Open Journal of Pain Medicine
Clinically important differences with standard medications used for the breakthrough pain in a hospital at home unit for patients with advanced cancer
Vicente Ruiz-García1*, Bernardo Valdivieso-Martínez1, Elisa Soriano-Melchor1, Mónica Albert-Coll1, Rosalía Domènech-Clar1, Rosa Navarro-Villanueva1, Ana Torrego-Giménez1, Sylvia Bort-Martí2, Nuria Garrido-Rodríguez1, Ángela Piqueras-Espallargás1
2HIPRA, S.L.U., Gerona (Spain)
Cite this as
Ruiz-García V, Valdivieso-Martínez B, Soriano-Melchor E, Albert-Coll M, Domènech-Clark R et al. (2020) Clinically important differences with standard medications used for the breakthrough pain in a hospital at home unit for patients with advanced cancer. Open J Pain Med 4(1): 038-043. DOI: 10.17352/ojpm.000022Background: One out of three cancer patients suffers pain, and half of those patients suffer Breakthrough Pain (BTP). In patients with advanced, metastatic, or terminal disease the number suffering BTP increases to two out of three patients.
BTP is defined as a peak of pain intensity of short duration in patients with stable and acceptable analgesia provided by analgesics given around the clock.
Context and purpose of the study: An observational study was performed at the Hospital at Home Unit at the University and Polytechnic Hospital La Fe of Valencia (Spain) to evaluate whether opioid therapy could significantly reduce BTP from baseline within 30 minutes of administration and achieve clinically meaningful differences.
Results: In total, 424 BTP episodes were recorded in the study. The incidence of BTP per patient over the study period was 13.7 episodes.
There were significant pre-and post-treatment differences in pain intensity as measured on a Visual Analogy Scale (VAS) of 0-100 points/100 (p<0.05). The mean difference of pain between pre- and post-treatment was 35.28 (CI95% 33.55-37.35). The intensity of BTP was highly variable for individual patients, with 70% of the variability being greater than 30 points/100 and 50% being greater than 40 points/100. The most frequent intensity of BTP was moderate. The most frequent time of BPT was the morning/early afternoon. The items related to clinically relevant pain relief after opioid therapy were: level of basal pain, type of basal medication (fentanyl patches), and gender (female).
Conclusions: It is recommended to check daily for the appearance of BTP in patients with advanced, metastatic or terminal cancer, especially in Units such as Hospital at Home Units.
We have observed that among opioids drugs, fentanyl patches and slow release morphine, which are the most frequently used opioids for the treatment of BTP, are the ones which achieved a clinically important difference in pain score, independently of the pre-treatment pain severity (mild, moderate or severe) of the patient.
The adverse events detected in the present study were few although we estimate that they were probably under-represented. Hence, we recommend that the doctors should ask on daily basis patients suffering from advanced cancer and being treated with opioid drugs for BTP for adverse events and/or insist the patients to report them.
Background
Cancer is an important cause of morbidity and mortality worldwide, being responsible for 9.6 million deaths in 2018 [1]. Pain is one of the most common cancer symptoms. Indeed, although pain relief and palliative care have been recognized as basic human rights and play an important role within the health care network [2], up to 39% of cancer patients experience pain [3,4]. Some patients suffer not only from a persistent well-controlled pain but also from severe transient exacerbation of their pain called Breakthrough Pain (BTP) [5,6].
Up to 66% of patients with advanced, metastatic or terminal disease, and 55% of patients with anti-cancer treatment claim to have suffered episodes of BPT [3]. It is worth noting that BTP prevalence in outpatient clinics is quite different to that reported in hospice patients (40% vs. 80%, respectively).
The subjectivity of pain perception helps explain previous results in a cohort of 100 patients, which showed that while 70% of doctors were satisfied with BTP control, only 35% of patients shared this point of view [7]. In this regard it is important to mention that the World Health Organization (WHO) suggested that the goal of optimum management of pain is to reduce pain to a level that allows an acceptable quality of life. Furthermore, the WHO emphasized that the BTP should be managed with an immediate-release and not a slow-release opioid.
Different formulations of the opioid drug fentanyl (i.e., oral, nasal, transmucosal) are the most prescribed drugs by Units of Pain and Oncology Departments in Spain [8,9], even though there is no clear evidence of their better efficacy compared to morphine [10,11]. Dose adjustment of opioid therapy for the relief of BTP should be performed according to the basal dose of analgesia [1].
In pain research, the most frequently reported pain data are based on statistical differences of pain between the pain treatments. Unfortunately, it is uncommon for pain relief effectivity to be reported according to Minimum Important Clinical Differences (MCD) for patients. However, Olsen [12,13], recently reported MCDs ranging from the highest to the lowest pain level. Olsen measured the pain with a Visual Analogue Scale (VAS) of 0-100 mm [14]. Mean differences of 4, 11 and 20 were the MCDs measured in patients with pain of <40 mm, 40-70 mm, and 70 mm, respectively. The overall mean difference for MCDs was 17 [15-19].
In the present study, the BTP relief was evaluated following Olsen criteria. The aim of the present study was to evaluate whether the opioid titration regimens used in standard clinical practice for the treatment of BTP in patients with advanced, metastatic or terminal cancer (“advanced”) induce an MCD.
Materials and methods
Study design and ethical standards
This paper reports an observational study of the clinical control of BTP in patients with advanced cancer using opioids. The study, entitled VRG_MOR-2018-01, was approved by the ethical committee (03.10.2018), by the local government of Health of Valencia (Spain) on 26.12.2018, and by the Spanish Agency of Medicines and Health Products on 31.05.2018. The study was performed with patients from the Unit of Hospital at Home (“Unit”), Hospital La Fe, Valencia (Spain). The Unit carries out control of symptoms in patients with advanced cancer and maintains symptomatic control at home on most occasions.
The study was completed following the International Society for Pharmacoepidemiology (ISPE) International Guidelines for Good Epidemiology Practice, the national guidelines on observational studies, and the basic ethical principles contained in the Declaration of Helsinki.
The first patient was included in the study on February 22, 2019. The last patient was included in the study on September 21, 2019. 30 patients participated in the study.
Patients with cancer were screened by consecutive sampling from among those referred to the Unit who met the screening criteria. We contacted the patients at home and offered them the possibility of inclusion in the study. The inclusion criteria were patients from the Home Hospitalization program with advanced cancer and controlled baseline pain. The exclusion criteria were: patients who did not accept inclusion in the study; patients who did not have a mobile phone or a fixed telephone line; uncooperative patients; or cognitively impaired patients who were unable to describe the pain severity using the VAS tool. Selected patients signed a written informed consent in order to participate in the study.
The definition of BTP was a peak of pain intensity of short duration in patients with stable and acceptable analgesia provided by analgesics given around the clock [5]. The pain intensity was rated according to the VAS tool. When patients experienced BTP, they wrote down the VAS score, took the rescue drug, which was an opioid, and after 30 minutes they wrote down the intensity of the pain according to the VAS. If the pain was not controlled, they took another dose of opioids.
Main outcome
Mean difference of pain intensity of BTP before and at 30 min after rescue with opioids, to assess whether it is perceived as a minimal clinically important improvement. The cut-off value was defined as a difference of 17 (15–19) mm in the pain perceived according to VAS.
Secondary outcomes
Mean difference of pain intensity of BTP before and at 30 min after rescue with opioids to assess whether it is perceived as a minimal clinically important improvement for three different levels of pain intensity, namely, mild (0-40), moderate (41-70), and severe (71-100). The cut-off values were 6(4-8), 13(11-14) and 21(20-23)/100 VAS, respectively.
Number of BTP episodes per patient.
Opioid drugs used to control BTP.
BTP differences depending on age and sex of patient.
BTP differences depending on time of day.
Adverse events.
Statistical analysis
The unit of analysis was a declared episode of BTP.
We calculated the study size with a confidence level of 95% and an accuracy of ±5% for at least 117 episodes of BTP. This study size was increased assuming subgroup analyses according to pain intensity. Assuming a good response in 60% of the mild, 50% of the moderate and 40% of the severe BTP episodes, it was estimated that data of 117 mild BTP episodes, 120 moderate BTP episodes and 117 serious BTP episodes were required.
We completed a descriptive analysis of the variables included in the present study. We calculated the main differences, with a 95% confidence interval (CI), between pain intensity of BTP before and after the use of opioids. We used Fisher’s test or the Chi-squared (χ2) test for comparisons between qualitative variables. In case statistically differences were detected, a forward conditional stepwise logistic regression was used to explain them.
Results
Thirty patients who signed the informed consent participated in the study.
Main outcome
Using a VAS of 0-100, the mean intensity of the BTP before the rescue was 57.80 (CI95% 56.15-59)/100. The mean intensity of the BTP at 30 min after rescue was 22.52 (CI95% 20.77-24.34)/100. The mean difference between these two intensities was 35.28 (CI95% 33.55-37.35)/100. The obtained mean difference was above 17 points (CI95% 15-19)/100, which is the cut-off value of a clinically different improvement (12). Even if the upper confidence interval of this difference, which is 20 points, were used, good clinical control of pain was achieved in 89% of BTPs.
Secondary outcomes
Table 1 below shows the percentage of BTPs displaying the mean difference for each grade of pain, i.e., mild, moderate and severe.
Individual patients reported high variability in BPT intensity ranging from mild to severe, with 70% of the variability being greater than 30 points and 50% being greater than 40 points. These results indicate that the same patient suffered mild, moderate and severe BTP. Most of the patients suffered BTP of moderate intensity.
A total of 424 BTP episodes were recorded in the study. The average incidence of BTP per patient over the period of the study was 13.7 episodes. The number of episodes of BTP that each patient suffered per day was highly variable from 1 to 6 episodes of BTP, with an average of 2-3 BTP episodes per patient and day.
The drugs used for the control of BTPs were oral fentanyl, transmucosal fentanyl, oral morphine, metamizole, paracetamol, buprenorphine, oxycodone, and hydromorphone. On the one hand, metamizol and paracetamol are not opioid drugs, so they were not considered in the present study. In the case of buprenorphine, oxycodone and hydromorphone, their use represented all together less than 3% of the overall cases, so they were disregarded from the analysis. Accordingly, only oral or transmucosal fentanyl (n=268; 63.2%) and oral morphine (n=144; 34%) were considered for the analysis, adding n= 412 episodes of BTP, which represented 97. 2% of the total number.
No adjuvant drugs were administered in the BTP.
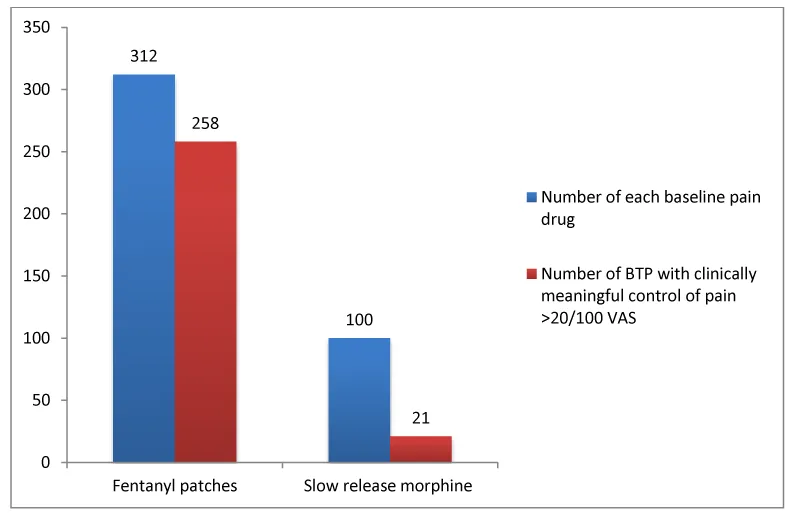
The medication for baseline pain (or basal drug) of the patients in the study was: fentanyl patch (n= 312; 73.6%); slow release morphine (n=100; 23.6%); buprenorphine patch (n=1; 0.2%); oxycodone (n=1; 0.2%); and hydromorphone (n=10; 2.4%). The mg of morphine equivalents used per patient in the baseline treatment was 208 mg (194-221) per day. The mg of morphine equivalents used per BTP was 30 mg (27-33).
Demographic data were collected for all 424 BTP cases. The mean age was 66 years (CI95% 65-67), ranging between 39 and 87 years. The gender proportion was women (21.9%)/men (78.1%).
There was better clinical improvement of pain in women (86%) than in men (62%) (χ2; p=0.0001).
Patients between 60-70 years reported the highest number of BTPs. See Table 2, below. The most frequent intensity of BTP was moderate (VAS 40-70) Table 2.
The most frequent time of day for BTP was between 08:00 a.m.-16:00 p.m. (n=158; 38.3%), followed by 16:00 p.m.-00:00 p.m. (n=142; 34.5%) and then 00:00 p.m.-07:59 a.m. (n=112; 27.2 %).
Regarding adverse events, the patients received a sheet with a table in which all the possible effects that could appear were detailed. The patients reported very few adverse events. In fact, only one adverse event was reported per patient. Among them, only excessive drowsiness and lethargy classified were reported as severe ones. The most frequent adverse effect reported [n=38 (9%)] was drowsiness, which was reported after taking rescue medication. There were no statistically significant differences between the appearance of drowsiness and lethargy between the different drugs used as rescue, i.e. fentanyl (27/258) and those that used morphine in oral solution (11/143), X2= NS, wherein NS stands for statistically non-significant.
In addition to all the above, we studied whether the basal drug (i.e., fentanyl patches or slow release morphine) played a role in the clinically meaningful control of the pain after BTP rescue. Table 3, Figure 1.
As shown in Table 3, we found a statistically significant difference between the use of fentanyl patches vs. the use of slow release morphine (p=0.000).
Additionally, we also observed that oral fentanyl was better than oral morphine (81% vs. 41%; χ2; p=0.000) as rescue drug to control BTP.
The above analyses were performed considering that a clinically meaning control of pain was achieved with a difference of pain of more than 20/100 VAS.
When we analyzed whether there were differences in the clinically meaningful control of pain between oral fentanyl and oral morphine for the different grades of BTP severity, surprisingly, we did not find any differences between them. So, we performed a logistic regression analysis, in particular, a forward conditional stepwise logistic regression analysis, to determine which variable(s) could explain a good control of BTP. In this analysis, the response variable was defined as good control of pain (yes/no), with an improvement of at least 8/100, 14/100 and 23/100 VAS points in the pain score for mild, moderate and severe BTP, respectively. The explanatory variables that were introduced into the model were the intensity of BTP, the medication used for the baseline pain (fentanyl patches or slow release morphine), the rescue medication (transmucosal fentanyl, oral fentanyl or oral morphine), the gender (F/M), and the equivalent dose of morphine in mg used in rescues, wherein transmucosal or oral fentanyl were used.
The above analysis was carried out assuming that the worst-case scenario was practically similar to a sensitivity analysis since the upper value of the 95% CI was taken as the cut-off value in the definition used for minimal clinically relevant improvement in each grade of pain. The variables that were found to be predictive in the model were the intensity of BTP, the gender and the total dose of opioids.
However, when the lower value of the 95% CI was taken as the cut-off value in the definition used for minimal clinically relevant improvement in each grade of pain, the variables associated with a clinically significant response extended to the type of baseline medication used (fentanyl patches better than slow release morphine). It is striking that the gender variable was maintained in all BTP grades Table 4.
Discussion
All the patients in the present study who had an advanced cancer disease reported BTPs. These results are consistent with recent systematic reviews that found high prevalence of BTPs in older patients and patients with advanced disease [2,15]. Among all the patients, only one had a hematologic cancer, namely, chronic myeloid leukemia. This patient experienced less BTPs compared to those patients who had solid tumors. This observation is also in line with the bibliography [16], which reports fewer incidences of BTPs in hematologic cancer compared to solid cancer.
Transdermal fentanyl was the most frequent opioid prescribed for the baseline pain and oral fentanyl (fentanyl buccal tablets) was also most frequently prescribed for the BTP than oral morphine. This is so despite there is neither clear evidence [10,11] nor a recommendation from WHO [1] that fentanyl is better that oral morphine.
In view of the results reported in the present study, patients with advanced cancer should be asked on daily basis for BTP. This recommendation is in line with those from WHO [1]. This is further supported by the literature [16], which shows that patients with advanced cancer who a priori did not report daily pain symptoms, when being actively asked, 82% of them were indeed suffering from pain [16]. Finally, the relevance to actively ask patients with advance cancer for pain episodes is that significant differences in global impressions of BTP have been detected between patients and doctors, and unfortunately, therapeutic decisions were more based on doctors’ perceptions than on patients’ perceptions [7].
The present study also shows that the intensity and number of BTPs per day and per patient were very similar to those reported in other cohorts [17].
Finally, the results of the present study agree with those reported in the literature regarding the most frequent intensity of BTP which is moderate and the most frequent age group which is 60-70 years [2]. There is no consensus in the literature regarding whether BTP is gender-related or not [2]. However, the results present here indicate that women have a better response to pain than men. This conclusion should be confirmed in further studies with larger samples and/or in studies addressing this specific research point.
One of the limitations of this study is that, despite exceeding the sample size needed for calculating changes in BTP control after rescue, the number of patients was low and may not represent the general population suffering from breakthrough cancer pain. Recruitment of patients with advanced cancer was low due to the appearance of other symptoms or to physical difficulties of the patients themselves. Most of the patients with advanced cancer who participated in the present study have died due to the advanced stage of their disease. This difficult recruitment situation has also been experienced in other studies despite having a multicenter design [15]. In view of the difficult recruitment situation of patients with advanced cancer, multicenter studies comprising more centers are needed to ensure higher rate of recruitment and be able to confirm the results reported in the present study.
Conclusions
It is recommended to check daily for the appearance of BTP in patients with advanced, metastatic or terminal cancer, especially in Units such as Hospital at Home Units.
Among opioids drugs, fentanyl patches and slow release morphine, which are the most frequently used opioids for the treatment of BTP, are the ones which achieved a clinically important difference in pain score, independently of the pre-treatment pain severity (mild, moderate or severe) of the patient.
The adverse events detected in the present study were few although we estimate that they were probably under-represented. Hence, we recommend that the doctors should ask on daily basis patients suffering from advanced cancer and being treated with opioid drugs for BTP for adverse events and/or insist the patients to report them.
The participation of the following researchers in the study is appreciated: Pablo Flors Villaverde, Silvia Forcano Sanjuan, Juan Ramón Domenech, Llanos Gómez, Carmen Gil Adiego, Alberto Muñoz Cano, Carolina Wollstein Lara.
Contribution
VRG: Conceptualization, Methodology, Software, Validation, Formal analysis, Data curation, Writing (original draft preparation), Writing (review and editing), Visualization, Supervision, Project administration, Funding acquisition.
BVM, ESM, MAC, RNV, ATG, NGR, APE: Writing (review and editing), Visualization, Supervision, Project administration.
SBM Writing (original draft preparation); Writing (review and editing).
Study VRG-MOR 2018-01.
Funding
Vicente Ruiz García MD received a grant from Kyowa Kirin Farmacéutica, S.L. Madrid, as an Investigator Sponsored Study, for the article processing charges.
KIowa Kyrin S.L. was not involved in the study design, data collection, or analysis. The decision to publish this work was the sole initiative of the authors. Also, there was no influence on the preparation, review or approval of the manuscript.
- World Health Organization (2018) WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Link: https://bit.ly/3rOWhHV
- Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, et al. (2018) Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report. Lancet 391: 1391-1454. Link: https://bit.ly/3aXgdlU
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al (2014) Prevalence of Breakthrough Cancer Pain: A Systematic Review and a Pooled Analysis of Published Literature. J Pain Symptom Manage 47: 57-76. Link: https://bit.ly/2WVmtlB
- van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA (2016) Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 51: 1070-1090.e9. Link: https://bit.ly/2XaYIGF
- Portenoy RK, Hagen NA(1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41: 273-281. Link: https://bit.ly/38ROAYH
- Mercadante S, Caraceni A, Masedu F, Scipioni T, Aielli F (2020) Breakthrough Cancer Pain in Patients Receiving Low Doses of Opioids for Background Pain. Oncologist 25: 156-160. Link: https://bit.ly/2KG4NIw .
- Webber K, Davies AN, Cowie MR (2016) Disparities Between Clinician and Patient Perception of Breakthrough Pain Control. J Pain Symptom Manage 51: 933-937.e2. Link: https://bit.ly/3pBiaZ5
- Estévez FV, Alarcón MDL, Mayoral V, de Madariaga M, Margarit C, et al. (2019) Current management of breakthrough cancer pain according to physicians from pain units in Spain. Clin Transl Oncol 21: 1168-1176. Link: https://bit.ly/3n09b1V
- Iglesias-Docampo L, Pimentel-Cáceres P, García-Coves MP, Firvida-Pérez JL, Lambea-Sorrosal JL, et al. (2019) Observational study to analyse the opioid titration process in the treatment of breakthrough pain of cancer patients in clinical practice. Open Journal of Pain Medicine 3: 001-007. Link: https://bit.ly/38ROd0f
- Zeppetella G, Davies AN (2015) Opioids for the management of breakthrough pain in cancer patients. En: Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. Link: https://bit.ly/3n28TaS
- Ruiz-Garcia V, Lopez-Briz E (2008) Morphine remains gold standard in breakthrough cancer pain. BMJ 337: a3104. Link: https://bit.ly/2LaYhsN
- Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, et al. (2017) Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Medicine 15: 35. Link: https://bit.ly/2JxtCFT
- Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, et al (2018). Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 101: 87-106.e2. Link: https://bit.ly/3pvBJSy
- Hawker GA, Mian S, Kendzerska T, French M (2011) Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF). Arthritis Care Research 63: S240-S252. Link:. https://bit.ly/3ofolSB
- Pérez-Hernández C, Blasco A, Gándara Á, Mañas A, Rodríguez-López MJ, et al. (2019) Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Scientific Reports 9: 17701. Link: https://go.nature.com/3htQMcA
- Herrero CC, Reina Zoilo JJ, Martín DM, Martínez FC, Porta VG, et al. (2019) Active study: undetected prevalence and clinical inertia in the treatment of breakthrough cancer pain(BTcP). Clin Transl Oncol 21: 380-390. Link: https://bit.ly/3pA3hX0
- Mercadante S, Marchetti P, Cuomo A, Caraceni A, Mediati RD, et al (2018) IOPS-MS Study Group. Factors Influencing the Clinical Presentation of Breakthrough Pain in Cancer Patients. Cancers (Basel) 10: 175. Link: https://bit.ly/3n1oirS
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
