Global Journal of Obesity, Diabetes and Metabolic Syndrome
Assessment of skin autofluorescence in children with diabetes mellitus type
Taranushenko Tatyana Evgenievna1*, Salmin Vladimir Valerievich2-4, Proskurina Margarita Viktorovna5 and Kiseleva Natalya Gennadievna6
2Doctor of Physics and Mathematics. Sciences, Professor of the Department of General Physics, Moscow Institute of Physics and Technology (National Research University) MIPT, Moscow, Russian Federation
3Professor of the Department of Fundamental Sciences-4, Moscow State Technical University named after N.E. Bauman (national research university) MSTU, Moscow, Russian Federation
4Professor of the Department Professor of the Department of Laser Micro-Nano and Biotechnologies; National Research Nuclear University MEPhI; Moscow, Russian Federation
5Graduate student of the Department of Pediatrics, IPO, Krasnoyarsk State Medical University named after Professor V.F. Voino-Yasenetsky, Krasnoyarsk, Russian Federation
6Medical Sciences, Associate Professor, Department of Pediatrics, Institute of Postgraduate Education, Krasnoyarsk State Medical University named after Professor V.F. Voino-Yasenetsky; Krasnoyarsk, Russian Federation
Cite this as
Evgenievna EE, Valerievich SV, Viktorovna PM, Gennadievna KN (2024) Assessment of skin autofluorescence in children with diabetes mellitus type. Glob J Obes Diabetes Metab Syndr 11(1): 009-014. DOI: 10.17352/2455-8583.000065Copyright License
© 2024 Evgenievna EE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: To measure skin autofluorescence in children and adolescents suffering from type 1 diabetes mellitus and evaluate its relationship with gender, age, experience, and chronic complications of the disease.
Materials and methods: The study group included 47 children and adolescents with type 1 diabetes. Autofluorescence of the skin from the inner surface of the shoulder and nail of patients was measured using an original compact spectrofluorimeter based on the STS-VIS OCEAN OPTICS © USA microspectrometer with UVA excitation. Statistical analysis was carried out using StatsoftStatistica 12.0 software. The data is presented as a two-dimensional array. The UV LED signal was averaged and smoothed using the moving average method with a 10 nm window. Then the spectra were renormalized taking into account the found coefficients. The result of applying additional normalization is a decrease in the standard deviation.
Results and discussion: Significant differences were revealed in the skin fluorescence spectra of children of different ages. Between age groups (5-7) and (8-12) is most significant in the region of the alpha band of oxyhemoglobin (540 nm) (p < 0.005). When using I-normalization, the NADH peak region (p < 0.02) is significant with increasing disease duration. When studying the influence of gender factors on the level of skin autofluorescence, the most significant differences are found in the area of only the isosbestic points of deoxy and oxyhemoglobin 442 nm (p < 10-7) and 491 nm (p < 10-8). Significant differences in skin autofluorescence at the reference length were also obtained waves in the autofluorescence spectrum of 500 nm correspond to p < 10-14, depending on the presence of complications.
Conclusion: In Russia, as well as throughout the world, there is an increase in the incidence of type 1 diabetes mellitus. For early diagnosis of changes in carbohydrate metabolism and complications of the disease, a simple, accessible, non-invasive research method is needed. Taking into account the results of our study, when creating non-invasive methods for monitoring the state of carbohydrate metabolism, it is necessary to take into account gender and age characteristics, experience, and the presence of complications of type 1 diabetes mellitus.
Abbreviations
DM 1t: Diabetes Mellitus type 1; DCCT: Diabetes Complications and Control Trial; EDIC: Epidemiology of Diabetes Interventions and Complications; AGEs: Advanced Glycation end Products; SEX: Gender; DD: Duration of Disease; CSII: Continuous Subcutaneous Insulin Infusion; CGM: Continuous Glucose Monitoring; SAP: Autofluorescence Spectra
Introduction
Type 1 Diabetes Mellitus (DM1t) in children and adolescents is a complex medical and social problem.
In 2021, 108,300 children and adolescents under the age of 15 with newly diagnosed type 1 diabetes and 651,700 children and adolescents with DM1t were registered worldwide [1]. The average increase in the incidence of DM1t is on average 3% - 4% per year. The study of the epidemiology of the disease in Russia is witnessing a steady increase in the incidence of type 1 diabetes mellitus. According to the results of the federal register, as of 01.01.2023, the total number of patients with diabetes in Russia who are registered at the dispensary according to the federal register of diabetes mellitus amounted to 4,962,762 people (3.31% of the population of the Russian Federation), of which: SD1 - 5.58% (277.1 thousand), the share of children and adolescents accounted for 48031 people [2]. The results of the clinical trial of DM1t Diabetes Complications and Control Trial (DCCT) and the subsequent Epidemiology of Diabetes Interventions and Complications (EDIC) study confirmed the association of chronic hyperglycemia with the risk of microvascular complications.
To date, it has been proven that the development of endothelial dysfunction is the basis for the development of vascular complications in diabetes mellitus. In addition to hyperglycemia and oxidative stress, the accumulation of end products of excessive glycation (AGE) plays an important role in its progression. The protein glycosylation reaction was first described by L. Maillard in 1913. Glycosylation is a non-enzymatic process in which glucose is combined with the residues of almost all proteins, which leads to a change in their structure and, as a result, function. To date, the process of glycation of hemoglobin has been well-studied. During the short-term incubation of the protein with glucose, intermediate unstable compounds called Schiff bases are formed. When the process continues for up to several weeks, they turn into more stable, but still reversible Amadori products. In the future, long-term hyperglycemia will lead to the conversion of ketomines into end products of excessive glycation (AGE). The end products of excessive glycation are a unique skin marker for diabetes, they accumulate in proteins with a longer half-life. The accumulation of AGE leads to a violation of the barrier function of the vascular wall, the accumulation of reactive oxygen species, the production of proinflammatory cytokines, and a number of other processes that contribute to the development of endothelial dysfunction. Accumulation of AGE in the skin causes an increase in autofluorescence of the skin, which correlates with microcirculatory disorders. The first evidence that SAF reflects the level of AGE in the skin and can be used as a biomarker for diseases associated with their accumulation, primarily in diabetes mellitus, was published in 2004. The study was conducted at the University Medical Center of Groningen, the Netherlands, where the AGE Reader device was created, which was used to measure SAF [3].
The level of autofluorescence of the skin is considered an integral indicator of dysmetabolic shifts in the development of diabetes mellitus, and pathology of the kidneys, brain, endocrine, vascular, and respiratory systems [4-6].
This research method is used in various fields of practical medicine [7,8]. Biopsy is considered the gold standard for measuring the end products of glycation associated with tissues. The advantage of measuring the SAF level is non-invasiveness, simplicity, and correlation with the reference skin biopsy method [9].
According to clinical recommendations, one of the main components of the treatment of DM1t is training in self-control of patients’ glycemia. The only method of preventing the development of microvascular complications is to achieve and maintain optimal glycemic targets [10,11]. Back in 1922, with the beginning of insulin therapy, Elliot Joslin was the first in medical practice to discuss the need to teach patients self-control at home.
A patient’s commitment to self-control depends primarily on the level of pain, accessibility, and simplicity of the glycemic examination method. Therefore, it is still relevant to create a non-invasive, accessible, accurate method for monitoring the state of carbohydrate metabolism.
Purpose of the study
To identify diagnostically significant indicators of skin autofluorescence in children suffering from T1DM depending on indicators such as Age (AGE), Gender (SEX), Disease Duration (DD), and the presence of complications. To evaluate correlations between skin autofluorescence parameters depending on age, duration of the disease, and complications.
Materials and methods
The study was conducted on the basis of FSBI KB No. 51 of the FMBA of Russia, a branch of the FSBI FSNCC of the FMBA of Russia KB No. 42.
All patients signed an informed consent to participate in the study. The study was approved by the Ethics Committee of the Federal State Budgetary Educational Institution of the Russian State Medical University named after Prof. V.F. Voino-Yasenetsky of the Ministry of Health of the Russian Federation (Protocol No. 114 dated 05.10.2022).
The study group included 47 patients with type 1 diabetes mellitus. Of these, the group of children consisted of 29 (61.7%), and the group of teenagers included 18 people (38.3%). At the same time, slightly more than half of the studied were boys 57.4% and 42.5% girls. The average length of illness of patients at the time of examination was 4.47, min. HbA1c 6.0, max 18.7%. All children have been on constant insulin replacement therapy since the disease was detected: 10 patients (21.2%) and 37 people (78.7%) on a syringe pen on continuous subcutaneous insulin infusion (NPII). Growth disorders were noted in one child (2.1%), weight deficiency was detected in 24 cases (51%), and excess weight in 3 cases (6.4%). All the subjects observed during the study period were on continuous glucose monitoring (NMH), with a predominance of flashmonitoring Libra. In 8 cases (17%), chronic complications in the form of diabetic neuropathy were detected in 6 cases, and diabetic nephropathy at the stage of microalbuminuria in two cases. Background diseases in the form of thyroid pathology were found in 9 patients.
Criteria for exclusion from the study group: children with newly diagnosed DM1t, the presence of ketoacidosis at the time of the study, mental illness, and lack of consent to participate in the study.
Statistical analysis was performed using the StatsoftStatistica 12.0 software. Statistically significant differences at p < 0.05.
Autofluorescence (SAF) spectra were recorded from the inner surface of the patient’s shoulder for 30 seconds using an original compact spectrofluorimeter based on the STS-VIS OCEAN OPTICS © USA microspectrometer with UVA excitation generated by an LED (375 nm) [12].
Results
Fluorescence spectra of skin from the inner surface of the forearm, obtained using the device, consist of two wide contours. The first, in the range of 400-700 nm, represents, in fact, the autofluorescence of the skin, and the second 700-820 nm, the spectrum of the UV LED excitation 375 nm, in the second order of diffraction of the diffraction grating (Figure 1).
For further analysis, the fluorescence spectra were normalized to the average value of the UV LED signal and smoothed using the moving average method with a 10 nm window. The resulting normalized spectra are presented in Figure 2.
This method of normalizing spectra will be further called D-normalization.
To more accurately compare the shapes of the spectra, we applied additional normalization. To do this, the average spectrum for the entire group of patients F (λ) was calculated and for each spectrum Fi(λ) the linear regression coefficients ai, bi were calculated using the least squares method so that after subsequent renormalization of the specified spectra were as close as possible to the average:
Then the spectra were renormalized taking into account the found coefficients:
The result of applying additional normalization is a decrease in the standard deviation of Figure 3. This normalization will be called I-normalization.
To assess the capabilities of UV-induced fluorescence spectroscopy in diagnosing the course of diabetes mellitus in children of different ages, we analyzed the significance of differences in fluorescence spectra between different age groups, gender differences, differences associated with the duration of the disease, as well as differences in metabolic indicators of the course of diabetes mellitus: the level of glycated hemoglobin, glycemic variability, average daily glycemia. For analysis, we plotted the dependence of the Z-score of differences between selected pairwise groups on the wavelength obtained using the Mann-Whitney test. Exceeding the specified indicator modulo the critical value of 1.96 corresponds to significant differences at the p < 0.05 level. This approach also allows you to select the most informative spectral regions for further analysis and the method of their normalization.
In the age groups that are traditionally accepted for pediatric practice, we have identified three age groups:
1.5-7 years; 2.8-12 years; 3.13-18 years
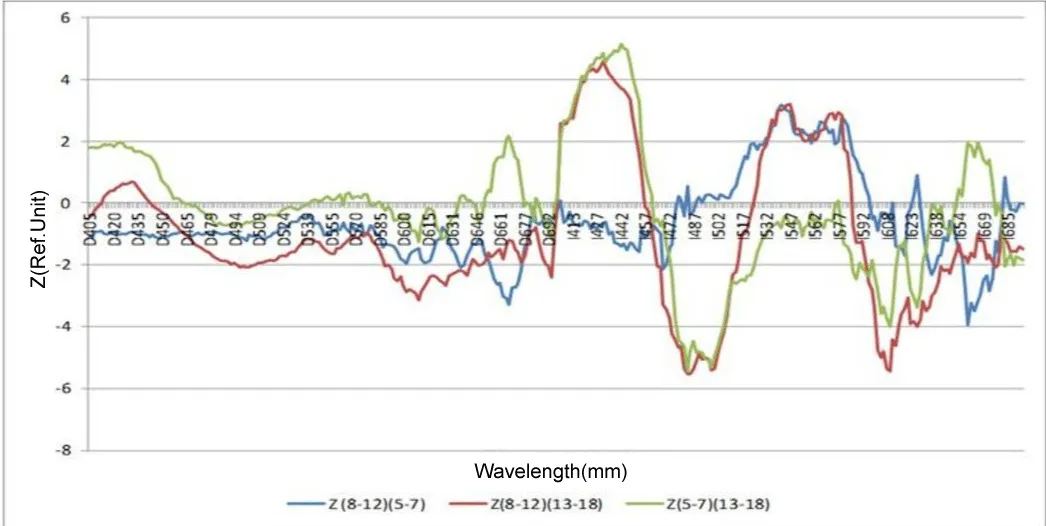
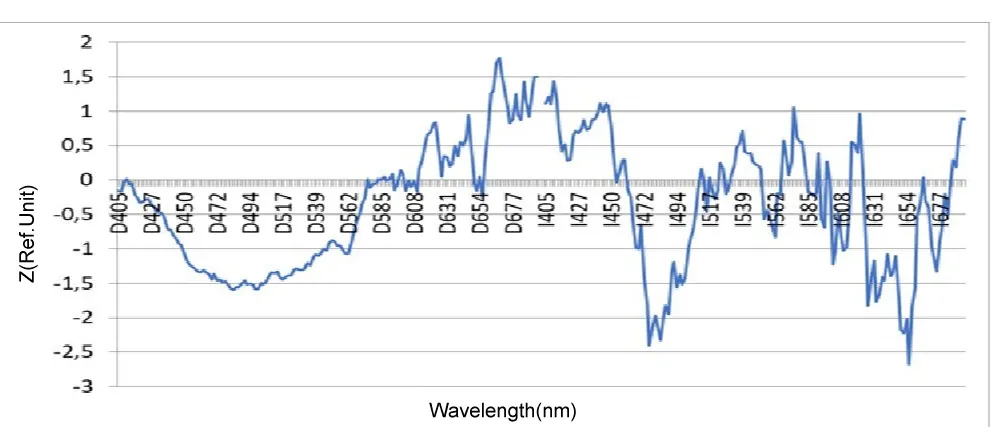
As can be seen from the presented diagram in Figure 4, the most pronounced age-related differences in the skin fluorescence spectra are recorded when using I-normalization, and these differences are maximum in the region of the peak of the Soret band of hemoglobin (410-445 nm) (p < 10-5) as well as alpha hemoglobin bands (520-590 nm) (p < 10-6). Also noteworthy is the presence of a significant difference between the older age group (13-18) years and the two younger ones (5-7) and (8-12) in the region of the fluorescence peak of the main tissue fluorophore NADH (460-510 nm) (p < 10 -7). The difference between age groups (5-7) and (8-12) is most significant in the region of the alpha band of oxyhemoglobin (540 nm) (p < 0.005) (Table 1).
Considering the influence of the duration of type 1 diabetes mellitus in the study group, two subgroups were identified:
1. up to 5 years 2. over 5 years.
In our study, as can be seen in the diagram in Figure 5, significant differences in the spectra are also found when using I-normalization, and just as for comparing age groups, the NADH peak area (p < 0.02) is significant, which confirms our assumption of an increase in hypoxic changes in the skin with increasing duration of type 1 diabetes in children (Table 2).
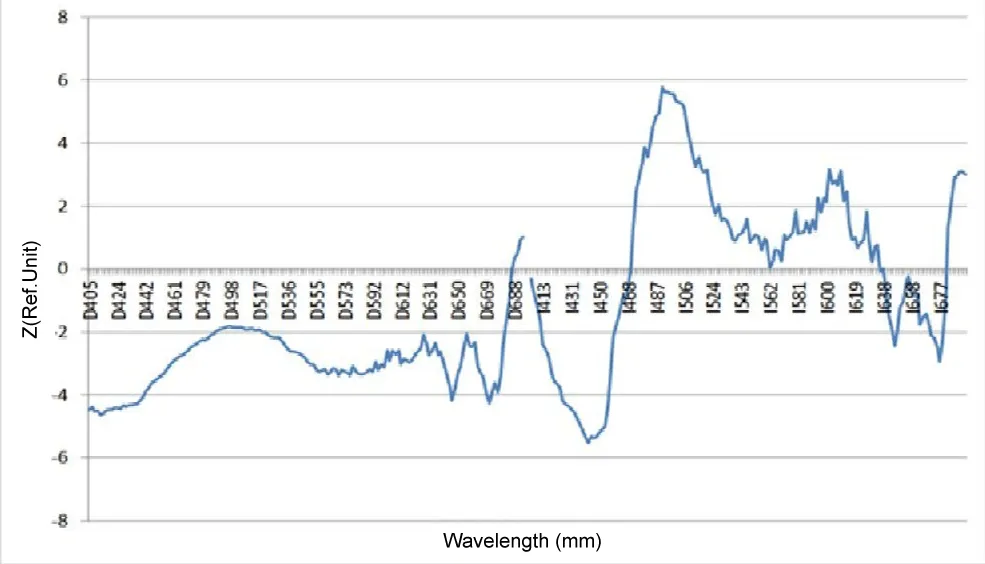
In addition, the work studied the influence of gender as one of the factors influencing the level of skin autofluorescence in diabetes mellitus. Not many studies have been devoted to studying the influence of gender factors on the level of autofluorescence in diabetes mellitus; their contribution is about 0.4% - 1.9%.
Based on the results of our work, a diagram demonstrating gender differences in skin fluorescence spectra is shown in Figure 6. As follows from the presented diagram, gender differences are significant with both types of spectra normalization, but also, as before, they become most significant with I-normalization (Table 3).
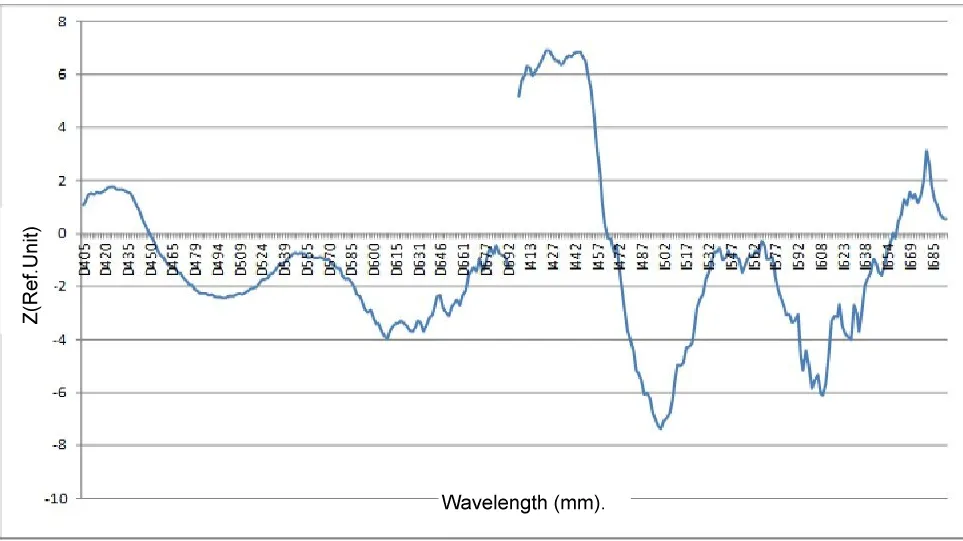
We also analyzed changes in skin autofluorescence in children and adolescents suffering from T1DM depending on the presence of microvascular complications.
As follows from the diagram above, significant areas of the spectrum that distinguish different groups of glycemic variability are present in both normalization methods.
As can be seen from the presented analyses, the most significant differences detected by spectrofluorimetry methods correspond to groups with complications of diabetes mellitus in comparison with their absence. The reference wavelength in the autofluorescence spectrum of 500 nm corresponds to p < 10-14, which can be characterized as a very sensitive criterion for assessing the development or course of complications (Figure 7) (Table 4).
Discussion
Thus, in children of different ages with type 1 diabetes mellitus, there are significant age-related differences in the skin fluorescence spectra. According to most authors, age is the most important factor influencing SAF, ranging from 23.8–28.5% [13-16]. These differences may be due to age-related anatomical changes in the skin, changes in total hemoglobin, and the development of irreversible microcirculatory changes, accompanied by an increase in hypoxic changes in the skin. Taking into account the most significant parts of the spectrum, we determined the intensities at the indicated wavelengths and compared the fluorescence intensities.
When studying the effect of diabetes experience on the level of autofluorescence, attention is drawn to the significant spectral region of 656 nm (p <0.007), which may be associated with the deposition of protoporphyrins in the skin [17].
The bulk of the work has proven a direct connection between the length of T1DM disease and the level of skin autofluorescence [18-21].
When considering the results of the identified publications, a gender-specific feature of SAF was revealed: the level was higher in girls compared to boys with diabetes mellitus [22-25].
In our study, the most significant gender differences are found in the region of only the isosbestic points of deoxy and oxyhemoglobin 442 nm (p < 10-7) higher in females and 491 nm (p < 10-8) in male patients, which is probably due to different level of total hemoglobin between the sexes.
When analyzing the level of skin autofluorescence depending on the presence of microvascular complications, a more pronounced structure of the spectrum is realized when using I-normalization. The most pronounced deviations in the spectrum of Z-scores are observed in the region of the Soret band of hemoglobin 424 nm (isosbestic point of oxy- and deoxyhemoglobin), 500 nm (also isosbestic point of oxy- and deoxyhemoglobin at the peak of NADH luminescence (the most significant changes), 610 nm - not interpreted peak. According to publications, the accumulation of glycation products in the skin of patients suffering from T1DM is described and their correlation with the progression of the disease is proven [26-29].
Conclusion
In our work, we found a significant increase in the level of autofluorescence in the skin of patients suffering from T1DM with age, duration of diabetes, female gender, and the presence of microvascular complications.
The detected connection between these same chromophores and fluorophores with gender and age differences and the duration of the disease should be taken into account when developing these methods.
It should be noted that the discovered relationship between fluorescence intensity at reference wavelengths corresponding to the main chromophores and fluorophores of the skin can be used in the development of non-invasive methods for analyzing laboratory parameters during diabetes mellitus.
- Liebman I, Haines A, Lyons S, Pradeep P, Rvagasor E, Tung Ji Yu, Jeffries SA, Oram RA, Dabelea D, Craig Ya. ISPAD Consensus Recommendations for Clinical Practice 2022: Definition, epidemiology and classification of diabetes mellitus in children and adolescents. Pediatr Diabetes. 2022; 23(8):1160-1174. doi: 10.1111/pedi.13454.
- And Grandfathers I, Shestakova M, Vikulova OK, Zheleznyakova AV, Isakov MA, Sazonova DV, Mokrysheva NG. Diabetes mellitus in the Russian Federation: dynamics of epidemiological indicators according to the Federal Register of Diabetes Mellitus for the period 2010-2022. Diabetes Mellitus. 2023; 26(2):104-123.
- Papachristou S, Pafili K, Papanas N. Skin AGEs and diabetic neuropathy. BMC Endocr Disord. 2021 Feb 23;21(1):28. doi: 10.1186/s12902-021-00697-7. PMID: 33622304; PMCID: PMC7903740.
- Popyhova EB, Stepanova TV, Lagutina DD, Kiriiazi TS, Ivanov AN. [The role of diabetes in the onset and development of endothelial dysfunction]. Probl Endokrinol (Mosk). 2020 Aug 4;66(1):47-55. Russian. doi: 10.14341/probl12212. PMID: 33351312.
- Leonova TS, Vikhnina MV, Grishina TV. The effect of the end products of deep glycation on cellular processes. Int Sci Res J. 2018; 12(78). doi: 10.23670/IRJ.2018.78.12.034
- Hosseini MS, Razavi Z, Ehsani AH, Firooz A, Afazeli S. Clinical Significance of Non-invasive Skin Autofluorescence Measurement in Patients with Diabetes: A Systematic Review and Meta-analysis. EClinicalMedicine. 2021 Nov 16;42:101194. doi: 10.1016/j.eclinm.2021.101194. PMID: 34841236; PMCID: PMC8605318.
- Perrone A, Giovino A, Benny J, Martinelli F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid Med Cell Longev. 2020 Mar 18;2020:3818196. doi: 10.1155/2020/3818196. PMID: 32256950; PMCID: PMC7104326.
- Passanisi S, Salzano G, Lombardo F. Skin Involvement in Paediatric Patients with Type 1 Diabetes. Curr Diabetes Rev. 2022;18(4):e030921196145. doi: 10.2174/1573399817666210903153837. PMID: 34477525.
- Stirban AO, Bondor CI, Florea B, Veresiu IA, Gavan NA. Skin autofluorescence: Correlation with measures of diabetic sensorimotor neuropathy. J Diabetes Complications. 2018 Sep;32(9):851-856. doi: 10.1016/j.jdiacomp.2018.06.014. Epub 2018 Jul 5. PMID: 30025785.
- Dedov II, Shestakova MV, Mayorov AYu, editors. Algorithms of specialized care for patients with diabetes mellitus. Issue 11. Moscow; 2023; 236.
- Peterkova VA, Bezlepkina OB, Laptev DN, et al. Diabetes mellitus in children. Clinical recommendations approved by the Scientific and Practical Council of the Ministry of Health of the Russian Federation. Moscow; 2022; 91.
- Salmin VV, Taranushenko TE, Kiseleva NG, Salmina AB. Noninvasive determination of serum sugar and glycated hemoglobin levels using UV-induced skin fluorescence. In: Biomedical Photonics for Diabetes Research. CRC Press; 2022; 155-176.
- Atzeni IM, van de Zande SC, Westra J, Zwerver J, Smit AJ, Mulder DJ. The AGE Reader: A non-invasive method to assess long-term tissue damage. Methods. 2022 Jul;203:533-541. doi: 10.1016/j.ymeth.2021.02.016. Epub 2021 Feb 23. PMID: 33636313.
- Januszewski AS, Xu D, Cho YH, Benitez-Aguirre PZ, O'Neal DN, Craig ME, Donaghue KC, Jenkins AJ. Skin autofluorescence in people with type 1 diabetes and people without diabetes: An eight-decade cross-sectional study with evidence of accelerated aging and associations with complications. Diabet Med. 2021 Jul;38(7):e14432. doi: 10.1111/dme.14432. Epub 2020 Nov 8. PMID: 33078416.
- Jiang T, Zhang Y, Dai F, Liu C, Hu H, Zhang Q. Advanced glycation end products and diabetes and other metabolic indicators. Diabetol Metab Syndr. 2022 Jul 25;14(1):104. doi: 10.1186/s13098-022-00873-2. PMID: 35879776; PMCID: PMC9310394.
- Choi LS, Ahmed K, Kim YS, Yim JE. Skin accumulation of advanced glycation end products and cardiovascular risk in Korean patients with type 2 diabetes mellitus. Heliyon. 2022 Jun 2;8(6):e09571. doi: 10.1016/j.heliyon.2022.e09571. PMID: 35711980; PMCID: PMC9192809.
- Fauaz G, Miranda AR, Gomes CZ, Courrol LC, Silva FR, Rocha FG, Schor N, Bellini MH. Erythrocyte protoporphyrin fluorescence as a potential marker of diabetes. Appl Spectrosc. 2010 Apr;64(4):391-5. doi: 10.1366/000370210791114248. PMID: 20412623.
- Shah S, Baez EA, Felipe DL, Maynard JD, Hempe JM, Chalew SA. Advanced glycation endproducts in children with diabetes. J Pediatr. 2013 Nov;163(5):1427-31. doi: 10.1016/j.jpeds.2013.06.044. Epub 2013 Aug 3. PMID: 23919908.
- Genevieve M, Vivot A, Gonzalez C, Raffaitin C, Barberger-Gateau P, Gin H, Rigalleau V. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab. 2013 Sep;39(4):349-54. doi: 10.1016/j.diabet.2013.03.003. Epub 2013 May 2. PMID: 23643347.
- Tomaszewski EL, Orchard TJ, Hawkins MS, Conway RBN, Buchanich JM, Maynard J, Songer T, Costacou T. Predictors of Change in Skin Intrinsic Fluorescence in Type 1 Diabetes: The Epidemiology of Diabetes Complications Study. J Diabetes Sci Technol. 2021 Nov;15(6):1368-1376. doi: 10.1177/19322968211014337. Epub 2021 May 15. PMID: 33993770; PMCID: PMC8655295.
- Banser A, Naafs JC, Hoorweg-Nijman JJ, van de Garde EM, van der Vorst MM. Advanced glycation end products, measured in skin, vs. HbA1c in children with type 1 diabetes mellitus. Pediatr Diabetes. 2016 Sep;17(6):426-32. doi: 10.1111/pedi.12311. Epub 2015 Sep 2. PMID: 26332801.
- Muk-Kanamori MU, Selim MM, Takiddin A, Al-Homsi H, Al-Mahmud K, Al-Obaidli A, Ziri MA, Rowe J, Herbie US, Chidiak OM, Kader SA, Al Muftah UA, McKeon S, Suhre K, Muk-Kanamori DO. Ethnic and gender differences in glycation end products obtained by skin autofluorescence. Dermatoendocrinol. 2013;5(2):325-30. doi: 10.4161/derm.26046.
- Boersma HE, van der Klauw MM, Smit AJ, Wolffenbuttel BHR. A non-invasive risk score including skin autofluorescence predicts diabetes risk in the general population. Sci Rep. 2022 Dec 16;12(1):21794. doi: 10.1038/s41598-022-26313-9. PMID: 36526712; PMCID: PMC9758123.
- Felipe DL, Hempe JM, Liu S, Matter N, Maynard J, Linares C, Chalew SA. Skin intrinsic fluorescence is associated with hemoglobin A(1c )and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care. 2011 Aug;34(8):1816-20. doi: 10.2337/dc11-0049. Epub 2011 Jun 2. Erratum in: Diabetes Care. 2013 Apr;36(4):1056. PMID: 21636794; PMCID: PMC3142049.
- Forbes DM, Le Bagge S, Rigi S, Fotheringham AK, Gallo LA, McCarthy DA, Leung S, Baskerville T, Nisbett J, Morton A, Tisdale S, D'Silva N, Barrett H, Jones T, Cooper J, Donahue K, Isbel N, Johnson DW, Donnellan L, Deo P, Akison LK, Moritz KM, O'Moore-Sullivan T. End products of increased glycation as predictors of kidney function in young people with type 1 diabetes mellitus. Sci Rep. 2021;11(1):9422. doi: 10.1038/s41598-021-88786-4.
- Christidis G, Kuppers F, Karataili SK, Karataili EW, Weber SN, Lammert F, Kravchik M. End products of glycation in the skin as indicators of the metabolic profile in diabetes mellitus: correlation with glycemic control, liver phenotypes and metabolic biomarkers. Endocr Imbalance BMC. 2024 Mar 5;24(1):31. doi: 10.1186/s12902-024-01558-9.
- Papachristou S, Paphili K, Papanas N. Skin age and diabetic neuropathy. Endocr Imbalance BMC. 2021 Feb 23;21(1):28. doi: 10.1186/s12902-021-00697-7.
- Ducos S, Rigaud M, Larrume A, Delifer MN, Korobelnik YF, Monlan M, Fussar N, Pupon P, Haissaguerre M, Blanco L, Mohammadi K, Rigallo V. Diabetic retinopathy in well-controlled type 2 diabetes mellitus: the role of glycemic memory. Diabetes Metab. 2021 Feb;47(1):101156. doi: 10.1016/j.diabet.2020.03.005.
- Wan L, Qin G, Yan W, Sun T. Skin Autofluorescence Is Associated with Diabetic Peripheral Neuropathy in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Genet Test Mol Biomarkers. 2019 Jun;23(6):387-392. doi: 10.1089/gtmb.2018.0328. PMID: 31161820; PMCID: PMC6555182.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
