Global Journal of Obesity, Diabetes and Metabolic Syndrome
Diabetic Neuropathy in Older Adults: Pathophysiological and Clinical Approach
Charles Johnson Sanabria Vera, Raquel Magali Jaramillo Simbaña*, Danna Mishell Vivanco Carrión, Gyslaine Betzabé Pachar Castro, Michael Gabriel Loja Nagua and Alison Marey Cabrera Valle
Technical University of Machala, Machala, El Oro, Ecuador
Cite this as
Sanabria Vera CJ, Jaramillo Simbaña RM, Vivanco Carrión DM, Pachar Castro GB, Loja Nagua MG, Cabrera Valle AM. Diabetic Neuropathy in Older Adults: Pathophysiological and Clinical Approach. Glob J Obes Diabetes Metab Syndr. 2025;12(1):008-015. DOI: 10.17352/2455-8583.000067Copyright License
© 2025 Sanabria Vera CJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder among older adults. Its prolonged and poorly controlled course promotes the development of microvascular and neurological complications, including peripheral diabetic neuropathy (PDN), which affects more than 50% of this population. It is characterized by neuropathic pain, sensory loss, and motor dysfunction.
Objective: To analyze the pathophysiological mechanisms involved in the development and progression of PDN in older adults with T2DM, as well as the main diagnostic approaches used for early detection.
Methodology: A narrative review of the scientific literature published between 2020 and 2025 was conducted through a critical analysis of 28 articles selected based on thematic relevance and methodological rigor. The search was performed in recognized databases (Google Scholar, Dialnet, Scielo, and PubMed), using descriptors in Spanish, English, and Portuguese related to PDN, T2DM, and aging.
Results: PDN in geriatric patients with T2DM is associated with multiple pathophysiological mechanisms, including neurotoxicity, oxidative stress, arteriosclerosis, peripheral nerve demyelination, and microvascular dysfunction. Key metabolic pathways are described (protein kinase C, hexosamine, and polyol pathways), along with the involvement of reactive oxygen species, dyslipidemia, and chronic inflammation. Diagnosis is supported by tools such as the Michigan Neuropathy Screening Instrument (MNSI) and the 10 g monofilament test, which show high sensitivity (79%) and specificity (94%). However, in Ecuador in 2022, only 33.3% of cases were confirmed using the monofilament test, highlighting gaps in timely diagnosis. PDN increases the risk of diabetic foot and amputations by 55%.
Conclusion: PDN represents one of the most common and disabling complications in older adults with T2DM, driven by multiple factors and pathophysiological processes. Understanding its underlying mechanisms and employing effective diagnostic tools are essential for early detection, comprehensive management, and prevention of severe complications such as diabetic foot.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most prevalent chronic metabolic disorders in older adults, characterized by sustained hyperglycemia resulting from multifactorial causes that impair the metabolism of sugars, proteins, lipids, and fats. This condition is caused either by a deficit in insulin synthesis by the pancreas or by resistance to its action in peripheral tissues, leading to progressive damage across multiple organs, including the peripheral nervous system and blood vessels [1].
Chronic hyperglycemia manifests through various symptoms such as excessive thirst, frequent urination, weight loss, and visual disturbances, and may lead to severe microvascular and neurological complications. One of the most common and disabling of these in the elderly population is peripheral diabetic neuropathy (PDN), affecting more than 50% of individuals aged 60 to 80 years with T2DM [2]. Aging is considered one of the most significant risk factors associated with its development [1].
PDN is characterized by the degeneration and disorganization of peripheral nerves, resulting in neuropathic pain, sensory loss, and motor dysfunction, which substantially increases the risk of limb ulceration, diabetic foot, and subsequent amputations. A major challenge in the management of patients with T2DM is the lack of educational interventions aimed at promoting healthy lifestyle habits and physical maintenance, which are essential to prevent the aggressive progression of the disease [2].
Despite its high prevalence and clinical impact, a substantial knowledge gap persists regarding the pathophysiological mechanisms and early diagnostic methods for PDN. Various studies have highlighted the heterogeneity of the metabolic pathways involved, the role of neuroinflammation, and the complex interplay between immunological and vascular factors in the progression of PDN. Moreover, there is variability in the use and effectiveness of diagnostic tools such as the Michigan.
Neuropathy Screening Instrument (MNSI) and the 10 g monofilament test, which are essential for early identification and proper clinical management, especially in Latin America.
In this context, an integrative analysis is needed to critically synthesize current scientific evidence regarding the pathophysiological mechanisms driving PDN and the most effective diagnostic strategies for its early detection in older adults with T2DM.
Therefore, this narrative review aims to analyze the pathophysiological mechanisms involved in the development and progression of PDN in older adults with T2DM, as well as the main diagnostic approaches used for its early identification.
Methodology
A narrative literature review was conducted following the PRISMA recommendations for non-systematic reviews [3] to critically analyze the pathophysiological mechanisms involved in PDN and the diagnostic approaches applied to older adults with T2DM. This type of review enables the integration of dispersed information, the identification of knowledge gaps, and the proposal of clinical management strategies for specific populations.
The search strategy was implemented from January 2020 to May 2025 across recognized scientific databases: PubMed, Scielo, Dialnet, and Google Scholar.
Descriptors were used in Spanish, English, and Portuguese, including: “Diabetic neuropathy,” “Diabetes complications,” “Aging and diabetes-related complications,” and “Prevalence of type 2 diabetes mellitus complications.” Boolean operators (AND, OR) were applied, and titles, abstracts, and full texts were reviewed for the selection of relevant documents.
The following inclusion criteria were established:
- Original articles, systematic or narrative reviews, and consensus documents addressing the pathophysiology or diagnosis of PDN.
- Studies involving geriatric populations (> 60 years old) diagnosed with T2DM.
- Publications with full-text availability and appropriate methodological quality.
Exclusion criteria were as follows:
- Publications lacking control groups.
- Scientific articles with redundant information or those not contributing direct evidence on the pathophysiology or diagnosis of PDN.
- Studies with non-representative sample sizes for the target age group (< 50 patients) or those lacking methodological rigor.
A total of 475 articles were identified through database search strategies. Of these, 301 were excluded due to non-relevant titles. Abstracts of 174 articles were evaluated, and 146 were excluded based on the inclusion and exclusion criteria. Ultimately, 28 articles meeting the quality standards and thematic relevance of the study were selected.
The synthesis of information was organized narratively and categorized into five main thematic axes: (1) epidemiology; (2) general physiology of T2DM; (3) specific pathophysiological mechanisms of PDN; (4) classification and clinical manifestations; and (5) diagnostic approaches in the geriatric population with T2DM.
Results and discussion
a. Epidemiology of T2DM and PDN
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by persistent hyperglycaemia and insulin resistance; when inadequately controlled, it can progress to severe microvascular and neurological complications. According to the World Health Organization (WHO), more than 463 million people worldwide were living with diabetes in 2023 [4]. Projections from the International Diabetes Federation (IDF) indicate that this figure will rise to 700 million by 2045, with 79.4% of cases occurring in low- and middle-income countries [5,6]; consequently, the condition is likely to rank among the leading global causes of mortality [6].
In Latin America, approximately 32 million people have T2DM, and nearly 50% are unaware of their diagnosis, which increases the risk of complications—including Peripheral Diabetic Neuropathy (PDN)—by 55% [6,7]. PDN presents with debilitating neuropathic pain, sensory loss, and nerve dysfunction, frequently leading to lower limb ulceration (diabetic foot) [8]. Reported prevalence ranges from 8% to 60% among patients with T2DM, with the most common form being symmetrical sensorimotor polyneuropathy [9]. Beyond physical disability, PDN is associated with emotional distress and anxiety-depressive symptoms, particularly in older adults [10].
Risk factors for T2DM in adults are closely linked to lifestyle—obesity, physical inactivity, and dyslipidaemia. The Pan American Health Organization recommends at least 30 minutes of daily walking as a minimum level of physical activity; however, 30% - 60% of the population in the Americas fails to meet this target [8]. Additional contributors include hypertension and other metabolic disorders.
Data from the Experimental and Applied Endocrinology Centre at the National University of La Plata indicate that, in 2021, 32% of individuals with T2DM died from causes directly or indirectly attributable to the disease. T2DM, therefore, constitutes not only a public-health issue with social implications but also a substantial economic burden. Annual global expenditure is estimated at approximately USD 966 billion, representing a 316% increase over the past two decades [11] (Table 1).
b. Pathophysiology of T2DM
The pathophysiology of T2DM encompasses a set of multifactorial metabolic disorders that are highly prevalent, affecting 10% - 25% of the entire geriatric population. T2DM is primarily characterised by insulin resistance and progressive deterioration of pancreatic β-cell function [12]. Physiologically, insulin facilitates glucose uptake into cells; when its synthesis is impaired, insulin-dependent cells experience metabolic disturbances. Insulin resistance arises when, despite normal or elevated circulating insulin, target cells cannot utilise glucose effectively because of impaired insulin–receptor binding or defects in intracellular signalling pathways [13]. In addition, hormones such as leptin and corticosteroids may inhibit insulin production [14].
Two fundamental mechanisms underlie T2DM:
- Defective synthesis of insulin-producing pancreatic β-cells [15].
- Reduced tissue sensitivity to insulin [12], leading to an inadequate cellular response to insulin secretion and hindering normal glucose–metabolism–derived energy production [15].
In older adults, ageing exacerbates these mechanisms owing to changes in body-fat distribution, physical inactivity, altered insulin sensitivity, and suboptimal dietary habits [16,17].
c. Pathophysiological mechanisms of PDN
Peripheral Diabetic Neuropathy (PDN) is one of the principal microvascular complications associated with T2DM, affecting sensory fibres and motor neurons within the peripheral nervous system. PDN is a major risk factor for the development of lower-limb ulcers that, in advanced stages, may necessitate amputation [18].
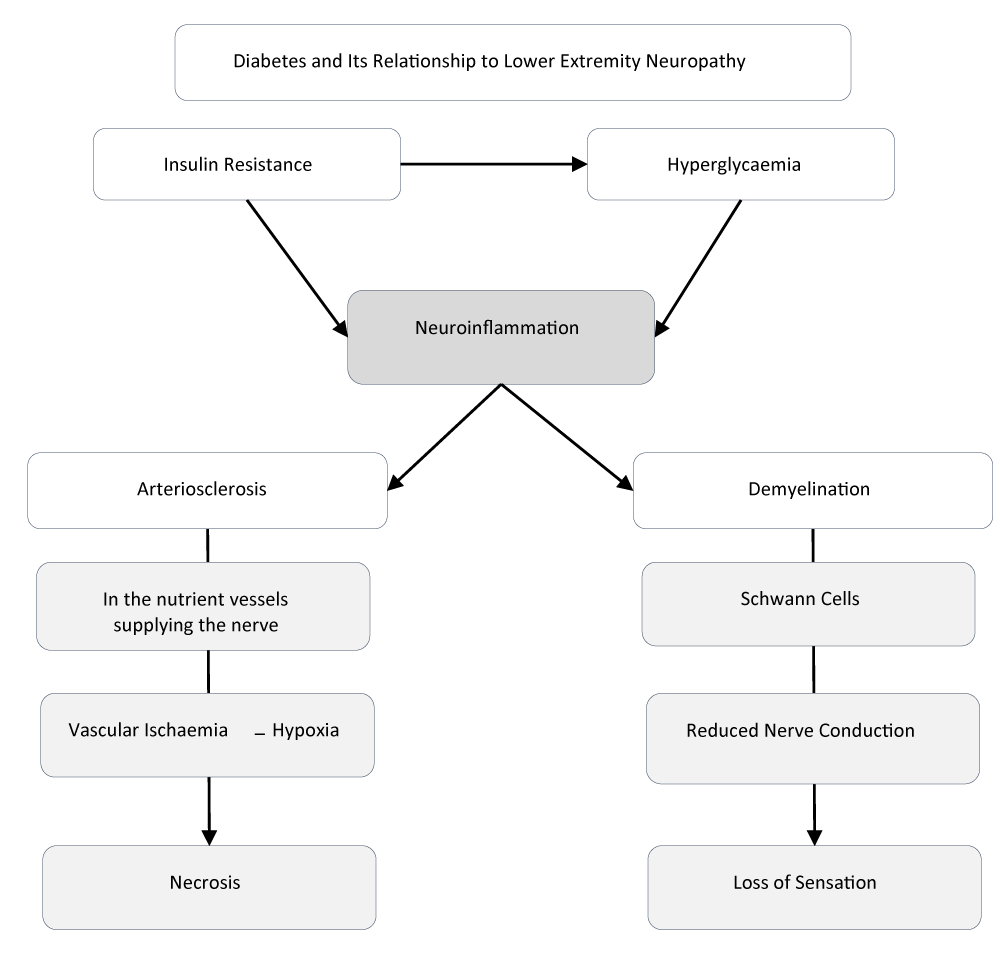
PDN can involve both the somatic and autonomic peripheral nervous systems. Although its exact aetiopathogenic mechanisms have not been fully elucidated, segmental demyelination and axonal degeneration are considered key contributors to nerve-fibre damage [14]. Chronic hyperglycaemia and insulin resistance predispose individuals to neuroinflammation and oxidative stress through the release of pro-inflammatory cytokines and free radicals, activating two interrelated pathophysiological processes: demyelination and microangiopathy [8].
Demyelination of Schwann cells—often observed in the lower extremities of geriatric patients—reduces nerve-conduction velocity and leads to one of the cardinal manifestations of PDN: sensory loss [9]. Microangiopathy, manifested as arteriosclerosis, induces vascular ischaemia and tissue hypoxia, thereby promoting necrosis in affected tissues.
Glucose enters the central and peripheral nervous systems via insulin-independent transporters. In this context, GLUT-1 facilitates glucose passage through microvascular and perineural structures of the blood–brain and blood–nerve barriers; GLUT-3 is predominantly located in peripheral neurons, whereas GLUT-4 is expressed in specific neuronal subpopulations [19].
The two aforementioned mechanisms define PDN, whose prevalence ranges from 8% to 60% in individuals with T2DM, approximately 30% higher than in type 1 diabetes mellitus (T1DM) [8,9]. The most common clinical presentation is symmetrical sensorimotor polyneuropathy, typically accompanied by neuropathic pain.
Multiple studies have documented the impact of T2DM on overall health. A meta-analysis conducted in Ireland over the past two decades reported a significant increase in morbidity and mortality attributable to T2DM, particularly due to its association with nephropathy, retinopathy, and cardiovascular disease—the leading causes of death in this population [7].
In the geriatric age group, susceptibility to these complications is heightened by physiological ageing. Age-related changes include progressive alterations in lipid metabolism—with elevated free-fatty-acid levels and visceral fat deposition—as well as diminished hepatic and renal function, resulting in reduced drug clearance and accumulation of potentially toxic compounds. Pancreatic β-cell mass also declines, further aggravating insulin resistance and impairing insulin secretion [16].
According to data from the Pan American Health Organization (PAHO), PDN shows a higher prevalence among older males, with a frequency of 78.3%, compared to 48.6% in females within the same age group [20]. These findings reinforce the direct relationship between the aging process, the presence of microvascular complications such as PDN, and the progression of T2DM. The etiology of this condition in older adults is widely recognized as multifactorial, with an estimated prevalence ranging from 10% to 25% in the general geriatric population. It is closely associated with changes in body composition, altered fat distribution, and reduced nutritional requirements—factors that further exacerbate the pathophysiology of the disease [17] (Figure 1).
Metabolic and oxidative pathways involved in PDN
- Protein Kinase C (PKC) pathway: Glycolysis plays a central role in the PKC and AGE pathways. Glucose is transported into cells via GLUT-1 and GLUT-3 transporters, then phosphorylated and metabolized into intermediates such as glyceraldehyde-3-phosphate, glucose-6-phosphate, fructose-6-phosphate, and pyruvate. Under hyperglycemic conditions, elevated levels of glyceraldehyde-3-phosphate lead to the formation of diacylglycerol, which activates the PKC pathway. In glial cells, this reduces glutamate uptake and increases the production of Reactive Oxygen Species (ROS), thereby promoting oxidative damage [21].
- Hexosamine pathway: Hyperglycemia also activates the hexosamine pathway by converting fructose-6-phosphate into uridine diphosphate N-acetylglucosamine (UDPGlcNAc). This metabolite contributes to tissue damage in neurons and Schwann cells. The degree of neuronal impairment depends on both the concentration and duration of blood glucose levels [22].
- Polyol pathway: Hyperglycemia activates aldose reductase, which reduces glucose to sorbitol. Sorbitol is subsequently metabolized by sorbitol dehydrogenase into fructose, converting NAD+ into NADH in the process. This NADH remains trapped within the cell, leading to cytotoxicity, increased oxidative stress, and osmotic imbalances [23].
- Microvascular damage: Microvascular injury in peripheral nerves is characterized by endothelial dysfunction, endoneurial capillary disorders, thickened basement membranes, reduced endoneurial perfusion, and ischemia. Activation of the PKC pathway exacerbates this process by inducing vasoconstriction and hypoxia. In addition, T2DM reduces the expression of angiogenic and neurotrophic factors, further compromising neuronal health [24].
- Reactive Oxygen Species (ROS): In hyperglycemic states, an imbalance in ROS production leads to oxidative stress detrimental to cellular function. Under normal physiological conditions, glucose is metabolized through glycolysis and the tricarboxylic acid (TCA) cycle in mitochondria. However, excessive free radical formation under chronic hyperglycemia promotes neuronal ischemia and subsequent functional impairments of nerve cells [25].
- Breakdown of the nerve barrier: The Blood–Nerve Barrier (BNB) and endoneurial vessels protect peripheral nerves. This barrier is composed of endothelial cells connected by tight junctions, pericytes, and a basal lamina. Disruption of the BNB is one of the primary contributors to PDN, as it increases permeability to proteins such as IgG and albumin, alters electrolyte transport, thickens perineural layers, and leads to edema, which, if persistent, contributes to ischemic nerve injury [26].
- Neuropathogenic role of dyslipidemia: In T2DM, dyslipoproteinemia is commonly observed and may influence the development of PDN [27]. Obesity significantly increases the likelihood of developing polyneuropathy, which in turn results in Autonomic Nervous System (ANS) dysfunction. This affects various organs due to impairments in sympathetic and parasympathetic nerve fibers, ultimately leading to peripheral nerve damage [28].
- Immunological and inflammatory factors: Inflammation is triggered by a range of molecules, including chemokines, cytokines (such as TNF-α and IL-1), and adhesion enzymes, which promote the binding of leukocytes to the endothelium and facilitate their migration to sites of injury. Inflammation is considered one of the most critical contributing factors in T2DM, as it promotes the onset of complications, particularly in this context, neuronal apoptosis [29] (Table 2).
d. Diagnosis of PDN in Older Adults with T2DM
According to the 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas, fewer than one-third of physicians diagnose Peripheral Diabetic Neuropathy (PDN) in the context of T2DM, even when patients present with evident clinical symptoms. This under-recognition contributes substantially to the high morbidity and mortality associated with the disease. The same source estimates that timely diagnosis—coupled with effective diabetic-foot prevention strategies—could reduce lower-limb amputations by up to 85% [6].
The American Diabetes Association (ADA) recommends that all individuals with T2DM undergo annual screening for PDN beginning in the fifth year after diagnosis [30]. Nevertheless, approximately 50% of PDN cases remain asymptomatic, thereby increasing the risk of irreversible complications.
In this context, diagnostic assessment must be thorough, particularly within primary-care settings. A visual inspection of the feet at every routine visit and a complete neurological examination at least once per year are advised to detect early lesions or functional disturbances [31].
Key components of the foot examination include:
- Assessment of foot structure and gait pattern
- Identification of erythema with warmth or fissures suggestive of tissue injury
- Pulse evaluation
- Detection of xerosis in distal limbs, particularly the feet
- Evaluation of deep-tendon reflexes
- Testing for loss of protective sensation
- Assessment of vibration perception and proprioception using a tuning fork
Studies conducted in primary-care units in Ecuador revealed a significant prevalence of PDN, predominantly in males, with a peak incidence between 60 and 69 years of age [16].
Diagnostic tools. The most commonly employed include the Michigan test, which comprises the Michigan Neuropathy Screening Instrument (MNSI) and the Michigan Diabetic Neuropathy Score (MDNS) – Feldman [32].
I. MNSI Test
The Michigan Neuropathy Screening Instrument (MNSI) is commonly used in the evaluation of peripheral neuropathy and assists in identifying clinical manifestations of nerve damage that may arise as complications of T2DM [32]. This tool consists of a symptom-based questionnaire containing targeted questions aimed at evaluating typical symptoms such as tingling, numbness, burning-type pain in the legs or feet, among others.
II. MDNS Test
- 10 g Monofilament Test – Semmes-Weinstein: This tool evaluates protective tactile sensation using a 10-gram nylon monofilament applied to specific pressure points on the foot [32].
- 128 Hz Tuning Fork: Used to assess vibratory sensation, typically applied to the hallux (big toe) [32].
These assessments are integrated into a scoring system to establish a reliable diagnosis. If the patient fails to perceive stimuli in key evaluated areas, resulting in a score greater than 7 points, the condition is classified as confirmed neuropathy. A sub-classification system further refines the diagnosis based on the following thresholds [32]:
- Mild neuropathy: 7–12 points
- Moderate neuropathy: 13–29 points
- Severe neuropathy: 30–46 points
These tools are widely used in primary care settings across Latin America to confirm the presence of diabetic neuropathy in patients with T2DM [33,34].
PDN is a major contributor to diabetic foot development. Loss of protective sensation reduces the ability to detect injuries, impairs motor control, and alters foot structure, all of which increase the risk of abnormal plantar pressure and ulcer formation [8].
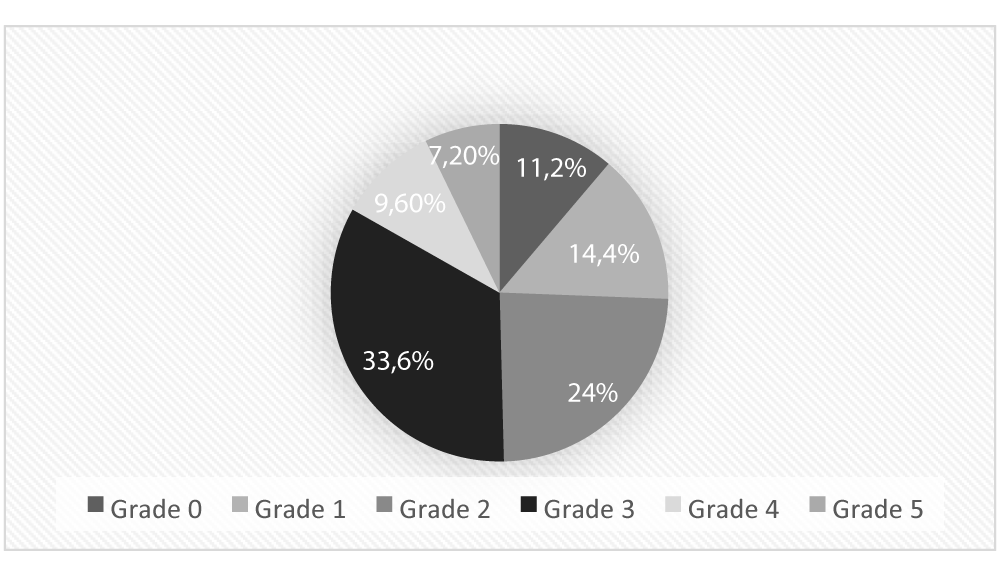
A study conducted by Toaza Karen [35] at the General Hospital of Babahoyo in 2023 revealed the following findings:
Distribution of diabetic foot prevalence by severity stages, as defined by the Meffitt–Wagner classification, in patients with T2DM. This scale categorizes foot lesions based on ulcer depth, presence of infection, and gangrene, ranging from Grade 0 (pre-ulcerative) to Grade 5 (extensive gangrene). The data reflect the clinical burden of diabetic foot complications among older adults with peripheral diabetic neuropathy in a hospital setting. To assess pain intensity, the Visual Analog Scale (VAS) is commonly used [36].
Results and interpretation
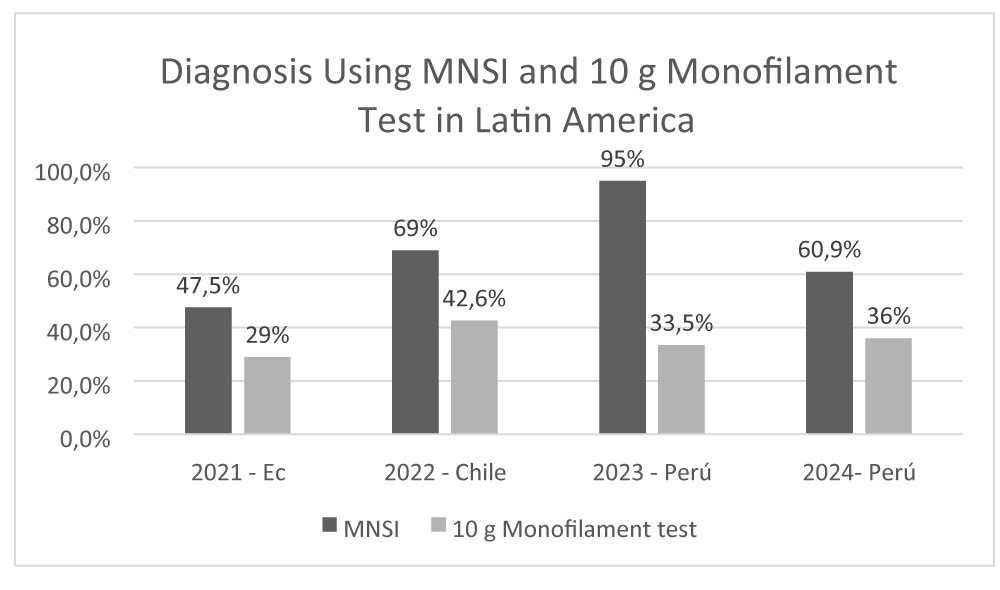
A bibliographic review revealed that in Latin America, the use of the Michigan test, with a reported sensitivity of 79% and specificity of 94%, along with the 10 g monofilament test, has been instrumental in the early diagnosis of this complication [37] (Figure 2).
In Ecuador, data from 2021 show that 47.5% of diabetic neuropathy diagnoses were made using the MNSI [35]. Similarly, a 2022 study conducted in Puerto Montt, Chile, confirmed that MNSI was the predominant diagnostic method in the majority of cases (69%) [38]. This trend continues in Peru, where health records from 2023 and 2024 demonstrate a sustained preference for MNSI, especially within primary care settings [37]. Notably, 65.5% of healthcare professionals reported discontinuing the use of the 10 g monofilament test as a diagnostic method [39].
PDN has been associated with a 55% increased risk of diabetic foot [40]. According to the study by Toaza [35], 33.6% of the patients in the study sample presented with Grade 3 diabetic foot as classified by the Meffitt–Wagner scale (Figure 3), indicating a moderate to severe degree of ulceration in distal extremities.
Discussion
Díaz J [41] contends that microvascular alteration is the most plausible theory explaining the pathophysiology of diabetic neuropathy, directly accounting for endothelial dysfunction that affects axons, Schwann cells, leading to demyelination and the perineural capillaries of peripheral nerves. Pro-inflammatory cytokine release, which generates oxidative stress, is considered a secondary immunological process.
This position contrasts with that of Jiménez et al. [9], who propose two independent mechanisms: (i) oxidative-stress-induced neurotoxicity and (ii) vascular damage that reduces nerve conduction. Despite these differences, both studies agree that severe, poorly controlled hyperglycaemia underlies these processes. According to ADA recommendations [30], neuropathic manifestations generally appear two to five years after diagnosis. Age is also a principal risk factor, because hyperglycaemia is compounded by age-related physiological changes that markedly increase neuropathic risk [42].
Microvascular insufficiency thus represents a key mechanism. Current evidence highlights atherosclerosis and hypoxia as leading causes of ischaemia and tissue necrosis, thereby promoting ulcer formation. In this context, Salinas-Hernández et al. describe vascular-wall thickening and basement-membrane hyalinisation as elements that reduce peripheral perfusion and create a pro-inflammatory environment detrimental to nerve fibres [43].
A variety of techniques are used to evaluate nerve function and complication risk in diabetic neuropathy. The Michigan Neuropathy Screening Instrument (MNSI)— combining a symptom questionnaire with a physical foot examination—remains one of the most widely applied tools. The Michigan Diabetic Neuropathy Score (MDNS) complements the MNSI by incorporating clinical findings and electrophysiological studies.
The 10 g monofilament test is the standard technique for assessing sensory loss. However, Purwanti Okti et al. highlight the Toronto Clinical Scoring System (TCSS) as an effective and straightforward method for detecting and grading diabetic neuropathy, correlating clinical findings with small-fibre alterations and electrophysiological data to optimise detection and follow-up [44].
Li et al. [45] report that loss of the Achilles tendon reflex is among the earliest clinical signs of PDN. By contrast, Carmichael J et al. [46] note that symptoms are more often related to distal hypoesthesia caused by small-fibre damage, presenting as numbness, neuropathic pain, and paraesthesias in distal extremities. This divergence underscores the need to employ multiple clinical tools for early diagnosis [47].
In Ecuador (2022), only 33.3% of diabetic-neuropathy diagnoses were confirmed with the 10 g monofilament test [35]. Conversely, in Peru (2024), 72.9% of diagnoses were confirmed using MNSI, which was applied in 45% of patients. Physical examination detected diabetic neuropathy in 60.9% of cases, and 50% of those assessed had moderate-grade neuropathy [37].
Among older adults, an imbalance of factors—unhealthy diet, physical inactivity, physiological ageing, limited self-care awareness, and restricted access to treatment—contributes substantially to the development of PDN. These conditions elevate blood-glucose levels, foster insulin resistance, and trigger multiple complications which, if not adequately controlled, severely impair quality of life [48].
Understanding the underlying pathophysiological mechanisms is essential for timely diagnosis and effective treatment. In patients with poorly controlled T2DM, loss of protective sensation is pivotal for determining the degree of nerve impairment and the risk of amputation, a highly prevalent complication [49]. Consequently, implementing appropriate screening tests and strengthening health-education programmes are fundamental strategies for reducing the burden of PDN in the geriatric population.
Conclusion
Peripheral diabetic neuropathy (PDN) is among the most common microvascular complications in geriatric patients with type 2 diabetes mellitus (T2DM). The symmetrical sensorimotor polyneuropathy variant is the predominant form of somatic peripheral neuropathy and is characterised by destruction and disorganisation of peripheral nerves, primarily associated with demyelination and microangiopathy that arise from physiological ageing and, consequently, the emergence of these complications.
Multiple mechanisms contribute to PDN pathogenesis, including chronic hyperglycaemia, oxidative stress, and—most critically—β-cell dysfunction. Hyperglycaemic metabolism drives the activation of several biochemical pathways, notably the protein kinase C, hexosamine, and polyol pathways, which generate toxic intermediates and Reactive Oxygen Species (ROS), leading to oxidative stress and neuronal tissue damage. Chronic inflammatory processes mediated by cytokines and growth factors further promote neuronal apoptosis.
Hyperglycaemia also favours the formation of advanced glycation end-products (AGEs), which bind to cellular membranes and activate pro-inflammatory receptors, perpetuating oxidative stress and damaging neural structures. Concurrent dyslipidaemia disrupts neuronal homeostasis and autonomic nervous system function, facilitating the entry of plasma proteins, impairing synaptic communication, and diminishing nerve conduction.
These findings underscore the importance of comprehensive clinical management and timely diagnosis of PDN in older adults with T2DM. Effective care must adopt an integrative approach that emphasises complication prevention and meticulous pain management.
- Harreiter J, Roden M. Diabetes mellitus – Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023) [Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien Klin Wochenschr. 2023;135(Suppl 1):7–17. German. Available from: https://doi.org/10.1007/s00508-022-02122-y
- Strand N, Anderson M, Attanti S, Gill B, Wie C, Dawodu A, et al. Diabetic neuropathy: pathophysiology review. Curr Pain Headache Rep. 2024;28(6):481–7. Available from: https://doi.org/10.1007/s11916-024-01243-5
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. Available from: https://www.acpjournals.org/doi/10.7326/M18-0850
- World Health Organization. Diabetes [Internet]. Geneva: WHO; 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Pan American Health Organization. Diabetes in the Americas. Washington, D.C.: PAHO; 2022. Available from: https://doi.org/10.37774/9789275126332
- Russo M, Grande M, Burgos M, Molaro A, Bonella M. Prevalence of diabetes, epidemiological characteristics and vascular complications. Arch Cardiol Mex. 2023;93(1):30–6. Available from: https://www.archivoscardiologia.com/frame_esp.php?id=551
- International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: IDF; 2021. Available from: https://fmdiabetes.org/wp-content/uploads/2022/01/IDF_Atlas_10th_Edition_2021-comprimido.pdf
- Pérez A, Feria A, Inclán A, Delgado J. Some up-to-date aspects on diabetic polyneuropathy. MEDISAN. 2022 Aug 1;26(4).
- Jiménez G, Martínez L, Anaya A. Diabetic neuropathy: a narrative review of pathophysiology, diagnosis, and treatment. Acta Méd Peru. 2023;40(3). Available from: https://amp.cmp.org.pe/index.php/AMP/article/view/2731
- Botero F, Cruz V, Cote D, Céspedes K, Smith S, Gómez C. Diabetic neuropathy and its association with anxiety symptoms. Universitas Médica. 2021;62(2). Available from: https://revistas.javeriana.edu.co/index.php/vnimedica/article/view/32042
- Bernardo M, Gagliardino J. Diabetes mellitus: magnitude of the problem, pathophysiology, diagnosis and treatment. Rev Soc Argent Diabetes. 2022 Nov 1;56(3 Suppl):18. Available from: https://doi.org/10.47196/diab.v56i3Sup.507
- Galicia U, Asier B, Jebari S, Larrea A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. Available from: https://www.mdpi.com/1422-0067/21/17/6275
- León H, Rojas M, Coy A. Pathophysiology and mechanisms of action of exercise in the management of type 2 diabetes mellitus. Rev Colomb Endocrinol Diabetes Metab. 2023;10(2). Available from: https://revistaendocrino.org/index.php/rcedm/article/view/790
- Staehelin T. The pathogenesis of painful diabetic neuropathy and clinical presentation. Diabetes Res Clin Pract. 2023;206:110753. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168822723005168
- Mizukami H. Pathological evaluation of the pathogenesis of diabetes mellitus and diabetic peripheral neuropathy. Pathol Int. 2024;74(8):438–53. Available from: https://onlinelibrary.wiley.com/doi/10.1111/pin.13458
- Gomezcoello V, Caza M, Jácome E. Prevalence of diabetes mellitus and its complications in older adults in a referral centre. Rev Med Vozandes. 2021;31(2):49–55.
- Romera L, Urbina A. Aging, frailty, and diabetes mellitus: what do they have in common? Diabetes Práctica. 2023. Available from: https://www.diabetespractica.com/files/120/art1.pdf
- Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, et al. New perspectives in diabetic neuropathy. Neuron. 2023;111(17):2623–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0896627323003458
- Calcutt NA. Diabetic neuropathy and neuropathic pain: a (con)fusion of pathogenic mechanisms? Pain. 2020;161(1):65–86.
- Agobian G, Traviezo L. Diabetic peripheral neuropathy in the type II urban outpatient clinic “Dr. Gualdrón” in Barquisimeto, Venezuela. Rev Med Sinergia. 2020;5(4):e448. Available from: https://revistamedicasinergia.com/index.php/rms/article/view/448
- Zhu J, Hu Z, Luo Y, Liu Y, Luo W, Du X. Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol. 2024;14:1265372.
- Cheng Y, Chen Y, Li K, Liu S, Pang C, Gao L, et al. How inflammation dictates diabetic peripheral neuropathy: an enlightening review. CNS Neurosci Ther. 2023;30(4). Available from: https://doi.org/10.1111/cns.14477
- Mizukami H, Osonoi S. Collateral glucose-utilizing pathways in diabetic polyneuropathy. Int J Mol Sci. 2021;22(1):94. Available from: https://doi.org/10.3390/ijms22010094
- Méndez-Morales ST, Pérez-De Marcos JC, Rodríguez-Cortés O, FloresMejía R, Martínez-Venegas M, Sánchez-Vera Y. Diabetic neuropathy: molecular approach—a treatment opportunity. Vasc Pharmacol. 2022;143:106954. Available from: https://doi.org/10.1016/j.vph.2022.106954
- Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus: a comprehensive review. J Diabetes Complications. 2020;34(8):107613. Available from: https://doi.org/10.1016/j.jdiacomp.2020.107613
- Galiero R, Caturano A, Vetrano E, Beccia D, Brin C, Alfano M, et al. Peripheral neuropathy in diabetes mellitus: pathogenetic mechanisms and diagnostic options. Int J Mol Sci. 2023;24(4):3554. Available from: https://www.mdpi.com/1422-0067/24/4/3554
- Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)—new aspects. Int J Mol Sci. 2021;22(19):10835. Available from: https://www.mdpi.com/1422-0067/22/19/10835
- Callaghan BC, Gallagher G, Fridman V, Feldman EL. Diabetic neuropathy: what does the future hold? Diabetologia. 2020;63(5):891–7. Available from: https://doi.org/10.1007/s00125-020-05085-9
- Haghgou A. Diabetic neuropathy from an updated pathophysiological perspective. ResearchGate. 2020. Available from: https://www.researchgate.net/publication/349058750
- ElSayed NA, Aleppo G, Bannuru RR, Beverly EA, Bruemmer D, Collins BS, et al. Summary of revisions: Standards of Care in Diabetes. Diabetes Care. 2024;47(Suppl 1):S5–10. Available from: https://doi.org/10.2337/dc24-SREV
- Arias-Rodríguez FD, Jiménez-Valdiviezo MA, Ríos-Criollo KC, Murillo-Araujo GP, Toapanta-Allauca DS, Rubio-Laverde KA. Diagnosis and treatment: bibliographic review. Angiología. 2023. Available from: http://www.revistaangiologia.es/articles/00474/show
- Senneville É, Albalawi Z, Asten S, Abbas Z. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections (IWGDF/IDSA 2023). Clin Infect Dis. 2023. Available from: https://doi.org/10.1093/cid/ciad527
- International Diabetes Federation. IDF Clinical Practice Recommendations on the Diabetic Foot 2022. Brussels: IDF; 2022.
- Vargas M. Relationship between 10 g monofilament and Michigan test results and metabolic control in diabetics at Health Centre No. 1, Loja. Loja: National University of Loja; 2020.
- Toaza K. Risk factors predisposing to foot-ulcer formation in patients with diabetes mellitus treated at the General Hospital of Babahoyo, January–December 2023 [thesis]. Machala: Technical University of Babahoyo; 2024. Available from: http://dspace.utb.edu.ec/handle/49000/17423
- Hernández M, Mendoza G. Frequency of peripheral neuropathy and diabetic foot in patients with type 2 diabetes mellitus. Innov Desarro Tecnol Rev Digit. 2024;16(1).
- Pacheco S. Clinical factors associated with peripheral neuropathy in patients with type 2 diabetes mellitus at Hospital II-2, Piura 2024. Lima: ALICIA Repository; 2024. Available from: https://hdl.handle.net/20.500.12759/29091
- Quintana C, Márquez JP, Kappes M, Silva MT, Navarro J. Estudio de prevalencia de retinopatía diabética en pacientes diabéticos tipo 2 de la comuna de Puerto Montt y sus factores asociados [Frequency of diabetic retinopathy and associated factors in Puerto Montt, Chile]. Rev Med Chil. 2023;151(1):7–14. Spanish. Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S003498872023000100007
- Longa-López J. Physicians’ attitudes toward the management of diabetic neuropathy in public and private health establishments, 2023. Rev Fac Med Hum. 2023;23(4):54–61.
- Ramírez-Perdomo CA, Perdomo-Romero AY, Rodríguez-Vélez ME. Association between self-care and diabetic-foot risk. SciELO Preprints. 2022. Available from: https://preprints.scielo.org/index.php/scielo/preprint/view/4454/version/4711
- Díaz J. Clinical and pathophysiologic aspects of the diabetic foot. Med Interna Méx. 2021;37:540–50. Available from: https://doi.org/10.24245/mim.v37i4.3298
- Mekuria Y, Tilahun N. Diabetic peripheral neuropathy among adult type 2 diabetes patients in Adama, Ethiopia: a health-facility-based study. Sci Rep. 2024;14(1):3844. Available from: https://doi.org/10.1038/s41598-024-53951-y
- Salinas L, Bustamante L, Trujillo V, Cuellar C. Diabetic neuropathy: pathophysiology, aetiology and diagnosis. Rev Med Investig UAEMéx. 2020;8(1):1–9. Available from: https://medicinainvestigacion.uaemex.mx/article/view/18819
- Purwanti O, Nursalam N, Pandin G. Early detection of diabetic neuropathy based on the health-belief model: a scoping review. Front Endocrinol. 2024;15:1369699. Available from: https://doi.org/10.3389/fendo.2024.1369699
- Li ZF, Niu XL, Nie LL, Chen LP, Cao CF, Guo L. Diagnostic value of clinical deep tendon reflexes in diabetic peripheral neuropathy. Arch Med Sci. 2020;19(5):1201–6. Available from: https://doi.org/10.5114/aoms.2020.100656
- Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne). 2021;12:671257. Available from: https://doi.org/10.3389/fendo.2021.671257
- Goldenberg DL. Small-fiber neuropathy: clinical scenarios and differential diagnoses. Curr Pain Headache Rep. 2021;21(6):38. Available from: https://www.medcentral.com/rheumatology/fibromyalgia/small-fiber-neuropathy-clinical-scenarios-differential-diagnoses
- Cruz A, Ríos R. Degree of diabetic neuropathy and glycated haemoglobin in diabetic patients at Family Medicine Unit 62. Rev Med Sinergia. 2024;9(8):e1157. Available from: https://revistamedicasinergia.com/index.php/rms/article/view/1157
- Pran L, Baijoo S, Harnanan D, Slim H, Maharaj R, Naraynsingh V. Quality of life experienced by major lower-extremity amputees. Cureus. 2021;13(8):e17440. Available from: https://doi.org/10.7759/cureus.17440
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
