect calorimeter on weight management. Global Journal of Obesity, diabetes, and Metabolic Syndrome.
- You Tube illustrative video of: Weight loss Plateau by Breezing Channel (accessed on Jan 2017).
- Seagle HM, Strain GW, Makris A, Reeves RS (2009) Position of the American Dietetic Association: Weight Management. J Am Diet Assoc 109: 330-346. Link: https://goo.gl/MBLKQr
- Xian X, Quach A, Bridgeman D, Tsow F, Forzani E, et al. (2015) Personalized Indirect Calorimeter for Energy Expenditure (EE) Measurement”. Glob J Obes Diabetes Metab Syndr 2: 107. Link: https://goo.gl/wmWhtt
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, et al. (2011) Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43: 1575-1581. Link: https://goo.gl/Y0URuo
- Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, et al. (2016) Evidence Analysis Library Review of Best Practices for Performing Indirect Calorimetry in Healthy and None Critically Ill Individuals, J Acad Nutr Diet 115: 1417-1446. Link: https://goo.gl/bw4Tls
- Arthur Harris J, Francis G (1918) A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A 4: 370–373. Link: https://goo.gl/WvP2Al
- Arthur Harris J, Francis G, Benedict A (1999) Biometric Study of Basal Metabolism in Man. Washington Carnegie Institution of Washington Link: https://goo.gl/M3HXtd
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, et al. (1990) A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247. Link: https://goo.gl/1cv2yB
- Apfelbaum M, Bostsarron J, Lacatis D (1971) Effect of caloric restriction and excessive caloric intake on energy expenditure. Am J Clin Nutr 24: 1405-1409. Link: https://goo.gl/nMPRnf
- McArdle W (2006) Essentials of Exercise Physiology. Lippincott Williams & Wilkins. Link: https://goo.gl/HLjN2A
- Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR (2005) Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 82: 941-948. Link: https://goo.gl/mSjxrv
- St Jeor S (1997) Obesity Assessment: Tools, Methods, Interpretations. Jones & Bartlett Learning. Link: https://goo.gl/pv8Dzq
- Hronek M, Zadak Z, Hrnciarikova D, Hyspler R, Ticha A (2009) New equation for the prediction of resting energy expenditure during pregnancy. Nutrition. 25: 947-953. Link: https://goo.gl/1jQwA9
- Evenson KR, Wen F (2011) Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med 53: 39-43. Link: https://goo.gl/Q6Y2Wu
- Evenson KR, Wen F (2010) National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999-2006. Prev Med 50: 123-128. Link: https://goo.gl/ejm8jq
- Chescheir NC (2011) Global obesity and the effect on women’s health. Obstet Gynecol 117: 1213–1222. Link: https://goo.gl/ZRZmAq
- Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, et al. (2015) Community Child Health Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol. 125: 144-152. Link: https://goo.gl/wb0JXL
- Gunderson EP, Abrams B, Selvin S (2000) The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord 24: 1660–1668. Link: https://goo.gl/lQPPNl
- Streuling I, Beyerlein A, von Kries R (2010) Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr 92: 678–687. Link: https://goo.gl/mpem0V
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, et al. (2011) Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr 93: 772-779. Link: https://goo.gl/nckCuc
- Vinter CA, Jenson DM, Ovesen P, Beck-Nielson H, Jorgensen JS (2011) A randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 34: 2502–2507. Link: https://goo.gl/61KpuW
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
