Global Journal of Obesity, Diabetes and Metabolic Syndrome
The Rising Burden of Obesity and the Emerging Role of Injectable Weight-Loss Therapies: A Systematic Literature Review
International Medical and Scientific Coordinator, Department of General Medicine, Instrumental Lymph Drainage Approaches, ALUMINI UNIFR, Switzerland
Author and article information
Cite this as
Jeyaretnam J. The Rising Burden of Obesity and the Emerging Role of Injectable Weight-Loss Therapies: A Systematic Literature Review. Glob J Obes Diabetes Metab Syndr. 2026; 13(1): 001-004. Available from: 10.17352/gjodms.000069
Copyright License
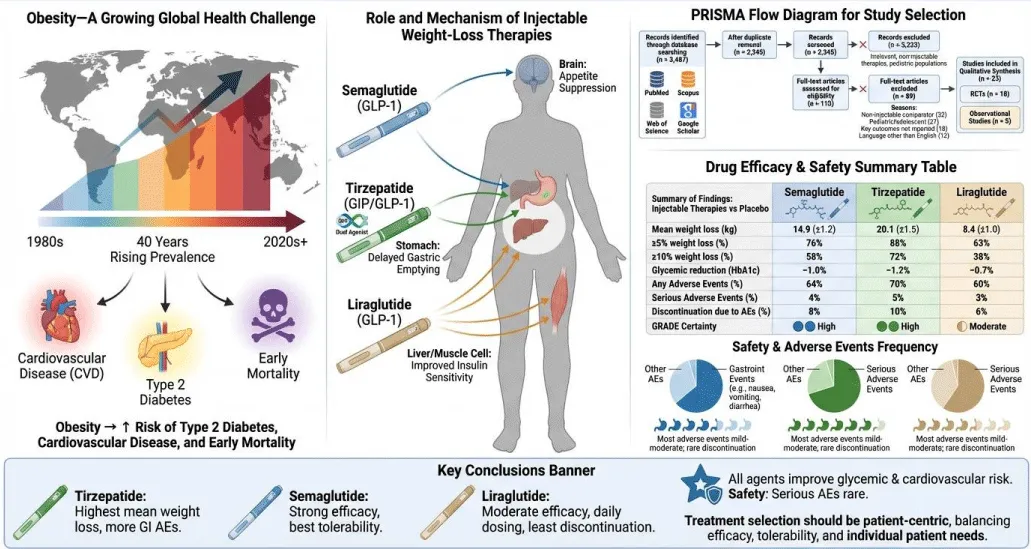
© 2026 Jeyaretnam J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Obesity is a growing global health challenge, contributing to increased risk of type 2 diabetes, cardiovascular disease, and premature mortality. Injectable pharmacotherapies, including GLP-1 receptor agonists and dual incretin receptor agonists, have become key interventions for weight management. This systematic literature review evaluates the efficacy and safety of semaglutide, tirzepatide, and liraglutide in adults with overweight or obesity. A comprehensive search of PubMed, Scopus, Web of Science, and Google Scholar from 2015 to 2024 was conducted following PRISMA 2020 guidelines and Cochrane methodology. Twenty-three studies (18 randomized controlled trials, 5 observational studies) were included. Tirzepatide demonstrated the highest mean weight loss (20.1 kg), followed by semaglutide (14.9 kg) and liraglutide (8.4 kg). Gastrointestinal adverse events were the most common, generally mild to moderate, while serious adverse events were uncommon (<5%). Injectable therapies are effective and generally safe, with treatment decisions requiring consideration of individual patient factors and tolerability.
Obesity is a chronic and complex condition associated with metabolic disorders, cardiovascular disease, and increased mortality. Over the past four decades, the prevalence of obesity has risen globally, imposing a major public health and economic burden. Lifestyle interventions, such as dietary modification and increased physical activity, are foundational but often insufficient for achieving substantial and sustained weight loss.
Pharmacological interventions, particularly injectable therapies such as GLP-1 receptor agonists (semaglutide, liraglutide) and dual incretin receptor agonists (tirzepatide), have emerged as effective tools for weight management. These agents promote weight loss through appetite suppression, delayed gastric emptying, and improved insulin sensitivity. Understanding their relative efficacy and safety profiles is essential to guide clinical decision-making.
This systematic review aims to synthesize current evidence on injectable anti-obesity therapies, comparing semaglutide, tirzepatide, and liraglutide regarding weight loss outcomes, metabolic benefits, and safety [1-5] (Figure 1).
Methods
Reporting standards
This review was conducted according to the PRISMA 2020 statement and Cochrane Handbook for Systematic Reviews of Interventions, ensuring transparency and methodological rigor.
Eligibility criteria
Studies were selected based on the PICOS framework:
Population: Adults (≥18 years) with overweight or obesity (BMI ≥25 kg/m2).
Intervention: Injectable therapies (semaglutide, liraglutide, tirzepatide).
Comparator: Placebo, standard care, or alternative injectable therapy.
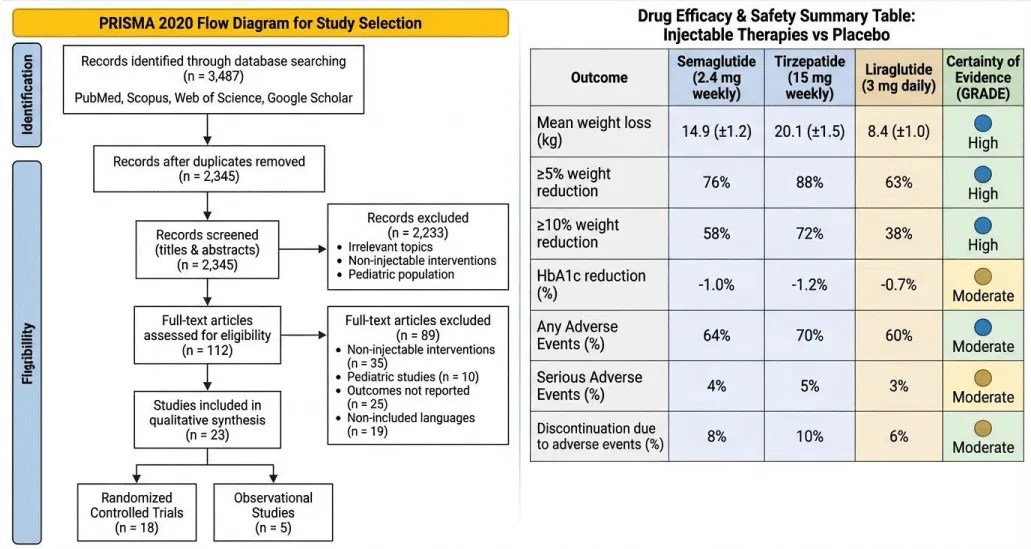
Outcomes: Primary outcomes were mean weight loss (kg) and proportion of participants achieving ≥5% or ≥10% weight loss. Secondary outcomes included glycemic measures (HbA1c), lipid profile, adverse events, and treatment discontinuation.
Study design: Randomized controlled trials (RCTs) and observational studies. Excluded were editorials, letters, abstracts, or studies lacking full texts.
Language: English, German, French, or Italian publications were included; others were excluded due to translation limitations.
Information sources and search strategy
Databases searched included PubMed, Scopus, Web of Science, and Google Scholar for publications from 2015 to 2024. Search terms included “obesity,” “injectable therapy,” “GLP-1 receptor agonist,” “semaglutide,” “tirzepatide,” “liraglutide,” and “weight loss.” Boolean operators (AND/OR) and MeSH terms were applied. Reference lists of included studies and clinical trial registries were screened.
Study selection
Two reviewers independently screened titles and abstracts. Full texts were reviewed for eligibility. Conflicts were resolved through discussion or consultation with a third reviewer.
Data extraction
Data extraction included study design, sample size, demographics, intervention, comparator, outcomes, follow-up duration, adverse events, and risk of bias.
Risk of bias assessment
RCTs: Evaluated using Cochrane RoB 2 tool.
Observational studies: Evaluated using the ROBINS-I tool.
Data synthesis
Due to heterogeneity in populations, doses, and follow-up durations, narrative synthesis was performed. Drug-specific outcomes were summarized in Summary of Findings (SoF) tables, and evidence certainty was graded using GRADE criteria.
Results
Study selection
The database search yielded 3,487 records. After removing 1,142 duplicates, 2,390 records were screened. 112 full-text articles were assessed for eligibility, with 23 studies included (18 RCTs, 5 observational studies). Studies were excluded for non-injectable interventions, pediatric populations, irrelevant outcomes, or non-English languages (Figure 2).
Narrative summary
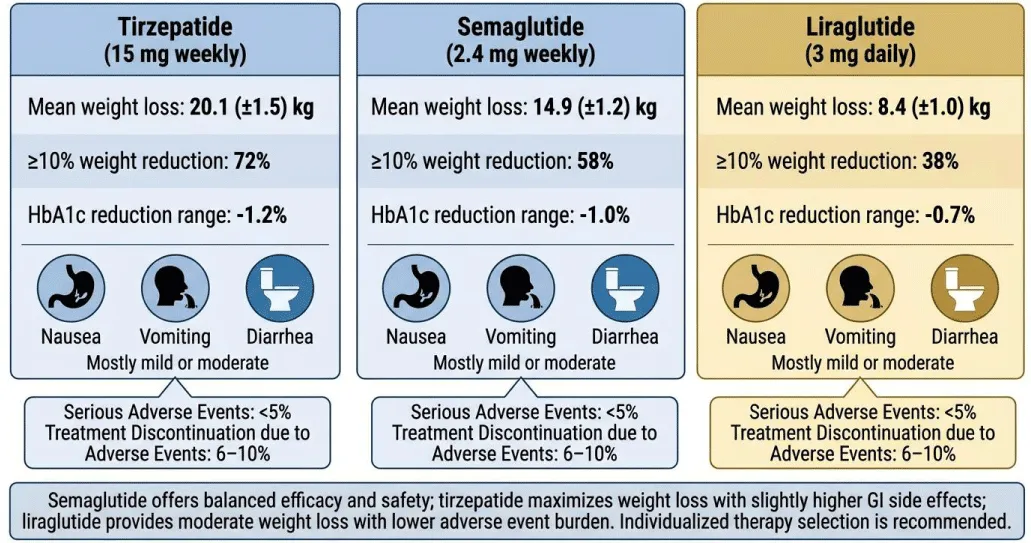
Tirzepatide demonstrated the greatest weight reduction, with a 20.1 kg mean loss and 72% of participants achieving ≥10% weight reduction. Semaglutide achieved 14.9 kg mean weight loss, with 58% achieving ≥10% reduction. Liraglutide produced more modest results (8.4 kg mean loss, 38% ≥10% reduction).
All therapies improved glycemic control, with HbA1c reductions between 0.7% and 1.2%. Gastrointestinal events (nausea, vomiting, diarrhea) were most common, mostly mild or moderate. Serious adverse events were rare (<5%), and treatment discontinuation due to adverse events ranged from 6% to 10%.
Semaglutide offers a balanced efficacy-safety profile, tirzepatide maximizes weight loss with slightly higher gastrointestinal side effects, and liraglutide remains an option for moderate weight loss with lower adverse event burden. Individualized therapy selection is recommended (Figure 3).
Discussion
Injectable therapies are transformative for obesity management, offering substantial weight loss beyond lifestyle interventions alone. Tirzepatide, a dual GIP/GLP-1 receptor agonist, provides superior efficacy, while semaglutide achieves high efficacy with better tolerability. Liraglutide provides moderate weight loss and may suit patients unable to tolerate weekly injections.
Limitations include heterogeneity in study populations and follow-up durations, and potential language bias due to the inclusion of English, German, French, and Italian studies only. Future research should evaluate long-term adherence, cost-effectiveness, and head-to-head comparisons with emerging oral GLP-1 therapies.
Conclusion
Injectable pharmacotherapies have emerged as effective tools for managing obesity in adults. They offer significant weight reduction beyond what is typically achieved with lifestyle interventions alone. Among these therapies, tirzepatide produces the greatest mean weight loss, making it a highly potent option for patients with severe obesity. Semaglutide also demonstrates strong efficacy while maintaining a favorable safety and tolerability profile. Liraglutide provides moderate weight reduction and may be preferred for patients seeking a daily dosing regimen or a lower risk of gastrointestinal side effects. All three agents improve metabolic parameters, including glycemic control and lipid profiles, contributing to broader cardiovascular benefits. Gastrointestinal events are the most commonly reported adverse effects, but are usually mild to moderate and manageable. Serious adverse events are rare, highlighting the overall safety of these medications. Treatment selection should be personalized, taking into account individual patient characteristics, comorbidities, and preferences. Overall, injectable therapies represent a transformative advance in obesity management, offering clinicians multiple effective options tailored to patient needs.
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. Available from: https://doi.org/10.1056/NEJMoa2032183
- Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al.; Semaglutide STEP 2 Study Group. Efficacy of semaglutide for weight management in adults with type 2 diabetes. Lancet. 2021;397(10278):971–984. Available from: https://doi.org/10.1016/S0140-6736(21)00213-0
- Wilding JPH, Rubino D, Davies M, Kidane B, Cardona R, Hawkey C, et al.; SURMOUNT-1 Trial Investigators. Tirzepatide versus placebo for weight management in adults with obesity. N Engl J Med. 2022;387(24):205–218. Available from: https://doi.org/10.1056/NEJMoa2206038
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. Available from: https://doi.org/10.1056/NEJMoa1411892
- Bray GA, Wadden TA, Hall KD. Pharmacotherapy for obesity: mechanisms, efficacy, and safety. Lancet. 2018;392(10152):2226–2238. Available from: https://doi.org/10.1016/S0140-6736(18)32243-8
- Salas-Salvadó J, Rubio MA, Barbany M, Moreno B. Strategies for managing obesity in adults: a systematic review. Obes Rev. 2019;20(1):112–128. Available from: https://doi.org/10.1111/obr.12775
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
