Archives of Clinical Gastroenterology
Antiulcerogenic activity of species Annona coriacea Mart. and Spiranthera odoratissima A. St. Hil
Fernanda Neves Estrela1, Keise Rodrigues Silva1, Álefe Cardoso Cruz1, Patrícia Fernandes de Souza1, Leonardo Oliveira Costa2, João Gabriel Moraes Junqueira2, Geraldo Sadoyama Leal1, Lucia de Paula1, Helder Nagai Consolaro1, Ana Paula Terezan2, Vanessa Gisele Pasqualotto Severino3 and Anderson Luiz-Ferreira1*
2Special Academic Unit of Physics and Chemistry, Federal University of Goiás (UFG), CEP 75704-020, Catalão, GO, Brazil
3Department of Organic Chemistry, Institute of Chemistry, Federal University of Goiás (UFG), CEP 74690-900, Goiânia, GO, Brazil
Cite this as
Estrela FN, Silva KR, Cruz AC, de Souza PF, Costa LO, et al. (2017) Antiulcerogenic activity of species Annona coriacea Mart. and Spiranthera odoratissima A. St. Hil. Arch Clin Gastroenterol 3(3): 080-084. DOI: 10.17352/2455-2283.000045Background: Medicinal plants from the Brazilian Cerrado are used in folk medicine to treat several diseases such as gastric disorders.
Purpose: The present work evaluated the antiulcerogenic and antimicrobial effects of the ethanolic extracts obtained from leaves of two Cerrado plant species.
Methods: The action of ethanolic extracts of Spiranthera odoratissima (SOL) and Annona coriacea (ACL) were evaluated in experimental in vivo models in rats that simulated this disease in human gastric mucosa. Additionally, a pharmacological study to test antimicrobial activity against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa were also evaluated by microdilution methods.
Results: The pretreated with SOL (100, 200 and 300 mg/kg, p.o.) promoted significant inhibition of mucosal injury (93, 99 and 98% inhibition; respectively). This inhibition also was detected in ACL treatment (50, 100, 200 and 300 mg/kg; 67, 52, 48 and 67% inhibition, respectively). The both extracts SOL (100 mg/kg) and ACL (50 mg/kg) also reduced significantly the formation of gastric lesions induced by indomethacin (73 and 63%, respectively) when compared to animals treated with respective vehicle. The phytochemical profile from SOL and ACL indicated the presence alkaloids as a main constituent of these extracts. ACL but not displayed antimicrobial activities in vitro.
Conclusion: Spiranthera odoratissima and Annona coriacea species present gastroprotective activity, supporting previous claims that its traditional use can treat gastrointestinal disorders.
Introduction
The peptic ulcers are a common disorder of the entire gastrointestinal tract that occur mainly in the stomach and the proximal duodenum. Traditionally, a hypersecretory acidic environment together with dietary factors or stress were thought to cause most peptic ulcer diseases, however the Helicobacter pylori infection and the widespread use of non-steroidal anti-inflammatory drugs (NSAIDs) are considered the main cause today [1].
Numerous natural products derived from plant sources have been evaluated as therapeutics for the treatment of various diseases, including peptic ulcers. In fact, several studies have been shown antiulcerogenic activities of medicinal plants [2-4].
Spiranthera odoratissima A. St.-Hil., (Rutaceae) is popularly known as “manacá” and used in folk medicine in the Brazil, for the treatment of several diseases such as renal and hepatic diseases, headaches, rheumatism and stomachache [5,6].
Annona coriacea Mart. (Annonaceae), popularly known as “marolo”, has been used as folk medicine for treating parasitoses, inflammation processes and ulcers [7,8].
Although, ethnopharmacology studies reveals that folk medicinal practices have been used to treat many illness, many still have not been subjected to scientific study to confirm their effectiveness [9]. Taking into account current concerns over the side effects that are often inevitable and limit clinical utility to treat gastric ulcer [10], the screening of potential new antiulcerogenic agents as therapeutic alternatives is an important issue. Therefore, we aimed to explore gastroprotective effects promoted by ethanolic extracts obtained from two species, used in folk medicine. Additionally, we undertook evaluation study of the antimicrobial activity.
Materials and Methods
Animals
Male Wistar rats (n = 7, 150–250 g) from Central Animal House of the Federal University of Uberlândia (CEBA-UFU; Uberlândia, Brazil) were used. The animals were fed a certified Presence (Purina®) diet with free access to tap water under standard conditions of 12 h dark – 12 h light, humidity (60 ± 1.0%) and temperature (21 ± 1°C). Fasting was used prior to all assays because standard drugs or extract treatment were always administered orally (by gavage). Moreover, the animals were kept in cages with raised floors of wide mesh to prevent coprophagy. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Federal University of Goiás (CEUA/UFG, no. 001/14).
Material plant
Both species were collected and identified by Dr. Helder Nagai Consolaro of Universidade Federal de Goiás (UFG). The Spiranthera odoratissima leaves (SOL) were collected in Julio Garcia Road (Brasilia, DF, Brazil) in June 2003, a Cerrado region, and a voucher was deposited under the number UB 5114 at Brasília University Herbarium (Brasília, DF, Brazil). The Annona coriacea leaves (ACL) were collected in the dependencies of the Federal University of Goiás, in June 2003, which is situated in a Cerrado region. The voucher was deposited under the number 47919 at Federal University of Goiás (Goiás, GO, Brazil).
Preparation of the extract and Nuclear Magnetic Resonance (NMR) analysis
The leaves from S. odoratissima (2.3 kg) and A. coriacea (0.62 kg) were dried carefully by forced air at 45 °C and reduced to powder. The powdered dried leaves were extracted with EtOH (3×9L, 7 days each) at room temperature and filtered. The filtered material was concentrated to yield the crude leaves extracts (270 g SOL and 57.5 g ACL).
The ethanolic extracts from leaves of S. odoratissima and A. coriacea were prepared with deutered solvent for nuclear magnetic resonance (NMR) analysis. The 1H NMR spectrum was obtained using Bruker DRX-500MHz spectrometer with dimethyl sulfoxide –d (DMSO-d) using TMS as internal standard.
Drugs and chemicals
The following drugs were used: Tween 80® (Sinth, SP, Brazil), absolute ethanol (Merk, Darmstadt, Germany); cimetidine, carbenoxolone and indomethacin were from Sigma Chemical Co. (St. Louis, USA). The chemicals used in the buffers and other solutions were all of analytical grade. All drugs and reagents were prepared immediately before use.
Antiulcerogenic activity
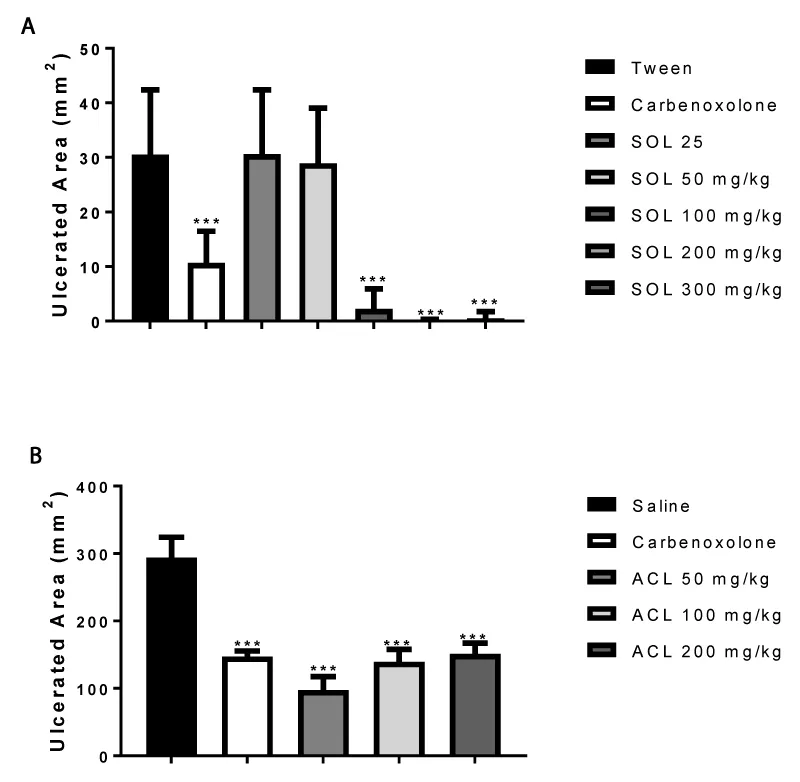
Ethanol-induced ulcer: After fasting for 24 h, the experimental groups were submitted to the treatments (p.o.) with vehicle (Tween 80 or saline; 10 mL/Kg), carbenoxolone (100 mg/Kg; positive control), SOL and ACL (25, 50, 100, 200 and 300 mg/Kg) 1 h before induction of gastric injury by absolute ethanol. Animals were euthanized, by CO gas, 1 h after ethanol administration (1mL); the stomachs were opened along the greater curvature, pressed onto a glass plate, and scanned. So that the lesions could be counted aided by the AVSoft program [2]. The results were expressed as total ulcerated area (mm2) [11].
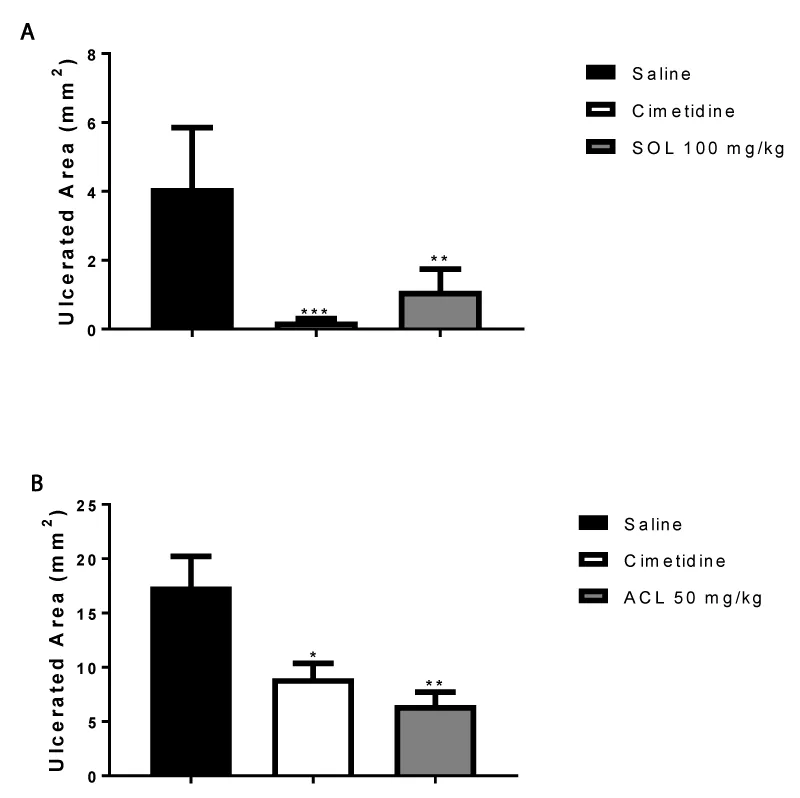
Nonsteroidal anti-inflammatory drugs (NSAID)-induced ulcer: Animals were fasted for 24 h. The gastric injuries were induced by oral administration of indomethacin 30 mg/Kg in male rats. The treatments (p.o.) with vehicle (Tween 80 or saline; 10 mL/Kg), cimetidine (100 mg/Kg; positive control), and SOL (100 mg/Kg) and ACL (50 mg/Kg) were carried out 30 min before administration of the NSAID. In this model, only the most effective doses of each species were tested. Four hours after the NSAID administration the animals were euthanized by CO gas and the stomachs were removed for lesion quantification [12].
Antimicrobial activity
Microbial susceptibility assays of the SOL and ACL using the Minimal Inhibitory Concentration (MIC) by microdilution in broth, was described by Clinical and Laboratory Satandards Institute [13]. The extracts were sterilized by filtration in nylon syringe filter 33 mm 0.45 µm and tested for successive 1:2 dilutions range 0.25 to 1024 μg/mL. The S. aureus ATTC 29213, E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were incubate in inoculated microdilution trays at 35±2°C for 16 to 20 hours. The MIC is the lowest concentration of antimicrobial agent that completely inhibits growth of the organism in the microdilution wells.
Statistical analysis
Results were expressed as mean ± S.E.M. and statistical significance was determined using the graphed Prism Software version 7.0 (GraphPad Software, Inc., San Diego, CA) by one-way analysis of variance followed by Tukey’s test with p < 0.05 defined as significant.
Results and Discussion
Natural products have been a rich source of compounds for drug discovery and the interest in natural products derived from medicinal plants has increased significantly worldwide [14,15]. Medicinal plants have made many contributions to commercial drug preparations manufactured today including ephedrine from Ephedra sinica, digitoxin from Digitalis purpurea, salicin from Salix alba and reserpine from Rauwolfia serpentina [16].
The animal models are useful to understand the pathogenesis of gastrointestinal tract and also to identify potential therapeutic agents, including for gastric ulcer. The two models used here, Ethanol and NSAIDs, are relevant because they mimic the ulcers present in humans.
The effects of the SOL and ACL on gastric ulceration induced by different necrotizing agents in gastric mucosa are summarized in figures 1,2.
Ethanol causes necrosis of superficial epithelial cells on gastric mucosa and erosion. In addition, it promotes damage in the mucosa result of decreased of the mucus [17,18].
The effects of SOL and ACL on ethanol model were tested in rats, and carbenoxolone, a stimulatory agent of the gastric mucus secretion, was used as a positive control. Oral administration of absolute ethanol led to severe lesions in the gastric mucosa in rats. On the other hand, in the SOL (100, 200 and 300) and ACL (50, 100, 200 and 300)-treated groups, protect the gastric mucosal lesions by inhibiting 93, 99 and 98% respectively 67, 52, 48 and 67%. Compared with the control group, the carbenoxolone group reduced the gastric lesions (65%).
Considering that the pathophysiology of this model is involved cytoprotection [3,11], SOL and ACL may increase the secretion levels of mucin in the stomach.
The gastric cytoprotection, which consists of mucus and bicarbonate secretion to the gastric lumen; the prostaglandins mediate various physiological aspects of this mucosal defense. Thus, its suppression in the stomach is a critical event to development of mucosal injury after administration of NSAID. The gastric damage is attributed to decreased mucus secretion, mucosal blood flow and bicarbonate secretion, enhanced acid back-diffusion, and inhibition of mucosal injury repair [2,3].
In NSAIDs-model, the lower effective dose for both species, SOL (100 mg/kg) and ACL (50 mg/kg) significantly reduced ulcer occurrence in the NSAIDs induced model (73 and 63% inhibition; respectively), supporting its cytoprotective effect, which may be mediated by prostaglandin production (Figure 2). Other species of the genus Annona (Annona muricata L. and Annona squamosa) were active against gastric ulcers, increased the mucus secretion and PGE production [19,20].
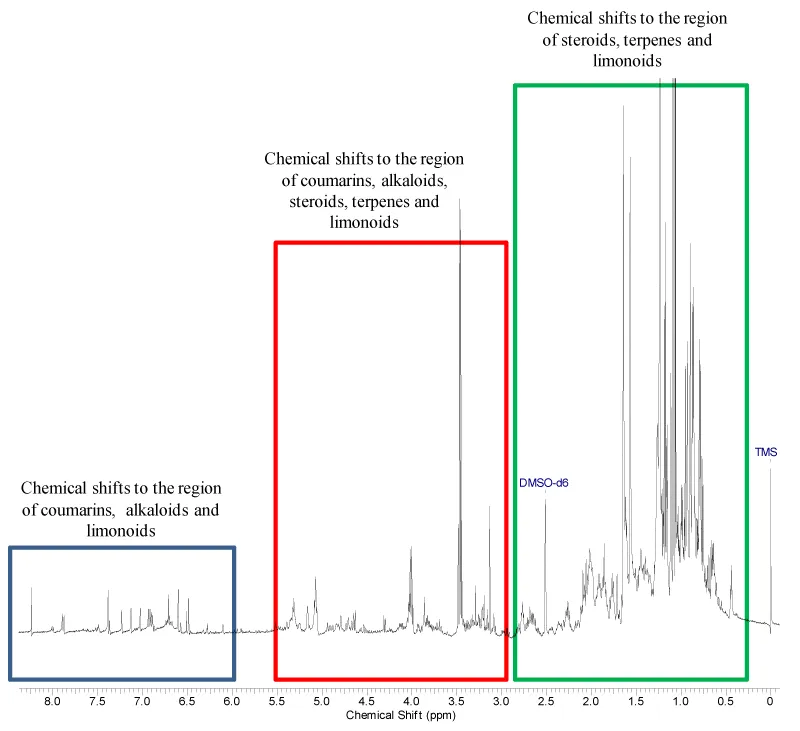
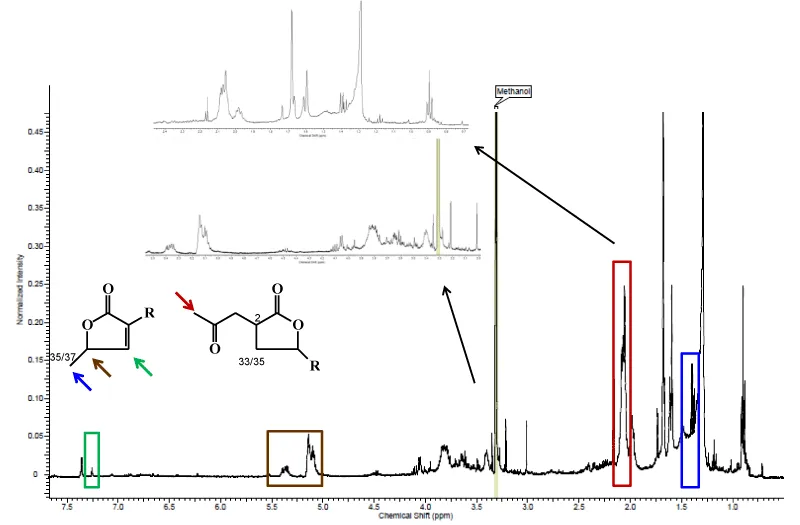
The identification by 1H NMR of secondary metabolites classes present on the ethanolic extracts from leaves of S. odoratissima and A. coriacea was based on characteristic chemical shifts (ppm) of them and literature data of isolated compounds from these species.
The qualitative phytochemical tests revealed the presence of alkaloids and nitrogen heterocyclic as a main constituent from ACL (Figure 3) and alkaloids, nitrogen heterocyclic and terpenoids from SOL (Figure 4). It is important to mention that several alkaloids present anti-ulcer activity [21]. Toma et al. [22], showed that the alkaloids present in Senecio brasiliensis are able to increase mucus secretion and prostaglandin production in gastric mucosa. Zanatta et al. [23], also showed which alkaloids from Galipea longiflora were able increase of mucus secretion.
There is a high prevalence of the non-H. pylori bacteria concurrent with H. pylori infection, and the non-H. pylori bacteria may also play important as-yet-undiscovered roles in the pathogenesis of stomach disorders [24]. E. coli infection promotes increased apoptotic activity of gastric cells [25]. Additionally, the most common intestinal infections that induce acute watery diarrhea in approximately 80% of people are caused by Escherichia coli and Staphylococcus aureus [26].
In this study, we also evaluated the in vitro antimicrobial activity of SOL and ACL. The antimicrobial assays showed that the ethanolic extract from leaves of S. odoratissima does not have antimicrobial activity presenting inhibitory concentrations ≥1000 μg/ml for the Gram-positive and Gram-negative bacteria. On the other hand, the ethanolic extract from leaves of A. coriacea showed antibacterial activity against Gram-positive bacteria (MIC = 256 μg/ml against Staphylococcus aureus) and Gram-negative bacteria (MIC = 512 against Pseudomonas aeruginosa) and (MIC = 512 against Escherichia coli) (Table 1). These results are in agreement with that found in the literature with other Annona species, Castillo-Juárez and collaborates (2009) showed that the Annona cherimola species has a high inhibitory effect of Helicobacter pylori.
Conclusions
The data obtained from ethanolic extracts from leaves of S. odoratissima and A. coriacea confirmed the traditional medicinal uses for the gastrointestinal disorders. These results further suggest the potential use of both species for its gastroprotective activities, probably by the action of alkaloids present in the composition that activated the defense mechanism of the gastric mucosa against aggressive factors. In regard to antimicrobial activity, only ACL was able to inhibit the growth of important bacteria in the pathogenesis of stomach disorders. In view of our results, more investigation of their function could provide insight into the mechanism of gastric cytoprotection and the role of active compounds presents in SOL and ACL.
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development (CNPq–grant number 010977/2013-9, 2014).
- Lanas A, Chan FKL (2017) Peptic ulcer disease. Lancet 390: 613-624. Link: https://goo.gl/fm8dd3
- Barbastefano V, Cola M, Luiz-Ferreira A, Farias-Silva E, Hiruma-Lima CA, et al. (2007) Vernonia polyanthes as a new source of antiulcer drugs. Fitoterapia 78: 545-551. Link: https://goo.gl/r9hxbV
- Luiz-Ferreira A, Almeida AC, Cola M, Barbastefano V, Almeida AB, et al. (2010) Mechanisms of the gastric antiulcerogenic activity of Anacardium humile St. Hil on ethanol-induced acute gastric mucosal injury in rats. Molecules 15: 7153-7166. Link: https://goo.gl/JAkxbw
- Périco LL, Heredia-Vieira SC, Beserra FP, de Cássia Dos Santos R, Weiss MB, et al. (2015) Does the gastroprotective action of a medicinal plant ensure healing effects? An integrative study of the biological effects of Serjania marginata Casar. (Sapindaceae) in rats. J Ethnopharmacol 172: 312-324. Link: https://goo.gl/vvqQ48
- Nascimento MV, Galdino PM, Florentino IF, de Brito AF, Vanderlinde FA, et al. (2012) Anti-inflammatory effect of Spiranthera odoratissima A. St.-Hil. leaves involves reduction of TNF-α. Nat Prod Res 26: 2274-2279. Link: https://goo.gl/VV6C7a
- Galdino PM, Nascimento MV, Florentino IF, Lino RC, Fajemiroye JO, et al. (2012) The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog Neuropsychopharmacol Biol Psychiatry 38: 276-284. Link: https://goo.gl/dmoZFK
- Sonnet PE, Jacobson M (1971) Tumor inhibitors. II. Cytotoxic alkaloids from Annona purpurea. J Pharm Sci 60: 1254-1256. Link: https://goo.gl/k65XG1
- Castillo-Juárez I, González V, Jaime-Aguilar H, Martínez G, Linares E, et al. (2009) Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J Ethnopharmacol 122: 402-405. Link: https://goo.gl/jTWcpT
- Cartaxo SL, Souza MM, de Albuquerque UP (2010) Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil J Ethnopharmacol 131: 326–342. Link: https://goo.gl/TwBhGT
- Bi WP, Man HB, Man MQ (2014) Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol 20: 17020-17028. Link: https://goo.gl/VbTeJq
- Morimoto Y, Shimohara K, Oshima S, Sukamoto T (1991) Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn J Pharmacol 57: 495–505. Link: https://goo.gl/t9e5ba
- Hayden LJ, Thomas G, West GB (1978) Inhibitors of gastric lesions in the rat. J Pharm Pharmacol 30: 244–246. Link: https://goo.gl/3rq4vf
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. CLSI document M07-A9. USA, 2012. Link: https://goo.gl/Zd8xri
- Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14: 111-129. Link: https://goo.gl/Q97Rbr
- Dutra RC, Campos MM, Santos AR, Calixto JB (2016) Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res 112: 4-29. Link: https://goo.gl/LAaBGE
- Rastogi S, Pandey MM, Rawat AK (2016) Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 23: 1082-1089. Link: https://goo.gl/UGTmon
- Abdel-Salam OM, Czimmer J, Debreceni A, Szolcsányi J, Mózsik G (2001) Gastric mucosal integrity: gastric mucosal blood flow and microcirculation. An overview. J Physiol 95: 105–127. Link: https://goo.gl/UiyECs
- de-Faria FM, Almeida AC, Luiz-Ferreira A, Takayama C, Dunder RJ, et al. (2012) Antioxidant action of mangrove polyphenols against gastric damage induced by absolute ethanol and ischemia-reperfusion in the rat. ScientificWorldJournal 2012: 327071. Link: https://goo.gl/FCv6Qw
- Yadav DK, Singh N, Dev K, Sharma R, Sahai M, et al. (2011) Anti-ulcer constituents of Annona squamosa twigs. Fitoterapia 82: 666-675. Link: https://goo.gl/CZ9XYX
- Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Abdulla MA, et al. (2014) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8: 2099-2110. Link: https://goo.gl/1mftWG
- de Sousa Falcão H, Leite JA, Barbosa-Filho JM, de Athayde-Filho PF, de Oliveira Chaves MC, et al. (2008) Gastric and duodenal antiulcer activity of alkaloids: a review. Molecules 13: 3198-3223. Link: https://goo.gl/84u2UU
- Toma W, Trigo JR, Bensuaski de Paula AC, Monteiro Souza Brito AR (2004) Modulation of gastrin and epidermal growth factor by pyrrolizidine alkaloids obtained from Senecio brasiliensis in acute and chronic induced gastric ulcers. Can J Physiol Pharmacol 82: 319-325. Link: https://goo.gl/FFDY2s
- Zanatta F, Gandolfi RB, Lemos M, Ticona JC, Gimenez A, et al. (2009) Gastroprotective activity of alkaloid extract and 2-phenylquinoline obtained from the bark of Galipea longiflora Krause (Rutaceae). Chem Biol Interact 180: 312-317. Link: https://goo.gl/4XNnSk
- Hu Y, He LH, Xiao D, Liu GD, Gu YX, et al. (2012) Bacterial flora concurrent with Helicobacter pylori in the stomach of patients with upper gastrointestinal diseases. World J Gastroenterol 18: 1257-1261. Link: https://goo.gl/fe3d4T
- Durkin E, Moran AP, Hanson PJ (2006) Apoptosis induction in gastric mucous cells in vitro: lesser potency of Helicobacter pylori than Escherichia coli lipopolysaccharide, but positive interaction with ibuprofen. J Endotoxin Res 12: 47-56. Link: https://goo.gl/eoeG8o
- Hunter PA, Dawson S, French GL, Goossens H, Hawkey PM, et al. (2010) Antimicrobial-resistant pathogens in animals and man: prescribing practices and policies. J Antimicrob Chemother 65: 13–17. Link: https://goo.gl/KzqqJw

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
