Global Journal of Obesity, Diabetes and Metabolic Syndrome
Comparison of Anti-Glycation Capacity of Two New Purple-Colored-Leaf Tea Cultivars with an Ordinary Green-Colored-Leaf Tea Cultivar in Taiwan
Su-Chen Ho1*, Min-Sheng Su1, Chih-Cheng Lin2 and Chui-Feng Chiu3
2Department of Biotechnology and Pharmaceutical Technology, Yuanpei University of Medical Technology, Hsinchu, Taiwan
3Tea Research and Extension Station, Taoyuan, Taiwan
Cite this as
Ho SC, Su MS, Lin CC, Chiu CF (2017) Comparison of Anti-Glycation Capacity of Two New Purple-Colored-Leaf Tea Cultivars with an Ordinary Green-Colored-Leaf Tea Cultivar in Taiwan. Glob J Obes Diabetes Metab Syndr 4(1): 009-015. DOI: 10.17352/2455-8583.000017The special tea varieties with purple- or red-colored leaves have been successfully bred in Taiwan and their health benefits are needed to be evaluated. The aim of this study was to evaluate the phytochemical content, the antioxidant properties, and the anti-glycation capacities of two new purple-colored-leaf tea cultivars (TTES No.113 & 117) and one of the most cultivated teas in Taiwan (TTES No.18). Green tea made from the two purple-colored-leaf tea cultivars, especially the TTES No.117 had significantly higher phenolic, flavonoid, anthocyanin, and catechin content than the TTES No.18. The purple-colored-leaf tea also had higher antioxidant activities including ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC). The formation of fluorescent advanced glycation end products (AGEs) and non-fluorescent AGEs (Nɛ-carboxymethyllysine; CML) in a glucose/ BSA system were significantly inhibited by teas. The inhibitory capacities of teas on fluorescent AGEs and CML were correlated with phenolics or flavonoids, rather than catechins or anthocyanins. These results implied that the potent antiglycation capacity of purple-colored-leaf tea was primarily attributed to the total phenolics. These findings highlight that the potential use of purple-colored-leaf tea bred in Taiwan as a functional beverage for preventing diabetic complications.
Introduction
Advanced glycation end-products (AGEs) which generated from the non-enzymatic glycation reaction between carbonyl group of physiological sugars and the amine group of proteins, nucleotides, and lipids are proposed to be implicated in the pathogenesis of diabetic complications.[1,2] AGEs can exert detrimental effects through receptor-independent and receptor-dependent mechanisms.[3] Covalently modified by AGEs, the physio-chemical properties and stereo-structure of proteins will be changed and therefore, the physiological functions, such as elasticity, enzyme activity and receptor recognition diminished.[4] On the other hand, AGEs can bind to specific receptors, especially the receptor for AGEs (RAGE) and activate the nuclear factor-κB (NF-κB) signaling pathway to induce oxidative stress, inflammatory response, and apoptosis contributing to the diabetic complications.[3,5] Undoubtedly, dietary agents may be valuable adjuvants to prevent diabetic complications by inhibition of AGEs formation.[6]
Tea is one of the most consumed beverage worldwide. Black tea consumed account for 80% and green tea is preferred in Asia, especially in Japan. Tea is documented to be good for diabetics in several ways including blood glucose level reduction, insulin resistance improvement, and antioxidant defense enhancement.[7-9] Additionally, not only AGEs formation in the in vitro bovine serum albumin (BSA)/glucose system could be inhibited by tea but also AGEs accumulation and development of diabetic complications in vivo could be attenuated. Most of these beneficial effects are attributed to the abundant phytochemicals of tea. [10,11].
Anthocyanins are water soluble plant pigment with red to blue color and have a wide spectrum of physiological activities including antioxidant, anti-inflammatory, anticarcinogenic, and antimicrobial activities.[12-15] Recently, the special tea varieties with purple- or red-colored buds and leaves containing high amount of anthocyanins have been successfully bred in many countries including Kenya, India, China, and Taiwan.[16-19] The purple-colored-leaf tea cultivar from Taiwan has been demonstrated to have higher anti-proliferative capacity on colorectal carcinoma cells than the ordinary green-colored-leaf tea cultivar (TTES No.18).[16] Tea anthocyanins could attenuate t-butylhydroperoxide induced oxidative stress in HEK 293 WT cells.[20] Despite this, little work has been carried out to evaluate the health benefits of the newly bred purple-colored-leaf tea cultivars in Taiwan. Thus, this study evaluated and compared anti-glycation capacities of tea from the new purple-colored-leaf tea cultivars (TTES No. 113 & 117) with the ordinary green-colored-left tea cultivar (TTES No.18) in Taiwan. Furthermore, correlation between the content of phenolics, flavonoids, anthocyanins, or catechins and anti-glycation capacity was analyzed to identify the active components that might responsible for the anti-glycation capacity.
Material and Methods
Chemicals
Bovine serum albumin (BSA), D-glucose, Folin–Ciocalteu phenol reagent, 2,4,6- tripyridyl-S-triazine (TPTZ), fluorescein, 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH), ferrozine, trolox, catechin, and gallic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Catechin standards: (+)-catechin, (–)-epicatechin, (–)-gallocatechin, (–)-epigallocation, (–)-epicatechin 3-gallate, (–)-catechin 3-gallate, (–)-epigallocatechin 3-gallate, (–)-gallocatechin 3-gallate, for HPLC analysis were obtained from Chromadex Co. (St. Louis, MO, USA). Anti-Nε-(carboxymethyl) lysine (CML) antibodies was purchased from Trans Genic Inc. (Tokyo, Japan). All other chemicals and solvents used were analytical grade.
Preparation of tea infusion
The green teas and black teas processed from two purple-colored-leaf tea cultivars (TTES No.113 & 117) as well as one green-colored-leaf tea cultivar (TTES No.18) were obtained from Tea Research and Extension Station (Taoyuan, Taiwan). The TTES No.18, a primarily used tea variety for black tea manufacture in Taiwan, was served as the control in this study. Ten grams of tea was steeped in 200 ml double-distillated water at 95 °C. After cooling to ambient temperature, infusions were filtered through Whatman No. 1 filter papers and the volume was adjusted to 200 ml with double-distillated water. The filtered infusion was aliquoted and stored at -20°C until analysis.
Determination of phenolic compounds, flavonoids and anthocyanins
The total phenolics content was determined by using the Folin–Ciocalteu method with gallic acid as the reference standard. The total flavonoids content was measured by using the aluminum chloride colorimetric method with catechin as the reference standard. Total anthocyanin content was measured according to the acid-differential method and the results were expressed as cyanidin-3-glucoside equivalents (CGEs) with corresponding molar absorptivity (26,900 l/cm·mole).
Analysis of catechins composition
The catechins composition was analyzed using HPLC. The HPLC system (Hitachi Pramaide 1100 liquid chromatographer) was equipped with a binary pump, an online degasser, an autosampler, a thermostatically controlled column oven and, a diode-array detector (Hitachi, Milford, MA, USA). Separation was performed using a 25 cm × 4.6 mm i.d., 5 μm C18 column (Thermo Fisher Scientific, Waltham, MA, USA) at a flow rate of 1 ml/min. The solvent A (0.1% formic acid/water) and solvent B (acetonitrile) were used. The gradient program was operated as follows: 0 min 10% B; 10 min, 10% B; 24 min, 20% B; 30 min, 22% B; 35 min, 25% B. The UV/vis spectra were recorded in the range of 200 - 400 nm and chromatograms were acquired at 280 nm. Injection volume was 20 μl. Quantification was conducted using the external standard method and the calibration graph was established with dilutions of each standard at concentrations in the range of 100 - 6.25 μg/ml.
Evaluation of anti-glycation capacity using a BSA/glucose system
Glucose-mediated protein glycation was carried out as follow. A 2.5 ml of glycation reaction solution includes diluted tea infusion (0.5 ml), 20 mg/ml BSA, 500 mM glucose, 0.02% (w/v) sodium azide, and 100 mM phosphate buffer (pH=7.4). After incubation at 37 °C for 1, 2, 3, and 3 weeks, the amount of fructosame, α-dicarbonyl compound, global fluorescent AGEs, and CML produced were determined, respectively.
Measurement of fructosamine: After 1 week of incubation, the concentration of fructosamine was measured by NBT assay. Briefly, 20 μl of glycation reaction solution was reacted with 180 μl of 300 µM NBT in 0.1 M carbonate buffer (pH=10.35) at 37 °C for 15 min. The absorbance was measured at 540 nm with a multimode reader (Infinite M200 PRO; Tecan Group Ltd., Männedorf, Switzerland).
Measurement of α-dicarbonyl compound: After 2 weeks of incubation, the concentration of α-dicarbonyl compound was measured by Girard-T assay. Briefly, 100 μl of glycation reaction solution was incubated with 50 μl of 500 mM Girard-T solution and 850 μl of 500 mM sodium formate (pH=2.9) at room temperature for one hour. The absorbance was read at 290 nm.
Measurement of fluorescent AGEs: After 3 weeks of incubation, the global fluorescent AGEs formed in the glycation reaction solution was measured using a multimode reader with an excitation and an emission wavelength at 370 and 440 nm, respectively.
Measurement of Nε-(carboxymethyl) lysine: After 3 weeks of incubation, Nε-(carboxymethyl) lysine (CML), a major antigenic AGE structure, was determined using immunoblotting. Equal amount of glycated samples were separated in 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane. After blocked overnight, the membrane was probed for 1h at ambient temperature with anti-CML monoclonal antibodies and alkaline phosphatase conjugated anti-mouse antibodies, respectively. After three times of washing, the specific bands on the membrane were visualized by incubating with the NBT/BCIP substrate solution. Gels stained with coomassie blue and photographed following electrophoresis were served as loading controls. Immunoreactive and coomassie stained bands were quantified by image densitometry (ImageJ software, National Institutes of Health, Bethesda, MD). After correcting for protein loading, we calculated percent inhibition relative to distillated water control.
The inhibitory capacity of each tea infusion on fructosamine, α-dicarbonyl compound, fluorescent AGEs, or CML formation was calculated using the following equation:
% inhibition = [1-(DVsample-DVsample blank)/ (DVcontrol-DVcontrol blank)]×100%, where DVsample-DVsample blank was the difference between the detected value of a tea sample incubated with glucose and that of one incubated without, and DVcontrol-DVcontrol blank was the difference between the detected value of the distillated water.
Evaluation of metal chelating capacity
Serial dilutions of the tea infusion were reacted with 40 μM ferrous chloride and 200 μM ferrozine at the ambient temperature for 10 min. Absorbance at 540 nm was then determined. The metal ion chelating capacity of tea infusion was expressed as IC50.
Evaluation of reducing power
The ferric ion reducing antioxidant power (FRAP) assay was adopted to measure the reducing power of the tea infusions. 50 μl of tea infusion was incubated with 50 μl of FeCl3 solution (3 mM in 5 mM HCl) at 37°C for 30 min. Then, 900 μl of TPTZ solution (1 mM in 0.05 M HCl) was added and the absorbance of the solution was measured at 620 nm. Ascorbic acid was used as the calibration standard and the results are expressed as micromole ascorbic acid equivalents (AAEs) per milliliter of tea infusion.
Evaluation of oxygen radical absorbance capacity (ORAC)
Tea sample and fluorescein solution were incubated at 37°C for 30 min in the dark. After that, 25 μL of 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH) solution was then added as a source of peroxyl radical. The solution’s fluorescence readings were taken every 2 min for 120 min. The difference between the area under the fluorescence decay curve for each sample, standard, and the corresponding area for the blank was determined to calculate the ORAC value. The results are expressed as millimole trolox equivalents (TEs) per milliliter of tea infusion.
Statistical analysis
The results are expressed as mean ± standard deviation from three independent tests. The significance of the differences between treatments was evaluated by one way ANOVA, followed by Duncan’s multiple range test to make multiple comparisons. Pearson’s correlation analyses were used to investigate relationships between different parameters. Differences were considered significant at p<0.05. All statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Total phenolics, flavonoids, and anthocyanins
Table 1 summarizes the total phenolics, flavonoids, and anthocyanins of green and black teas made from different varieties. In agreement with Hsu et al.[16], green teas prepared from the purple-colored-leaf tea cultivars, especially the TTES No.117, had more phytochemicals than the ordinary green-colored-leaf tea. The phenolics, flavonoids, and anthocyanins of the green tea made from the TTES No.117 were 2.41-, 3.10-, and 27.9-fold of those made from the TTES No.18, respectively. However, the black teas had less content of the phenolics, flavonoids, and anthocyanins than those of their corresponding green teas processed from the same variety. This result is also in agreement with Kerio et al., [17] who suggested that oxidative fermentation during black tea processing will cause significant destruction of these antioxidant phytochemicals. Anthocyanins are especially susceptible to oxidative destruction. The accelerated degradation of anthocyanins has been proposed to be related to catechins. However, the health benefit of anthocyanins is higher. We suggest that the purple-colored-leaf tea leaves were not suitable to process into black tea. As compared with the TTES No.18, black teas made from the TTES No.113 and 117 still had more phenolics and flavonoids. Based on these results, purple-colored-leaf tea cultivar is still a better choice than the ordinary green-colored-leaf tea cultivar to process into either green tea or black tea.
Catechin composition
Due to catechins constitute approximately 80–90% of the total flavonoids and are considered as the pharmacologically active compounds of green tea. It is necessary to know the catechins composition of purple-colored-leaf tea cultivars from Taiwan. According to the results of HPLC analysis, six catechins including epigallocatechin (EGC), catechin (C), epicatechin (EC), epigallocatechin gallate (EGCG), gallocatechin gallate (GCG), and epicatechin-3-gallate (ECG) were identified in the tea infusion. As listed in Table 2, the total catechin content of green tea made from purple-colored-leaf tea cultivars (TTES No.113 & 117) were higher than that of from green-colored-leaf tea cultivar (TTES No.18). As expected, due to oxidation and polymerization into theaflavins and thearubigins, the total catechin content of black tea was lower than that of corresponding green tea. Additionally, the catechin profiles of green tea made from different cultivars were different. The green tea from the purple-colored-leaf tea cultivars had higher EGCG, EGC, ECG, or EC content. The EGCG (TTES No.117) was the most abundant compound. In contrast, only EGCG and EGC had a concentration of over 100 μg/ml in the green tea from TTES No.18. Trace amount of C and GCG were detected in green tea from TTES No.113 and TTES No.117. The EGCG content of black tea was significant lower than that of corresponding green tea. The results might be attributed to the different redox potential of catechins. The B-ring trihydroxylated catechins (EGCG and EGC) have a lower redox potential and oxidize more rapidly than the B-ring dihydroxylated catechins (C, EC and ECG).[21,22]
Antioxidant capacities
The metal chelating capacity, FRAP and ORAC of the tea infusions were listed in Table 3. The metal chelating capacities were not significantly different among all of the green teas tested. Green teas from the purple-colored-leaf tea cultivars, especially the TTES No.117, had higher FRAP and ORAC than the TTES No.18. The FRAP and ORAC of green tea from TTES No.117 were 2.35- and 3.10-folds of that from the TTES No. 18, respectively. The FRAP and ORAC of black teas from purple-colored-leaf tea cultivars were significantly lower than the corresponding green teas. The FRAP and ORAC of teas highly correlated with total phenolics, flavonoids, anthocyanins and catechins. The results implied that the phytochemicals contribute to the reducing power and radical scavenging capacities of teas tested. A similar conclusion was reported by Joshi et al.[19] Moreover, Kerio et al. indicated that the DDPH-radical scavenging ability of purple- and green- colored-leaf teas significantly correlated with anthocyanin or catechin content. Also, they considered that the antioxidant capacity of tea is dependent mainly on the flavonoids, tea cultivar, and processing method.[23] Noteworthy, due to the anthocyanin content was negative related to the catechin content, there is not much increase in the antioxidant capacity of purple-colored-leaf teas grown in Kenya and India. [19,23] However, the antioxidant capacity of purple-colored-leaf teas bred in Taiwan enhanced largely because both of the catechins and anthocyanins were increased.
Anti-glycation capacities
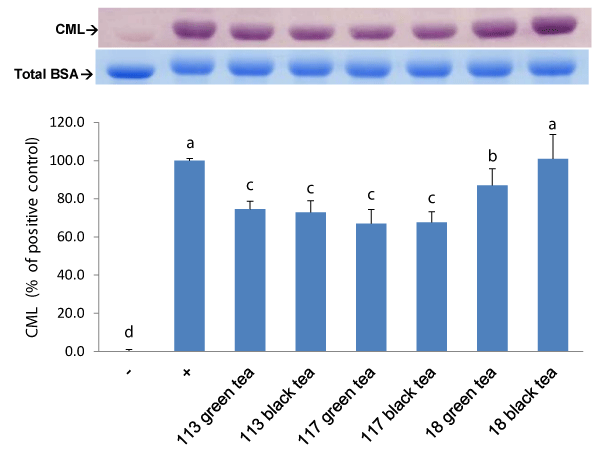
Glycation reaction can be divided into three stages. In the initial-stage, a carbonyl of the physiological sugars condenses with an amine moiety to form a Schiff base adduct and transform to Amadori products by rearrangement. In the intermediate stage, these Amadori products irreversibly degraded into reactive α-dicarbonyl compounds such as methylglyoxal (MGO). In the final stage, these reactive intermediates react with lysine, arginine, or cysteine residues of proteins and yield numerous crosslinked and non-crosslinked AGEs w/o fluorescence via a serial cascade of complex reactions including enolization, dehydration, cyclization, fragmentation, and oxidation.[24] In this study, fructosamine and α-dicarbonyl compound were served as an indicator of early and intermediate stage, respectively. The fluorescent AGEs and crosslinked CML were served as indicators of final stage of glycation. As listed in Table 4, fructosamine formation was inhibited by all tea infusions in a dose-dependent manner at dilutions from 2-fold to 8-fold. At the 2-fold dilution, 25.3%~14.0 % of fructosamine formation in the BSA/glucose system was inhibited. The green tea prepared from TTES No.117 had higher inhibitory capacity, while the green tea from TTES No.18 had the lowest inhibitory capacity. At the 2-fold dilution, about 60% of the α-dicarbonyl compound formation was inhibited by the green tea prepared from TTES No.113 or 117 while only 20% was inhibited by the TTES No.18. Apparently, the inhibitory effect of the teas tested on the α-dicarbonyl compound formation was obviously higher than the fructosamine formation. The glucose-mediated formation of fluorescent AGEs was also inhibited by all tea infusions in a dose-dependent manner at dilutions from 2-fold to 8-fold. At the 2-fold dilution, the inhibitory capacity of tea infusions were as follow: green tea (TTES No.117) > green tea (TTES No.113) > black tea (TTES No.117 & 113) > green tea and black tea (TTES No.18). In contrast to the high inhibitory effect on global fluorescent AGEs, the CML formation was only mildly inhibited by the tea infusions tested. After treated with the green teas made from TTES No.117, No.113 and No.18, the CML formation in a BSA/glucose system decreased 33.1, 25.5, and 13.0 %, respectively (Figure 1). The results show that the green tea made from TTES No.117 had higher inhibitory capacity on the AGEs formation among all of the teas tested.
Except for fructosamine, the phenolic or flavonoid content of tea infusion was highly correlated with the inhibitory capacity on the α-dicarbonyl compound, fluorescent AGEs, or CML formation. The catechin content only correlated with the inhibitory capacity on the fluorescent AGEs formation. The highest correlation between the phenolics and all of the anti-glycation indicators were observed. These results implied that the anti-glycation capacity of tea was mainly attributed to the total phenolics and the inhibitory actions of the phytochemicals were mainly at the intermediate and final stages of glycation. In fact, the significant correlation between anti-glycation activity and phenolics as well as flavonoids of several plant foods such as spices, herbal teas, and medicinal plants have been reported. [25-27] The inhibitory mechanism of phenolics and flavonoids against glycation was partly due to their scavenging effect on free radicals derived from the glycoxidation process.[28] Moreover, anthocyanin-rich extract from berries, red grape skin and butterfly pea flower petals have also been reported to exhibit anti-glycation capacity, and total phenolics rather than anthocyanins seems to be primarily responsible for the anti-glycation capacity. The anti-glycation capacity is closely related to the antioxidant activity. [29-31].
In addition to antioxidant activity, reactive dicarbonyl species trapping capacity is considered to be one of the mechanisms contributing to the anti-glycation of tea catechins. Four tea flavanol compounds (EGCG, EGC, ECG, and EC) and three theaflavins (TF1, TF2 and TF3) have been reported to have methylglyoxal trapping capacity.[32] Furthermore, catechins have been reported that they could attenuate AGEs formation, reduce inflammatory response, and consequently ameliorate partially diabetic nephropathy in type 2 diabetic mice.[33] Recently, Zhu et al. indicated that catechins could significantly trap dehydroascorbic acid, physiological reactive dicarbonyl species, and consequently attenuate ascorbic acid-caused protein glycation.[34] Sri Harsha et al. found that the reactive carbonyl species trapping ability correlated significantly with total phenolic content and antioxidant capacity of red grape skin extract but not white grape skin extract and they suggested that anthocyanins were involved in the trapping activity.[35] Indeed, delphinidin-3-rutinoside and cyanidin-3-rutinoside, the major anthocyanins of blackcurrant, were recently reported to be capable of trapping methylglyoxal and consequently to prevent AGEs formation.[36] The results of this study show that the anti-glycation capacity of TTES No.117 green tea was significantly higher than the TTES No.118 green tea.
In addition to anti-glycation, tea and catechins have been reported that they could ameliorate the progress of diabetic complications by the antioxidant enzyme system enhancement, inhibitory of RAGE expression, and inhibitory of AGEs-mediated oxidation and inflammation.[37-39] Anthocyanins are able to reduce blood sugar, attenuate insulin resistance, strengthen antioxidant defense, and help to prevent diabetic complications.[40,41] Because of high contents of catechins and anthocyanins, the Taiwanese purple-color-leaf tea has a good ability to prevent diabetic complications.
Conclusion
The functional tea from Taiwanese purple-color-leaf tea represents a promising dietary intervention to prevent AGEs-induced diabetic complication based on their potent anti-glycation and antioxidant capacities.
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under Contract No. MOST 104-2320-B-264-001-MY3.
- Thornalley PJ (1999) Clinical significance of glycation. Clin Lab 45: 263-273. Link: https://goo.gl/0DpM6A
- Ahmed N (2005) Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Res Clin Pract 67: 3–21. Link: https://goo.gl/vwKpUC
- Stirban A, Gawlowski T, Roden M (2014) Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol. Metab 3: 94-108. Link: https://goo.gl/KRX97e
- Brownlee M, Cerami A, Vlassara H (1988) Advanced glycosylation end products in tissue and biochemical basis of diabetic complications. N Engl J Med 318: 1315–1321. Link: https://goo.gl/hkU9fR
- Stern DM, Yan SD, Yan SF, Schmidt AM (2002) Receptors for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 1: 1–15. Link: https://goo.gl/YOJF1Z
- Elosta A, Ghous T, Ahmed N (2012) Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8: 92-108. Link: https://goo.gl/T2NJod
- Babu PV, Sabitha KE, Shyamaladevi CS (2006) Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem Biol Interact 162: 114-120. Link: https://goo.gl/NIoSk9
- Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS (2004) Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem 52: 643-648. Link: https://goo.gl/5lZqKk
- Liu K, Zhou R, Wang B, Chen K, Shi LY, et al. (2013) Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr 98: 340-348. Link: https://goo.gl/rh9acQ
- Babu PV, Sabitha KE, Shyamaladevi CS (2008) Effect of green tea extract on advanced glycation and cross-linking of tail tendon collagen in streptozotocin induced diabetic rats. Food Chem Toxicol 46: 280-285. Link: https://goo.gl/53vE9p
- Nakagawa T, Yokozawa T, Terasawa K, Shu S, Juneja LR (2002) Protective activity of green tea against free radical- and glucose-mediated protein damage. J Agric Food Chem 50: 2418-2422. Link: https://goo.gl/ZeCwo6
- Lyall KA, Hurst SM, Cooney J, Jensen D, Lo K, et al. (2009) Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am J Physiol Regul Integr Comp Physiol 297: R70-R81. Link: https://goo.gl/Yhk91w
- Norberto S, Silva S, Meireles M, Faria A, Pintado, M, et al.(2013) Blueberry anthocyanins in health promotion: a metabolic overview. J Funct Foods 5: 1518-1528. Link: https://goo.gl/4HWQqI
- Prior R.L, Cao G, Martin A, Sofic E, McEwen J, et al. (1998) Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem 46: 2686-2693. Link: https://goo.gl/hnJi0p
- Satué-Gracia MT, Heinonen M, Frankel EN (1997) Anthocyanins as antioxidants on human low-density lipoprotein and lecithin-liposome systems. J Agric Food Chem 45: 3362-3367. Link: https://goo.gl/UCNA8l
- Hsu CP, Shih YT, Lin BR, Chiu CF, Lin CC (2012) Inhibitory effect and mechanisms of an anthocyanins- and anthocyanidins-rich extract from purple shoot tea on colorectal carcinoma cell proliferation. J Agric Food Chem 60: 3686-3692. Link: https://goo.gl/o59lBt
- Kerio LC, Wachira FN, Wanyoko JK, Rotich MK (2012) Characterization of anthocyanins in Kenyan teas: Extraction and identification. Food Chem 131: 31-38. Link: https://goo.gl/PJ2fEm
- Kilel EC, Faraj AK, Wanyoko JK, Wachira FN, Mwingirwa V (2013) Green tea from purple leaf coloured tea clones in Kenya - their quality characteristics. Food Chem 141: 769-775. Link: https://goo.gl/iAMJjP
- Joshi R, Rana A, Gulati A (2015) Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem 28: 135-143. Link: https://goo.gl/X6QzvE
- Kerio LC, Bend JR, Wachira FN, Wanyoko J.K, Rotich M.K (2011) Attenuation of t-butylhydroperoxide induced oxidative stress in HEK 293 WT cells by tea catechins and anthocyanins. J. Toxicol. Environ. Health Sci 3: 367-375. Link: https://goo.gl/olcLsQ
- Owuor PO, M Obanda (2007) The use of greentea (Camellia sinensis) leaf flavan-3-ol composition in predicting plain black tea quality potential. Food Chem 100: 873-884. Link: https://goo.gl/utPr6K
- Ngure FM, Wanyoko JK, Mahungu SM, Shitandi AA (2009) Catechins depletion patterns in relation to theaflavin and thearubigins formation. Food Chem 115: 8-14. Link: https://goo.gl/fzbVdy
- Kerio LC, Wachira FN, Wanyoko JK, Rotich MK (2013) Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem 136: 1405-1413. Link: https://goo.gl/v9ZLMz
- Lapolla A, Traldi P, Fedele D (2005) Importance of measuring products of nonenzymatic glycation of proteins. Clin Biochem 38: 103–115. Link: https://goo.gl/pmdqYr
- Dearlove RP, Greenspan P, Hartle DK, Swanson RB, Hargrove JL (2008) Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food 11:275-281. Link: https://goo.gl/LuI0Pb
- Ho SC, Wu SP, Lin SM, Tang YL (2010) Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem. 122: 768-774. Link: https://goo.gl/DIwcst
- Ramkissoon JS, Mahomoodally MF, Ahmed N and Subratty AH (2013) Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med 6: 561-569. Link: https://goo.gl/tms797
- Wu CH, Yen GC (2005) Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem 53: 3167-3173. Link: https://goo.gl/NFd4wd
- Ramkissoon JS, Mahomoodally MF, Ahmed N and Subratty AH (2014) Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: chemical correlates of in vitro antiglycation activity. Asian Pac J Trop Med 69: 71-77. Link: https://goo.gl/M642b9
- Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S (2013) Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med 13: 171. Link: https://goo.gl/MHhJQt
- Chayaratanasin P, Barbieri MA, Suanpairintr N, Adisakwattana S (2015) Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement Altern Med 15: 27. Link: https://goo.gl/sYiomZ
- Lo CY, Li S, Tan D, Pan MH, Sang S, et al. (2006) C.T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol Nutr Food Res 50: 1118-1128. Link: https://goo.gl/pQF0aX
- Zhu D, Wang L, Zhou Q, Yan S, Li Z, et al. (2014) (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol Nutr Food Res 58: 2249-2260. Link: https://goo.gl/SfhJ0b
- Zhu Y, Zhao Y, Wang P, Ahmedna M, Ho CT, et al. (2015) Tea flavanols block advanced glycation of lens crystallins induced by dehydroascorbic acid. Chem Res Toxicol. 28: 135-143. Link: https://goo.gl/uF8vVY
- Sri Harsha PS, Mesias M, Lavelli V, Morales FJ (2016) Grape skin extracts from winemaking by-products as a source of trapping agents for reactive carbonyl species. J Sci Food Agric 96: 656-663. Link: https://goo.gl/kRK5eq
- Chen XY, Huang IM, Hwang LS, Ho CT, Li S, et al. (2014) Anthocyanins in blackcurrant effectively prevent the formation of advanced glycation end products by trapping methylglyoxal. J. Funct Foods 8: 259-268. Link: https://goo.gl/77O1hI
- Sabu MC, Smitha K, Kuttan R (2002) Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol 83: 109-116. Link: https://goo.gl/y8dTbe
- Yan J, Zhao Y, Suo S, Liu Y, Zhao B (2012) Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic Biol Med 52: 1648-1657. Link: https://goo.gl/oEz3a0
- Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, et al. (2009) Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther 11: R71-R83. Link: https://goo.gl/a775Rq
- Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, et al. (2009) The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res 53: 1419-1429. Link: https://goo.gl/tqddGM
- Li D, Zhang Y, Liu Y, Sun R, Xia M (2015) Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 145: 742-748. Link: https://goo.gl/bfVxyt
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
