Journal of Clinical Research and Ophthalmology
A rare case of bilateral acute retinal necrosis due to varicella zoster virus in a patient of Multiple myeloma
Priyanka1*, Jyotirmay Biswas2 and Bhavana Sharma3
2Senior consultant and Head, Department of Uveitis and Ocular Pathology, Sankara Nethralaya, Chennai, Tamil Nadu, India
3Professsor & Head, Department of Ophthalmology, AIIMS, Bhopal, Madhya Pradesh, India
Cite this as
Priyanka, Biswas J, Sharma B (2017) A rare case of bilateral acute retinal necrosis due to varicella zoster virus in a patient of Multiple myeloma. J Clin Res Ophthalmol 4(2): 037-039. DOI: 10.17352/2455-1414.000042Acute retinal necrosis syndrome (ARN) is a well-defined entity with characteristic clinical picture of progressive intraretinal inflammation and necrosis and is caused by one of the members of the Herpes group of viruses. The condition occurs typically in otherwise healthy patients. Rarely ARN has been reported in immunosuppressed patients with AIDS. We report a rare case of Multiple myeloma patient presenting with bilateral ARN, which to our knowledge is the first such case reported from India.
Introduction
Clinically, ARN is characterized by anterior uveitis, vitritis, retinal necrosis beginning in the peripheral retina, and occlusive vasculitis involving the retina and choroid [1,2]. Onset is typically unilateral with visual loss, often with ocular or periocular discomfort. Sequential bilateral involvement occurs in up to one-third of cases, usually within 3 months, but may be delayed for several years. The diagnosis of ARN syndrome is based on a clinical examination and a characteristic fundoscopic appearance. Diagnostic vitrectomy or retinal biopsy may be indicated in some atypical cases.
Case Report
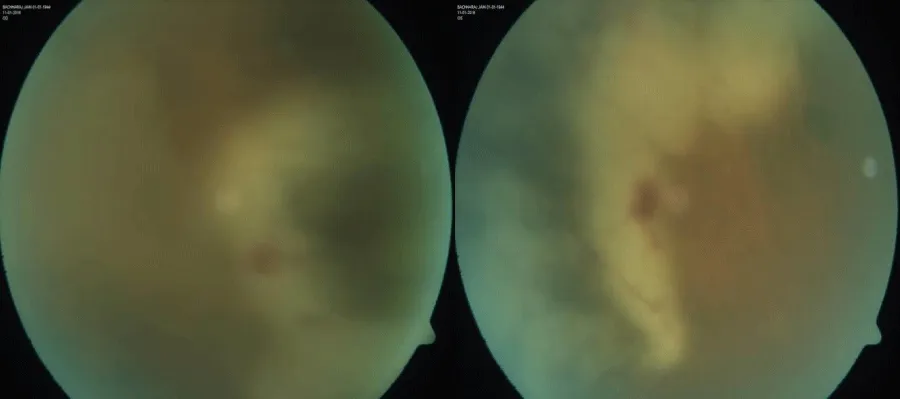
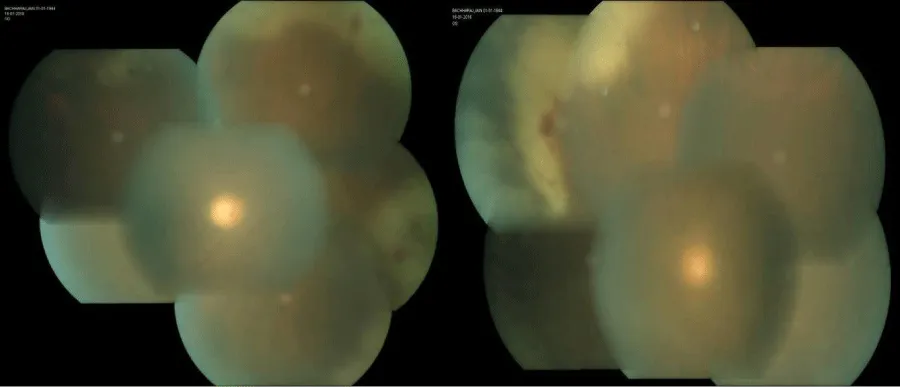
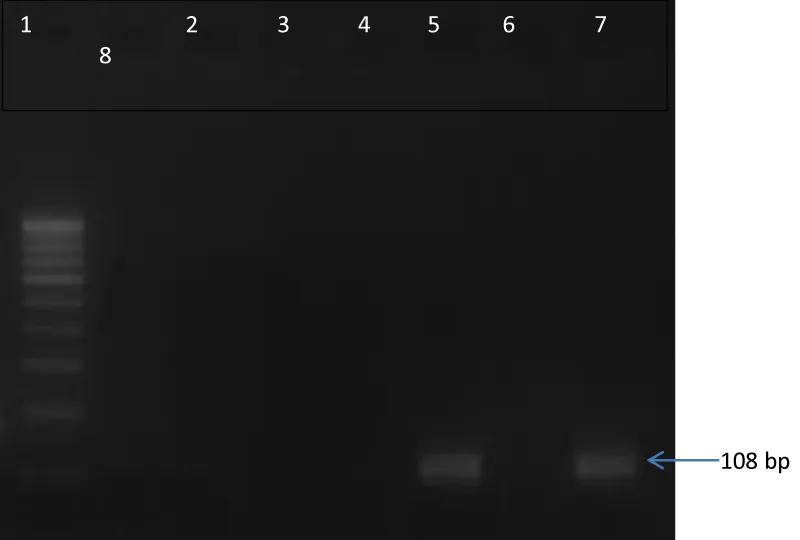
A 68-year-old male diagnosed case of Multiple myeloma, presented with gradual & progressive diminution of vision in both eye for last 6 weeks. He had received four cycle of chemotherapy before presentation. On ocular examination, visual acuity in the right eye was 6/60 and 6/36 in left eye. Intraocular pressures in both eyes were normal (10 mm Hg) by Goldman applanation tonometry. Slit lamp examination showed anterior chamber cells 1+ with vitreous cell 1+ in both eye [3]. Fundus examination revealed Vitreous haze 2+ with extensive areas of whitening and retinal necrosis involving the peripheral retina in both eye (Figures 1,2). Right eye anterior chamber tap done for Polymerase chain reaction (PCR) for herpes simplex (HSV), herpes zoster, varicella zoster (VZV), cytomegalovirus (CMV). Agarose Gel Electrophoretogram showed positive result of VZV PCR on aqueous aspirate specimen (Figure 3). A diagnosis of bilateral Acute Retinal Necrosis was made. Patient received intravenous acyclovir for 7 days with topical corticosteroids. Prednisolone 60mg/day was started after 48 hours of antiviral treatment. At day 7 of follow up fundus examination showed healing retinitis with vitreous haze 1+ in both eye. No obvious vitreous traction was noted. Intravenous Acyclovir changed to oral valacyclovir 1 gram three times a day and oral steroid tapered. One month follow-up showed similar findings. Unfortunately patient did not turned up for further follow-up.
Discussion
To the best of our knowledge, this is the first reported case of acute retinal necrosis syndrome in a patient of multiple myeloma from India. The other reported case from western world was a case of multiple myeloma with bilateral panuveitis from human herpes virus [4].
The ARN syndrome may be insidious and presents with floaters, blurred vision, or rarely with decreased peripheral vision. Features of ARN syndrome include a vaso-occlusive angitis of both retinal and choroidal vessels, a necrotizing retinitis that preferentially involves the peripheral retina, and significant intraocular inflammation. Peripheral retinal whitening is typically present, and the entire peripheral retina may be involved. Vitritis and optic disc swelling either hyperemic or pallid, are common features of the ARN syndrome.
Diagnosis
The American Uveitis Society released its criteria for diagnosis of acute retinal necrosis syndrome in 1994 [1]. Under this definition, diagnosis of ARN syndrome is dependent solely on the observed clinical findings and their progression. Recent developments leads to Polymerase chain reaction (PCR) as the preferred method of viral diagnosis now a days [5-7]. PCR analysis from anterior chamber fluid or vitreous fluid can identify the particular virus causing ARN. Multiple studies support the utility of PCR analysis with a sensitivity and specificity of greater than 90% in detecting VZV, HSV, and CMV. PCR assays are capable of detecting a single varicella zoster virion from vitreous biopsy. It has been proposed to include laboratory data in the diagnostic criteria for ARN [8]. Both qualitative and quantitative PCR testing may be used to know the aetiology of ARN and may also be utilized to monitor response of therapy [9,10].
Treatment
Earlier treatment was supportive with or without corticosteroids, which commonly led to progression of the retinitis, fellow eye involvement, and poor visual outcomes. With the knowledge of the herpes family causing the retinitis, antiviral therapy emerged as mainstay of treatment with other adjunctive therapies includes corticosteroids, prophylactic laser, and vitrectomy. The main aim of systemic therapy is to inhibit viral replication and halt disease progression in the affected eye and prevent involvement of the unaffected eye. Options for systemic therapy are intravenous and oral acyclovir, oral valacyclovir, famciclovir, valganciclovir, intravenous foscarnet and ganciclovir. Earlier, ARN was treated initially with intravenous acyclovir (1500 mg/m2/day) for 1 week followed by oral acyclovir (800 mg 5 times/day) for 6 weeks. Acyclovir therapy has been shown to decrease viral progression and prevent bilateral eye involvement. Now a day after the development of newer oral agents with improved vitreous penetration, especially valacyclovir may be preferable as first-line therapy in ARN. Oral acyclovir has poor bioavailability in plasma when compared to valacyclovir (acyclovir pro-drug). Valacyclovir is capable of achieving plasma levels comparable to intravenous acyclovir making valacyclovir an excellent oral option for the treatment of ARN [11-12]. Huynh et al. demonstrated that oral valacyclovir (1 gram TID) can reach concentrations in the vitreous and achieve inhibitory ranges of HSV-1, HSV-2, and VZV [13]. Furthermore, multiple case reports support valacyclovir use in ARN and it has been shown to be effective in halting disease progression and preventing second eye involvement [11,14-15]. Intravitreal foscarnet and ganciclovir provides direct and immediate therapy to the area of active infection to rapidly halt viral multiplication particularly in an immune suppressed host, which may be necessary in view of aggressive nature of ARN.
Corticosteroids, prophylactic laser, and vitrectomy are proposed adjuvant therapies for this condition. Oral corticosteroids are typically added to the treatment regimen at 48 hours after initiation of antiviral therapy. If initiated too early or without co-administration of systemic antiviral treatment it may potentiate viral replication and cause rapid progression of the retinitis [16]. A case series of 4 patients treated with valacyclovir (2 gram TID) and oral corticosteroids (1 kg/mg/day) with supplemental intravitreal triamcinolone acetonide showed decrease in the vitritis and improved vision in 3 of 4 patients after 1 week of treatment [17]. Prophylactic barrier laser around lesions and early vitrectomy prior to retinal detachment should be considered to lower the risk of retinal detachment in ARN.
Conclusion
ARN should also be considered in the differential for infectious uveitis in immunocompromised hosts, although it commonly occurs in immunocompetent individual. PCR from intraocular fluid have rapidly improved our ability to identify the precise herpetic aetiology of ARN and initiate prompt therapy. The valacyclovir and ganciclovir achieve high vitreous levels when administered orally and have been increasingly used in clinical practice. Careful consideration must be given to the timing of corticosteroid administration. Severe exacerbation of ARN following systemic and local corticosteroid administration is reminder of the importance of judicious use of corticosteroid-based therapies whenever an infectious viral uveitis is a diagnostic consideration. The destructive nature of the disease process may limit visual acuity outcomes and predispose patients to Retinal detachment if they are not rapidly treated. Further large randomized clinical trials are needed to evaluate the efficacy of the treatment in bilateral ARN syndrome.
- Holland GN (1994) Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol 117: 663–667. Link: https://goo.gl/BF2eiS
- Muthiah MN, Michaelides M, Child CS, Mitchell SM (2007) Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol 91:1452–1455. Link: https://goo.gl/R1WB8H
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International workshop. Am J Ophthalmol140: 509–516. Link: https://goo.gl/ANvk7U
- Bajric J, Wendy M Smith (2014) A case of bilateral human herpes virus 6 panuveitis with genomic viral DNA integration: J Ophthalmic Inflamm Infect 4: 16. Link: https://goo.gl/Sc53XA
- Short GA, Margolis TP, Kuppermann BD, Irvine AR, Martin DF, et al. (1997) A polymerase chain reaction-based assay for diagnosing varicella-zoster virus retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol 123:157–164. Link: https://goo.gl/iMwesa
- Cunningham ET Jr, Short GA, Irvine AR, Duker JS, Margolis TP (1996) Acquired immunodeficiency syndrome--associated herpes simplex virus retinitis. Clinical description and use of a polymerase chain reaction--based assay as a diagnostic tool. Arch Ophthalmol 114: 834–840. Link: https://goo.gl/wbNtLz
- Dabil H, Boley ML, Schmitz TM, Van Gelder RN (2001) Validation of a diagnostic multiplex polymerase chain reaction assay for infectious posterior uveitis. Arch Ophthalmol 119: 1315–1322. Link: https://goo.gl/ZoxHx2
- Takase H, Okada AA, Goto H, Mizuki N, Namba K, et al. (2015) Development and validation of new diagnostic criteria for acute retinal necrosis. Jpn J Ophthalmol 59: 14–20. Link: https://goo.gl/M2FAAN
- Bernheim D, Germi R, Labetoulle M, Romanet JP, Morand P, et al. (2013) Time profile of viral DNA in aqueous humor samples of patients treated for varicella-zoster virus acute retinal necrosis by use of quantitative real-time PCR. J Clin Microbiol 51: 2160–2166. Link: https://goo.gl/9DehTc
- Sugita S, Shimizu N, Watanabe K, et al. (2008) Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol 92: 928–932. Link: https://goo.gl/o8afvR
- Aizman A, Johnson MW, Elner SG (2007) Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology 114: 307–312. Link: https://goo.gl/meXYbj
- Beutner KR (1995) Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res 28: 281–290. Link: https://goo.gl/eLc25y
- Huynh TH, Johnson MW, Comer GM, Fish DN (2008) Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol 145: 682–686. Link: https://goo.gl/FGXUeC
- Taylor SR, Hamilton R, Hooper CY, Joshi L, Morarji J, et al. (2012) Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmol 12: 48. Link: https://goo.gl/BtU8fZ
- Aslanides IM, De Souza S, Wong DT, Giavedoni LR, Altomare F, et al. (2002) Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina 22: 352–354. Link: https://goo.gl/mdfh1E
- Satoh N, Abe T, Nakajima A, Sakuragi S (1998) Recurrent varicella-zoster virus retinitis in a patient treated with systemic corticosteroids. Ocul Immunol Inflamm 6: 185–188. Link: https://goo.gl/4HxbNs
- Kishore K, Jain S, Zarbin MA (2011) Intravitreal ganciclovir and dexamethasone as adjunctive therapy in the management of acute retinal necrosis caused by varicella zoster virus. Ophthalmic Surg Lasers Imaging 42: e87–90. Link: https://goo.gl/jGsbxs

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
