Analysis of care for cancer patients with opioid-induced constipation in clinical practice: EIO-PRAXIS Project
1MD, PhD, ECO Foundation, Medical Oncology Department, Hospital Reina Sofía, Córdoba, Spain
2MD, PhD, ECO Foundation, Medical Oncology Department, Fundación Jiménez Díaz, Madrid, Spain
3MD, PhD, ECO Foundation, Medical Oncology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain
4MD, PhD, ECO Foundation, Medical Oncology Department, Hospital General de Valencia, Universidad de Valencia, Ciberonc, Spain
5MD, PhD, ECO Foundation, Medical Oncology Service, Alcalá University, Hospital Ramón y Cajal, Madrid, Irycis, CIBERONC, Spain
6MD, PhD, ECO Foundation, Medical Oncology Department, Complejo Hospitalario Universitario de Pontevedra, Spain
7MD, PhD, ECO Foundation, Medical Oncology Department, Hospital Clínico Universitario de Salamanca, Spain
8MD, PhD, ECO Foundation, Vice Presidency of the Royal National Academy of Medicine, IdISCC, CIBERONC, Spain
9MD, PhD, ECO Foundation, Medical Oncology Department, Hospital Universitari Clínic i Provincial, Barcelona, Spain
10MD, PhD, ECO Foundation, Medical Oncology Instituto Valenciano de Oncología, Valencia, Spain
11MD, PhD, ECO Foundation, Medical Oncology Service, Hospital Clínico Universitario of Santiago de Compostela, Spain
12MD, PhD, ECO Foundation, Medical Oncology Hospital Universitario La Moraleja, Sanitas, Madrid, Spain
13MD, Medical Department, E-C-BIO, SL, Las Rozas (Madrid), Spain
14MD, PhD, ECO Foundation, Medical Oncology Department, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), CIBERONC, Madrid, Spain
Author and article information
Cite this as
Aranda E, García-Foncillas J, Antón A, Camps C, Carrato A, et al. (2019) Analysis of care for cancer patients with opioid-induced constipation in clinical practice: EIO-PRAXIS Project. Open J Pain Med. 2019; 3(1): 027-033. Available from: 10.17352/ojpm.000015
Copyright License
© 2019 Aranda E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Purpose: The objective of the study was to analyses how Opioid-Induced Constipation (OIC) is managed in cancer patients in clinical practice for comparison against the current recommendations for the management of patients with OIC.
More than half of cancer patients suffer pain for months or even years during the course of their disease, so it is common for opioids to be used for adequate pain control once the previous alternatives recommended in the WHO cancer pain ladder have been exhausted [1,2]. Opioids are known to cause different adverse effects, the most common being dry mouth, oesophageal reflux, nausea, vomiting, abdominal discomfort, swelling, abdominal pain, and constipation [3-5]. Of these, constipation is the most common, and is generally the most bothersome for patients, affecting their quality of life [4,6,7]. Since unique pathophysiological causes to constipation caused by opioids have been described, this type of constipation has been recognized as a specific clinical condition called Opioid-Induced Constipation (OIC) to differentiate it from constipation from other causes [4,8-11].
OIC had no medical definition until the recent publication of the Rome IV criteria, defined as: A change in bowel habits and defecation patterns when initiating opioid therapy characterized by any of the following conditions: decreased frequency of defecation; development or worsening of straining; sensation of incomplete evacuation; or patient-perceived alterations related to bowel habits [8,12,13].
OIC may occur from the start of opioid treatment and persist throughout the period of use. OIC occurs with low doses of opioids and is also independent of their strength [9,14]. Unlike other adverse effects caused by opioids, such as nausea, vomiting and sedation that disappear over time, OIC does not disappear without treatment [10].
The constipation seen in cancer patients, in addition to being caused by the use of opioids, is multifactorial [6,7,15]. The use of medications such as antacids, antitussives, anticholinergics, antidepressants, antiemetics, neuroleptics, iron, diuretics and chemotherapeutic agents is associated with an increased incidence of constipation. Metabolic problems such as dehydration, hypercalcemia, hypokalaemia, uraemia, diabetes mellitus, and hypothyroidism are common in cancer patients, and are associated with constipation. Neuromuscular and neurological changes may also account for the presence of constipation, as well as physical factors such as the presence of tumour masses or radiation fibrosis. Pain in itself is also related to the presence of constipation both at the local anorectal level and to the general pain caused by cancer. A low-fibre diet, anorexia, or inadequate food or fluid intake contributes to the occurrence of constipation. Factors such as lack of privacy when defecating for hospitalized patients, inactivity, or the older age of the patient, also play a role in its occurrence. In cancer patients, in addition to the use of opioids for the treatment of pain, vinca alkaloid-like chemotherapeutic agents produce severe and prolonged neuropathic effects at the gastrointestinal level, which are more severe with vincristine and vindesine, and milder with vinblastine or vinorelbine [16].
Clinical practice guidelines are available in Spain and have already been updated with the new Rome IV criteria for the diagnosis of OIC [13]. The European Society for Medical Oncology (ESMO) has recently published a guide for the management of constipation in cancer patients [16]. In 2019, the American Gastroenterological Association published guidelines for the management of OIC, also updated with the Rome IV criteria [17]. That same year, a consensus of European experts on pathophysiology and management of OIC was also published [18].
The primary objective of the study was to analyse how OIC is managed in cancer patients in medical oncology units in order to identify critical points in relation to the most recent recommendations.
Methods
Study design and ethical standards
A retrospective observational study was designed called the EIO-PRAXIS Project. At each site, information was collected from the clinical histories of 10 consecutive cancer patients who received opioid treatment and developed opioid-induced constipation according to Rome IV criteria [8]: New or worsening symptoms of constipation when initiating, changing, or increasing opioid therapy that must include ≥2 of the following: a) Straining in at least 25% of defecations; b) Lumpy or hard stools in at least 25% of defecations (Bristol type 1-2); c) Sensation of incomplete evacuation in at least 25% of defecations; d) Sensation of anorectal obstruction or blockage in at least 25% of defecations; e) Manual manoeuvres to facilitate defecation in at least 25% of defecations; f) Fewer than 3 spontaneous bowel movements per week. The presence of soft stools is rare without the use of laxatives.
The clinical histories had to be from before 1 March 2018, prior to the design of this study. Data from the 10 cases selected at each site were collected on an aggregate basis, registering the number of patients meeting the indicator, not meeting it, and cases where it was not applicable or not recorded in the clinical history. Therefore, no individual information is available for the patients from whom the data was collected. The data was transcribed to a restricted-access website specifically designed for the study.
The Scientific Committee of the study selected structure, process and outcome indicators related to the diagnosis and treatment of patients with OIC.
The Ethics Committee of Hospital Universitario Puerta de Hierro (Majadahonda, Madrid, Spain) approved the study on 5 April 2018. The study was completed following the guidelines of the International Society for Pharmacoepidemiology (ISPE), the International Guidelines for Good Epidemiology Practice and the latest version of the Declaration of Helsinki. The study was completed between 16 April and 26 July 2018.
Calculation of sample size
A sample of 90 medical oncology units was estimated to be enough to assess the selected process and outcome indicators with an estimated precision of ±11% in the confidence intervals of the percentages for each indicator, with a statistical power of 87% and a two-tailed alpha error of 0.05. Final participation was 85.5% (77 sites), with an 82% power to obtain the established objectives (Sample Power SPSS).
Statistical analysis
A descriptive analysis of frequencies for qualitative variables, and the calculation of the usual values for quantitative variables: mean, 95% confidence interval, median, and minimum and maximum values, were performed. SPSS 25.0 software was used for the statistical analysis. Due to the study design with pooled data, no comparison tests were performed between variables.
A descriptive analysis was made of the applicability and quality of data recording in the clinical histories, for the process and outcome indicators. The analysis was performed by calculating the percentage of patients in whom the indicator was not applicable or was not recorded in the clinical history. If the non-applicability exceeded 10% of the cases, an analysis of the possible reasons justifying it would be necessary.
Results
A total of 77 oncologists from 26 Spanish provinces participated in the study, who included information from 770 clinical histories.
Structure indicators
A total of 96.1% (74) of the sites to which the investigators belonged were public hospitals or centres, 1.3% (1) were private clinics, and 2.6% (2) were mixed centres.
In the participating investigators’ consultation offices, 47.5% (95% CI 41.7-53.2) of cancer patients received opioid treatment, with percentages ranging from 10% to 100% of patients.
The percentage of cancer patients treated with opioids who developed OIC according to Rome IV criteria [8], was 44.9% (95% CI 39.3-50.5).
A written protocol for the diagnosis, treatment, and prevention of OIC at the clinic was not available for 89.6% (69) of sites.
A total of 84.4% (65) of investigators had no educational programs for patients with OIC in their departments.
A total of 51.9% (40) of the investigators did not follow any guidelines for the management of patients with OIC.
Description of patients
Mean patient age was 61.6 years, with 57.1% of patients (n=440) aged 50-75 years, 25.6% (n=197) aged over 75 years, 15.8% (n=122) aged 26-50 years, and 1.4% (n=11) aged 18-25 years.
Process indicators
Actions at the start of treatment with opioids: A total of 62.8% (n=424) of patients were evaluated for functional constipation before starting treatment with opioids.
The mean opioid dose in morphine milligram equivalents/day received by patients at the time of diagnosis of OIC was 65.9 morphine milligram equivalents (MME)/day (95% CI 55.4-76.5), with a median of 60, ranging from 2 and 260 MME/day. UK Medicines Information conversion measures were used to calculate morphine milligram equivalents (https://www.sps.nhs.uk/articles/what-are-the-equivalent-doses-of-oral-morphine-to-other-oral-opioids-when-used-as-analgesics-in-adult-palliative-care-2/).
Mean duration of treatment with opioids was 4.9 months, ranging from one month to 3 months in 29.5% of patients (n=227), from 3 to 6 months in 28.6% (n=220), from 6 to 12 months in 19.1% (n=147), from one month or less in 16.1% (n=124), and for more than 12 months in 6.8% (n=52) of patients.
As regards the therapeutic measures for OIC applied at the time of opioid prescription, healthy habits were prescribed in 78.9% of patients (n=551); laxatives were prescribed in 69.6% of patients (n=502); other therapeutic measures were indicated in 23.1% of patients (n=151).
Diagnosis of OIC: In 32.7% of the patients (n=213), nursing staff were involved in facilitating the diagnosis of OIC.
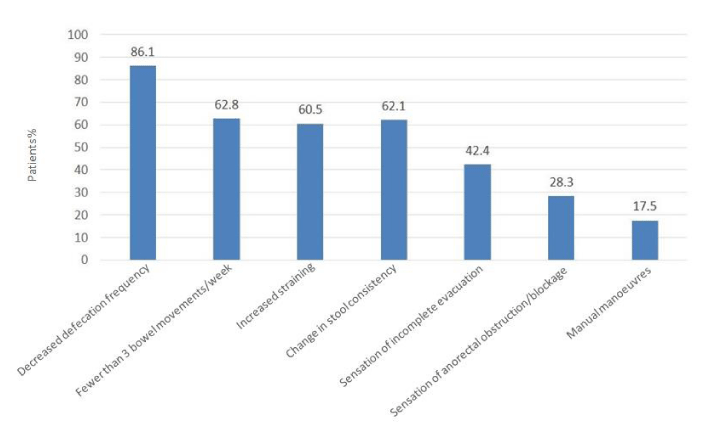
Figure 1 shows the percentage of patients with each OIC symptom as described by the Rome IV criteria for the diagnosis of OIC.
The mean time from the start of opioid treatment to the first symptoms of OIC was 16.5 days (95% CI 13.1-19.9), with a median of 12 days, ranging from 0 to 80 days.
No additional tests were requested for the differential diagnosis of OIC in 57.9% of patients (n=414). Radiological imaging tests were requested in 27.7% of patients (n=191), anorectal examination in 20.8% (n=143), laboratory tests in 19.6% (n=137), ultrasound in 5.9% (n=40), and other tests in 5.1% (n=35).
Constipation severity was assessed according to CTCAE version 5.0 [19]. No information was collected on the severity of OIC in 27.3% of patients (n=210). In 46.4% of patients (n=260) OIC was Grade 2 (moderate), in 33.4% (n=187) OIC was Grade 1 (mild), in 18.9% (n=106) OIC was Grade 3 (severe), and in 1.3% (n=7) OIC was Grade 4 (with risk of mortality or disability).
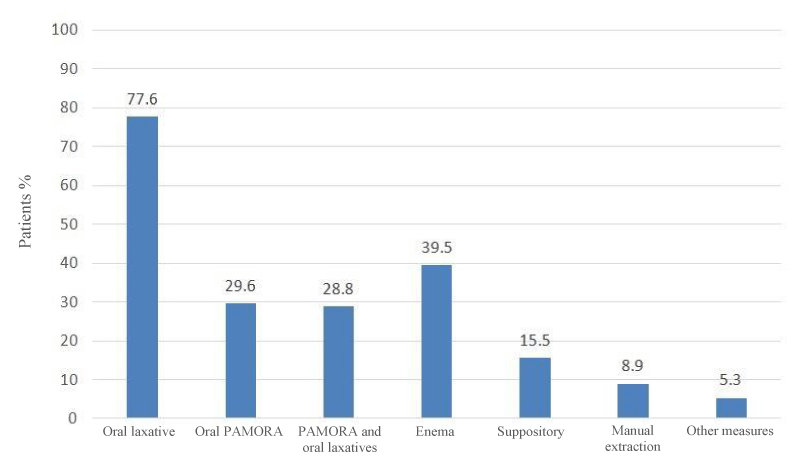
Treatment of OIC: Figure 2 shows the percentage of patients who received treatment for OIC with each therapeutic measure or rescue manoeuvre until the time of review of their clinical history.
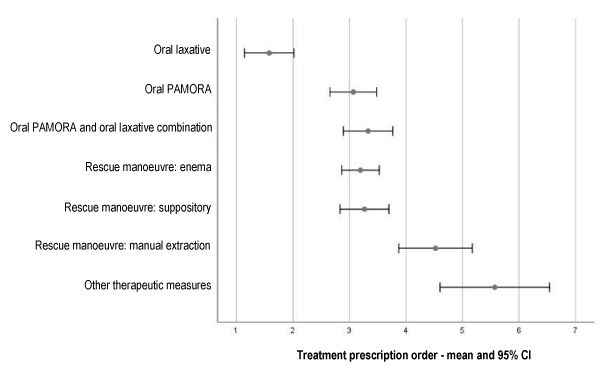
Figure 3 shows the order of prescription of each therapeutic measure or rescue manoeuvre in patients included in the study. Laxatives were prescribed in first place significantly more than all other therapeutic measures. In a clear second place in prescription order were oral PAMORA (Peripherally Active µ-Opioid Receptor Antagonists), a combination of PAMORA and oral laxatives, enemas and suppositories. In last place were manual extraction manoeuvres.
Outcome indicators
Efficacy assessment: The mean time from the start of treatment with the oral laxative to the finding of lack of efficacy was 10.7 days (95% CI 8.6-12.9), with a median of 7 days, ranging from 1 to 57 days.
In patients treated with oral PAMORA, the mean time from diagnosis of OIC to the start of treatment with oral PAMORA was 15.8 days (95% CI 12.5-19.2), with a median of 12 days, ranging from 0 to 60 days.
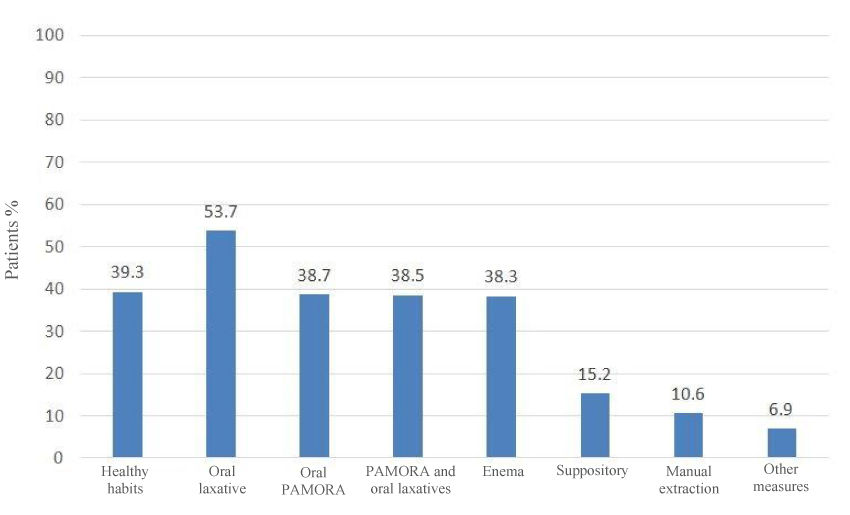
Figure 4 shows the percentage of patients in whom each treatment or intervention was effective for the treatment of opioid-induced constipation.
Analysis of the applicability of the indicators
Table 1 details the analysis of the applicability and quality of recording of process and outcome indicators in the clinical histories.
Discussion
Medical oncologists belonging to public centres (96.1%), participated in the EIO-PRAXIS Project, so the results are applicable to most oncology centres in Spain. It was observed that in oncology clinics, approximately half of the patients (47.5%) received treatment with opioids, and of these, 44.9% developed OIC. This result agrees with that reported in the most recent literature, where 40%-80% of patients treated with opioids present OIC [4,7].
It was found that many sites (89.6%) had no written protocol for the management of patients with OIC, and that one-half did not follow a specific guideline, mainly because none had been published yet at the time of study conduct or because they followed more general guidelines for the treatment of constipation.
Most patients included were over 50 years of age (72.9%), which coincides with the age group with the greatest incidence of cancer in our setting [20]. Before starting treatment with opioids, functional constipation was assessed in only 62.8% of patients, despite the fact that it is known that response to OIC treatment may be conditioned by this previous clinical condition. The importance of this assessment should therefore be emphasized and be documented in the clinical history, since this information was not detailed in 12.3% of cases (Table 1).
Healthy lifestyle habits were prescribed at the time of opioid prescription in 78.9% of patients, and laxatives were prescribed to 69.6% of patients at that time, although both recommendations should be applied to 100% of patients as a preventive measure of OIC. Considering that half of the patients will develop OIC, it is critical to emphasize the importance of these preventive measures that should be considered in all patients who will receive opioid treatment [9,16].
Although the role of nurses in the follow-up of symptoms of cancer patients is recognized, they were only involved in the diagnosis of 32.7% of patients, so it is the oncologist who determines whether the patient has OIC.
The patients included in the study had to meet Rome IV criteria for the diagnosis of OIC [8]. Figure 1 shows the percentage of patients with each criterion, with at least two being required to confirm the diagnosis. Decreased defecation frequency is a Rome IV criterion for diagnosis of OIC. The study found that 86.1% of patients met this criterion, with a reduction in the number of bowel movements to fewer than three per week in 62.8% of patients. It should be noted that the increase in straining observed in 60.5% of patients, and the change in stool consistency in 62.1% of cases, were symptoms as common as the reduction in defecation frequency (Figure 1). The sensation of incomplete evacuation reported by 42.4% of patients, the sensation of obstruction or blockage (28.3% of patients), and the need to use manual manoeuvres for stool extraction (17.5% of patients) were criteria of OIC that occurred in a lower percentage of cases, but their presence has an equivalent diagnostic value according to the recent definition of OIC. It is therefore very important to collect information about the occurrence of these disorders in the clinical history [8,12,13].
Table 1 presents an analysis of the percentage of patients in whom the presence or absence of each symptom was not documented in the clinical history. In 13% of the cases, the change in stool consistency or the sensation of incomplete evacuation was not documented, and in 19%, the sensation of anorectal obstruction or blockage was not documented.
The need for manual manoeuvres to facilitate bowel movements was not documented in 22%. It will be necessary to reiterate the need for recording all symptoms of OIC to be able to carry out an adequate diagnosis and follow-up of the patient, particularly considering that the use of specific questionnaires outside the clinical trial setting is not common, such as the use of the Bowel Function Index (BFI) recommended in the guidelines [9,21].
The time from the start of opioid treatment to the onset of the first symptoms of OIC was seen to be approximately 16 days, but it is possible that the symptoms had appeared earlier, as has been seen in previous studies, and this data was recorded at the time when the patient reported to the clinic for the next chemotherapy cycle or the next visit [18,22].
No complementary tests were requested for differential diagnosis of OIC in 57.9% of patients. Due to the complexity of cancer patients, the rate at which laboratory and radiological tests were requested is justifiable.
OIC severity was assessed using the CTCAE v.5 criteria [19]. However, 27.3% of patients had no information about the severity of OIC in their clinical history. As an internal consistency measure for the data collected in the study, it was found that the 18.9% of patients seen with Grade 3 severity of OIC (severe) coincided with the percentage of patients requiring manual manoeuvres at diagnosis (Figure 1). There is currently no specific definition of the severity of OIC related to the Rome IV diagnostic criteria.
The percentage of patients who received treatment with laxatives was 77.6% (Figure 2) during the observation period since administration of the opioid, which on average was approximately 5 months in the patients included in the study, and they were administered as first-line treatment (Figure 3) as recommended by current guidelines [11,12,21,23]. As second-line treatment, the administration of PAMORAs with or without laxatives was reported for 58.4% of patients. The use of enemas in 39.5% of patients is quite striking. However, we do not know the clinical conditions of the cancer patients included in the study and whether their use could be justified. Use of enemas is not recommended because of the risk of bowel perforation and secondary infection, in patients at risk of thrombocytopenia, leukopenia, if they have received recent radiotherapy in the pelvic area, or colorectal or gynaecological surgery, in paralytic ileus, intestinal obstruction, and rectal or anal trauma in severe colitis, inflammation or infection in the abdomen, toxic megacolon, abdominal pain of unknown origin, or recent radiotherapy in the pelvic area [16,23].
The mean time seen in the study from the start of treatment with oral laxatives and the finding of lack of efficacy of 10.7 days is within the recommended time. Although there is no consensus definition of laxative-refractory OIC, the most commonly used is: the presence of moderate to severe OIC symptoms despite the use of one or more classes of laxatives for at least four days in a two-week period. [17,18]. The time at which treatment with oral PAMORAs is started since the diagnosis of OIC depends on the previous outcome of laxative use and the time of assessment of its efficacy.
Each case should be assessed individually as regards the waiting period until administration of the next line of treatment, and patient well-being should be a priority. PAMORAs should be administered as soon as the lack of efficacy of laxatives is confirmed [17,18].
The percentages of patients in whom efficacy was observed with each treatment (Figure 4) depended on the definition of efficacy, which was not specified for this study. The efficacy of the treatments analysed should not be considered comparative, since second-line treatment includes patients refractory to treatment with laxatives and perhaps in a more advanced clinical situation.
The limitations of this study are mainly found in its retrospective design and the pooled collection of patient data, which does not allow for comparing variables between patient groups or establishing relationships between them. No random sampling was performed for site selection, so it cannot be guaranteed that the results are representative at national level; however, the distribution of sites by province was seen to be proportional to the population of each area. The definition of treatment efficacy was not established because retrospective data collection would not allow for a valid definition, so the overall efficacy assessment performed for each case was collected.
Conclusion
Although no protocols for the management of cancer patients with OIC were available in a high percentage of sites, compliance with current recommendations was considered consistent with the group of cancer patients in clinical practice. Emphasis should be placed on the need to document complete information about the symptoms and treatments received by cancer patients for OIC in the clinical histories in order to ensure adequate patient follow-up. We think that greater dissemination of the new recommendations on the treatment of OIC is needed, emphasizing the current diagnostic criteria, the importance of laxative administration at the time the opioid is prescribed, and the need to rapidly recognize the inefficacy of this treatment to use the PAMORAs, OIC-specific treatments, recommended as second-line treatment [6,9,16-18,21].
The participation of the following researchers in the study is appreciated.
Investigators EIO-PRAXIS project: Carlos Aguado; Santiago Aguín; Claudio Avila Andrade; Aitor Azkárate Martínez; Javier Ballesteros Bargues; José Balsalobre Yago; Juan Antonio Barrera Ramírez; Gretel Benítez López; Mohamed Hassan Bennis; Reyes Bernabé Caro; Elena María Brozos Vázquez; José Manuel Cabrera Romero; Jon Cacicedo; Hector Randhall Callata Carhuapoma; Alberto Carral Maseda; Alberto Carretero González; Estefanía Casaut Lora; Isaac Ceballos Lenza; Pablo Cerezuela Fuentes; Raquel Cervera Calero; Juan Felipe Cordoba Ortega; Alfonso Cortes Salgado; Patricia Cruz Castellanos; José David Cumplido Burón; Benjamín Domingo Arrué; Eduardo Feliciangeli Moreno; Ovidio Fernández Calvo; Gonzalo Fernández Hinojal; Natalia Fernández Núñez; Lydia Gaba Garcia; Laura Gálvez Carvajal; Carme García Lorenzo; Ana Godoy; Roberto Gómez Díaz; María José Gómez Reina; Aranzazu González Vicente; Carlos Grande Ventura; Maria Guirado Risueño; Henry Hernández Mejia; Raquel Hernández San Gil; Sergio Hoyos Simón; Cruz Josefina; Jairo Legaspi Folgueira; Iker López Calderero; Javier López Gallego; José Luis López González; María Dolores Mediano Rambla; José Carlos Méndez; Antonio José Mérida García; Ekaterina Meshoulam Nikolaeva; Julián Mínguez Manrique; Kevin Molina Mata; Silvia Muñoz Borrajo; Eva Muñoz Couselo; Adolfo Murias Rosales; Edurne Muruzábal Huarte; Esteban Nogales Fernández; Berta Obispo Portero; Ana Otero Romero; Javier Pérez Altozano; Leyre Pérez Ricarte; Nuria Piera Molon; Estela Pineda; María Teresa Quintanar Verdúguez; Avinash Ramchandani Vaswani; Rubén Darío Ramirez Vargas; Eduardo David Roberts Cervantes; Alberto Rodrigo Cáceres; Dulce Rodriguez Mesa; Ángel Rodríguez Sánchez; Hugo Javier Rosales Gonzalez; Sandra Rubiales Trujillano; Renato Salguero Aguilar; Miguel Soria Tristán; Miguel Sotelo Lezama; María Teresa Taberner Bonastre; Remei Tell Busquets.
Ethical standards
The study was approved by an Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
This study was sponsored by ECO Foundation (Excellence and Quality in Oncology), Fundación ECO, Madrid, Spain that received a grant from Kyowa Kirin Farmacéutica, S.L.U. for the study.
EA declared advisory role outside the submitted work with Amgen, Bayer, Celgene, Merck, Roche and Sanofi. CC declared advisory role outside the submitted work with BMS, Roche, Bayer and Angelini, and research funding by BMS, Astra Zeneca and Sysmex. EDR declared advisory role outside the submitted work with Merck-Serono, Amgen, Bayer, Genomica, Servier and MSD.
BS was contracted by Fundación ECO, to conduct the design, monitoring, statistical analysis, medical writing and management of the publications of the study.
Remaining authors received a payment from the sponsor for their participation in the study.
- Jara C, Del Barco S, Grávalos C, Hoyos S, Hernández B, et al. (2017) SEOM clinical guideline for treatment of cancer pain. Clin Transl Oncol 20: 97-107. Link: http://bit.ly/34XlR1y
- Organización Mundial de la Salud (OMS) (1996) Alivio del dolor en el cáncer: Con una guía sobre la disponibilidad de opioides, 2a ed. Link: http://bit.ly/362Tzm5
- Sykes NP (1998) The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 12: 375-382. Link: http://bit.ly/2PfRQDh
- Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, et al. (2009) The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 10: 35-42. Link: http://bit.ly/2YmZ24D
- Galvez R, Provencio M, Cobo M, Pérez C, Pérez C, et al. (2014) Prevalencia y severidad de la disfunción intestinal inducida por opioides. Atención Primaria 46: 32-39. Link: http://bit.ly/3889Ub5
- Rumman A, Gallinger ZR, Liu LWC (2016) Opioid induced constipation in cancer patients: pathophysiology, diagnosis and treatment. Expert Review of Quality of Life in Cancer Care 1: 25-35. Link: http://bit.ly/2sKAd6Z
- Abramowitz L, Beziaud N, Labreze L, Giardina V, Caussé C, et al. (2013) Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ 16: 1423-1433. Link: http://bit.ly/33Pegk9
- Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, et al. (2016) Bowel Disorders. Gastroenterology 150: 1393-1407. Link: http://bit.ly/2RlkKEJ
- Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, et al. (2014) Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 26: 1386-1395. Link: http://bit.ly/2LE52RF
- Panchal SJ, Schwefe MP, Wurzelmann JI (2007) Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 61: 1181-1187. Link: http://bit.ly/35YBzJE
- Kumar L, Barker C, Emmanuel A (2014) Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract 2014: 141737. Link: http://bit.ly/2Pad5GE
- Drossman DA, Hasler WL (2016) Rome IV-Functional GI Disorders: Disorders Gut-Brain Interaction. Gastroenterology 150: 1257-1261. Link: http://bit.ly/34RPP6Y
- Mearin F, Ciriza C, Mínguez M, Rey E, Mascort JJ, et al. (2016) Clinical Practice Guideline: Irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig (Madrid) 108: 323-363. Link: http://bit.ly/2Rl9eJu
- Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM (2015) Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol 8: 360-372. Link: http://bit.ly/33NBaZe
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, et al. (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93: 247-257. Link: http://bit.ly/2YgzZ38
- Larkin PJ , Cherny NI, La Carpia D, Guglielmo M, Ostgathe C, et al. (2018) Ripamonti CI. ESMO Guidelines Committee; Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Annals of Oncology 29 iv111-iv125. Link: http://bit.ly/2YhknfX
- Crockett SD, Greer KB, Heidelbaugh JJ, Falck-Ytter Y, Hanson BJ, et al. (2019) American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the medical Management of opioid-induced constipation. Gastroenterol 156: 218-226. Link: http://bit.ly/2DNNNsC
- Farmer AD, Drewes AM, Chiarioni G, De Giorgio R, O'Brien T, et al. (2019) Pathophysiology and Management of opioid-induced constipation: European expert consensus statement. United European Gastroenterology J 7: 7-20. Link: http://bit.ly/33Lwd2Y
- U.S. Department of Health and Human Services (2017) National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE. Link: http://bit.ly/365GEQE
- Galceran J, Ameijide A, Carulla M, Mateos A, Quirós JR, et al. (2017) Cancer incidence in Spain, 2015. Clin Transl Oncol 19: 799-825. Link: http://bit.ly/2YmTiIm
- Argoff CE, Brennan MJ, Camilleri M, Davies A, Fudin J, et al. (2015) Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med 16: 2324-2337. Link: http://bit.ly/2qmcFVb
- Poulsen J, Nilsson M, Brock C, Sandberg TH, Krogh K, et al. (2016) The impact of opioid treatment on regional transit. J Neurogastroenterol Motil 22: 282-291. Link: http://bit.ly/2Yijpjq
- Instituto Nacional del Cáncer (INC) (2016) Complicaciones gastrointestinales (PDQ®)–Versión para profesionales de salud. Link: http://bit.ly/2LmJHM4
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
