Open Journal of Pain Medicine
Methods for saving opioids in the perioperative period via intravenous, via neuraxial and in nervous blocks
Borja Mugabure Bujedo*
Cite this as
Bujedo BM (2019) Methods for saving opioids in the perioperative period via intravenous, via neuraxial and in nervous blocks. Open J Pain Med 3(1): 015-020. DOI: 10.17352/ojpm.000012The greatest challenge facing anesthesiologists in the current clinical context is to provide adequate perioperative care, achieving sufficient control of acute pain and at the same time make responsible use of intraoperative opioids. There is a trending topic in our times that is based on saving perioperative opioids called Opioid free anesthesia (OFA) or to a lesser extent Opioid less anesthesia (OFA) to avoid the so-called opioid epidemic. All this is framed within appropriate protocols of care and post-operative recovery (ERAS).
In this sense, it has been proven that there is clinical evidence of several drugs as a single therapy against intravenous placebo. These include dexmedetomidine, ketamine, lidocaine, gabapentinoids, and esmolol. However, there are no studies that support the combination of several of these drugs in the same patient during the same perioperative period. On the other hand, intrathecal morphine in doses lower than 300 mcg has shown high effectiveness in saving opioids after major surgery. There are also useful adjuvants in this field for perineural administration. These include dexamethasone, dexmedetomidine, and buprenorphine.
Introduction
The opioid epidemic they suffer in the United States is hardly justifiable and certainly not acceptable to health agencies. Multiple factors have been studied to eliminate the risk of overdose and unintentional deaths related to their use and estimated at around 20,000 people per year. They have entailed a personal and social cost that surpasses that of the Vietnam War. That is why the U.S. government declared a state of health emergency [1]. Therefore, health organizations such as the FDA (Food and drug administration) and CDC (Center control of diseases) have advocated new guidelines on the management of opioids in the context of chronic pain to reduce this socio-health scourge. Likewise, they have developed a training program for health professionals in the reasonable prescription of opioids, initiated in December 2011, called REMS, to evaluate, reduce and mitigate the risk associated with its use [2]. In response to these calls for sanity, all health professionals dedicated to the management of acute postoperative pain have been involved in developing postoperative analgesia programs based on a reduced use called opioid less anesthesia (OLA) or even abolished opioid-free anesthesia (OFA). All this framed in a global program of post-operative recovery, including rehabilitation, early mobilization, and sensibly returns to their hospital environment. Globally they have been called ERAS, and have become the cornerstone of perioperative patient management [3]. In a recent article on the chronic use of patients who underwent surgery in the U.S., and who had not used opioids before surgery, it was found that 7% of them maintained them as chronic therapy three months later. More striking was the fact that they were mostly considered minor surgeries such as varicose veins surgery, appendectomy or inguinal hernia repair surgery. There were previous comorbidities that favor this fact, such as chronic pain prior lumbar or cervical, psychological disorders such as anxiety and cross addictions such as smoking or alcohol consumption [4].
Therefore, we the anesthesiologists have in our hand the power to initiate in our surgical environment a modality of multimodal analgesia not based on opioids and thus reduce later consumption. For this, we have regional techniques and a multitude of non-opioid drugs, starting with paracetamol and anti-inflammatories, and followed by gabapentinoids (pregabalin and gabapentin), alpha-2 agonists such as dexmedetomidine, lidocaine, and ketamine, beta-blockers such as esmolol and glucocorticoids. We are entering a new era of reinvention of postoperative analgesia, in which anesthesiologists must adapt to the changes, because as Charles Darwin said “the species that best suits to the environment around it, is the one that survives and endures over time [5].
Material and Methods
To this end, the author has carried out a non-systematic review, relating understandably what each of the drugs, known as opioid savers, provide, both intravenously and in association with local anesthetics via neuraxial and nervous blocks. To this end, a search has been done for articles in English found in Pub Med/ Ovid Medline until December 2018 with the keywords; opioid-free analgesia, postoperative pain, postoperative recovery, dexmedetomidine, clonidine, ketamine, pregabalin, lidocaine, esmolol. Systematic reviews were mainly recruited with or without meta-analysis, Cochrane reviews, controlled clinical trials, and finally expert opinion articles on the subject.
Findings
The use of multimodal or balanced analgesia is a generalized fact. Its practice is supported by a multitude of controlled clinical trials, clinical practice guidelines, and recommendations from scientific societies. The efficacy, in terms of saving milligrams of morphine in the first 24 hours, is higher for intrathecal morphine in abdominal surgery. Significant savings are also achieved with the use of intravenous perfusions of dexmedetomidine and lidocaine, especially in abdominal surgery. Ketamine is useful both as a bolus and as a perioperative infusion. Dexamethasone and buprenorphine have also been shown to be useful as perineural adjuvants. All studies are corroborated by comparisons to placebo, not in the form of combination therapy, on which further clinical experience is needed.
Intravenous coadjutants useful in OFA / OLA
Dexmedetomidine: Dexmedetomidine is an adrenoreceptor α-2 agonist. This drug is approved for use as a sedative in patients in intensive care units, so its use as an intraoperative analgesic adjuvant is out of technical specifications in most countries. However, its action on the alpha-2 receptors of the posterior medullary horn and locus ceruleus makes it very attractive for this use.
A Cochrane review [6], that included 422 patients included in 7 RCTs concluded that their use in continuous intraoperative infusion at a dose of 0.5-1 μg/kg in bolus ± intraoperative infusion produced a statistically significant reduction in opioid consumption in the first 24 hours postoperatively despite not decreasing pain intensity measured on the analog visual scale (VAS). Most recent studies included patients in abdominal surgery [7], although subsequent studies have demonstrated its efficacy in gynecologic [8], orthopedic [9], and neurosurgery surgery [10].
Ketamine: Ketamine is an (NMDA) N-methyl-d-receptor aspartate antagonist, which has been used in the context of chronic oncological, neuropathic, and also post-surgical pain. The blocking of the NMDA receiver allegedly results in a reduction in nociceptive transmission and inflammatory pain. Besides, preclinical evidence suggests that ketamine may also exert its analgesic effect by interacting with other opioid receptors (μ, δ). The Consensual Guidelines on the Use of Intravenous Ketamine for the Management of Acute Pain, developed by a panel of experts from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain, and the American Society of Anesthesiology, could hardly be published at a more appropriate time than the present. Overall, they concluded that there is moderate evidence supporting the use of subanesthetic doses of ketamine bolus IV (up to 0.35 mg/kg) and infusions (up to 1 mg/kg per hour) as opioid supplements for perioperative analgesia (moderate level of certainty, grade B recommendation) [11].
Gabapentinoids (pregabalin and gabapentin): The gabapentinoids (gabapentin and pregabalin) are structural analogs of the neurotransmitter inhibitor GABA, but their mechanism of action consists of inhibiting nerve transmission by attaching to the α-2-δ subunit of voltage-dependent calcium channels. Gabapentin was first used at doses of 300-400 mg/8h, and although expectations were high, recent reviews focusing on studies with low levels of the statistical error have not confirmed this potential as a perioperative opioid saver [12,13].
Pregabalin acts through the same mechanism, but its pharmacokinetics is more predictable and linear and in turn, is not influenced by the high dependence on renal function such as gabapentin. In a recent systematic review of 4155 patients included in 55 RCTs, an apparent opioid-sparing effect was demonstrated in the first 24 hours compared to placebo, and a decrease in the VAS score both at rest and in motion. However, there was no difference between single-dose (75-300 mg) preoperative administration regimens or those of prolonged duration several postoperative days [14]. Two subsequent meta-analyses confirmed this good efficacy profile of pregabalin [15,16]. However, a recent systematic review [17], which included 7201 patients out of 97 RCTs, applied the GRADE system of statistical analysis (Grading of Recommendations, Assessment, Development and Evaluation) and assessed both the efficacy and savings of perioperative opioid of pregabalin, as well as the occurrence of adverse effects considered necessary for having to discontinue therapy, and found little encouraging results. Overall, morphine savings in the first 24 hours were only included in 11 RCTs and were calculated at 5.8 mg (3.2-8.5, confidence interval: 3,2-8,5). A correlation was also found between the dose administered and the effect measured, being higher for high doses (300 mg) but with a significant increase in adverse effects such as sedation, dizziness, and mental confusion. As a conclusion, based on the low risk of bias trials, the pregabalin may have a minimal preservation effect of opioids, but the risk of severe adverse effects seems to be increasing. Therefore, the GRADE assessments showed evidence of moderate to deficient quality.
Lidocaine: This ancient drug has anti-arrhythmic, analgesic, and cell membrane stabilizing properties as a local anesthetic, through various mechanisms of action in which the blockage of sodium channels predominates. However, other steps have been taken, such as interleukin release inhibitor and NMDA antagonist.
Its effectiveness as a perioperative opioid saver has been demonstrated in a Cochrane review [18], that included 1700 patients of 43 RCTs versus placebo. It was shown that bolus administration followed by continuous infusion for 24 h (100 mg or 1-3 mg/kg, followed by an infusion from 1-5 mg/kg/h, to 2-4 mg/min), decreased opioid consumption and VAS score during this period, but this effect was not prolonged at 48 h. The most significant impact was found in patients with abdominal surgery in which the paralytic ileus, as well as the PONV index, decreased, which contributed to an earlier hospital discharge estimated at 8 hours.
Esmolol: Esmolol is a drug from the beta-blocking group with selective action on the β-1 receptor. Its duration of action is ultra-short due to its elimination by plasma cholinesterase enzymes, so it is desirable to avoid hemodynamic response due to tracheal intubation. In the form of continuous infusion, it has been used to prevent hypertensive response to insufflation of the pneumoperitoneum in laparoscopic surgery and recently to avoid perioperative consumption of opioids by modulating the central adrenergic response.
A meta-analysis including 936 patients from 19 RCTs [19], concluded that continuous infusion of esmolol (5-500 μg/kg/min), preceded in some cases by a bolus of intravenous loading dose (0.5-1 mg/kg), achieved a 69% decrease in postoperative opioid consumption during the first 24 h estimated at 5.1 mg of morphine, compared to the placebo group. This fact leads to a 61% decrease in the incidence of PONV. The reduction in postoperative pain scores was also statistically significant, while the opioid saving effect appears promising, was clinically modest due to the available studies show critical methodological deficiencies [19].
Magnesium: Systemic magnesium has been used to minimize postoperative pain with conflicting results through clinical studies. The objective of the current research was to assess the effect of systemic magnesium on postoperative pain outcomes. An extensive search was conducted to identify randomized controlled trials that evaluated the effects of systemic magnesium on postoperative pain outcomes in surgical procedures performed under general anesthesia. Meta-analysis was performed using a random-effects model [20]. Twenty randomized clinical trials with 1,257 subjects were included. The weighted mean difference (99% CI) of the combined effects favored magnesium control for pain at rest (≤ 4 h, -0.74 [-1.08 to -0.48], 24 h, -0.36 [-0.63 to -0.09]) and with movement at 24 h, -0.73 (-1.37 to -0.1). Opioid consumption decreased greatly in the systemic magnesium group compared to control, a weighted mean difference (99% CI) of -10.52 (-13.50 to -7.54) mg. Morphine. Significant heterogeneity was observed in some analyses but could be explained in part by intraoperative administration of single magnesium compared to intraoperative and postoperative administration in other studies. None of the studies reported clinical toxicity related to toxic serum levels of magnesium. The main conclusion was that systemic administration of perioperative magnesium reduces postoperative pain and rescue opioid consumption. Therefore, administration of magnesium should be considered as a strategy for relieving postoperative pain in surgical patients.
Regional techniques
There are numerous articles with a high degree of scientific evidence that support the use of regional techniques, both central as the epidural or intrathecal way, or peripheral as blocks of nerve plexuses or peripheral nerves, to reduce the use of postoperative opioids. The use of a local anesthetic before surgical incision in a multitude of surgical procedures has demonstrated a significant reduction in postoperative opioid consumption, a shorter hospital stay, as well as a decrease in the incidence of chronic pain. All of this results in higher patient satisfaction and lower healthcare costs. These techniques are currently implemented in the ERAS protocols in most hospitals throughout the world.
Opioid-sparing adjuvants in Neuraxial Analgesia: Neuraxial, epidural or intrathecal anesthesia has also been shown to be very useful in decreasing postoperative opioid consumption. The combination of a local anesthetic with an opioid, especially of a hydrophilic nature such as morphine, has been very effective in this purpose in a wide range of types of surgery, but at the expense of a higher number of side effects such as hypotension, sedation, pruritus, or technical failures such as the accidental removal of the epidural catheter. Morphine has been the most widely used epidural opioid and could be considered the “gold standard” of spinal drugs, since due to their selectivity the used epidural dose is much lower than the parenteral (1/10), with a recommended maximum dose of 10 mg daily. It can be administered in either bolus form (30-100 μg/kg) or as in continuous infusion, as the latter seems to induce a higher quality analgesic, and as a drug alone or together with local anesthetics, as the latter enhances the global effect of the by means of a synergistic effect [21]. Therefore, the use of neuraxial morphine (intrathecal and epidural) has been shown to decrease consumption of systemic perioperative opioids in a variety of forms of thoracic and abdominal procedures. In a meta-analysis of 15 RCTs on patients undergoing coronary revascularization surgery, it was proven how neuraxial anesthesia achieved an estimated saving of 11 mg of morphine/day compared to the control group [22]. Similar results have been observed with epidural analgesia after abdominal and cesarean section surgery [23,24].
Concerning intrathecal administration of morphine, a meta-analysis including 645 patients from 27 RCTs [25], undergoing major surgery (15 cardiothoracic surgery, 9 abdominal surgery, and 3 spine surgery), received a preoperative dose of 100-1000 μg, demonstrated postoperative morphine saving estimated at an average of 19.6 mg. It was also proven that the VAS resting scale was 2 cm lesser at four h later and 1 cm lower at 12 and 24 h, on a range over 10 cm in the intrathecal morphine group. Moreover, this effect was more pronounced against the movement, since the improvement was maintained at 2 cm throughout the control time. Postoperative opioid requirements up to 48 h were lower in the intrathecal morphine group, and morphine consumption at 24 h was significantly lower in the abdominal surgery group (-24.2 mg) compared to the cardiothoracic surgery group (-9.7 mg).
More recently, a meta-analysis of 393 patients undergoing vertebral scoliosis correction surgery included in 8 RCTs also showed a reduction in both the VAS scale and postoperative opioid consumption opposite to placebo group [26].
Useful additives added to local anesthetics in the plexus blocks: Within the current trend of performing correct postoperative analgesia within an ERAS program, the performance of continuous regional techniques, maintained in the immediate postoperative in the hospital and later in the outpatient setting, took a big boom among health professionals. However, the difficulty in controlling these analgesic devices in the patient’s home environment entails a series of additional challenges and an increase in the number of personnel needed to perform it within adequate safety margins. For this reason, pharmacological adjuvants have been studied in this field that prolongs the effect of local anesthetics and help us to maintain excellent postoperative analgesia without the aid of continuous infusion systems with associated opioid-sparing effects [27].
Dexamethasone: There is considerable controversy about the use of dexamethasone as an adjuvant to local anesthetics in nerve blocks. On the one hand, there is a recent meta-analysis that showed a dose of 4 to 10 mg of this drug doubled the time of postoperative analgesia until 22 h, compared to the group that received an only local anesthetic in the blockade of the brachial plexus [28]. In a later study, it was found that this analgesic blocking potentiating effect, in this case in a supra-scapular plexus approach, was achieved with doses as low as 1-4 mg of dexamethasone, the duration of bupivacaine analgesia was prolonged from 12 to 22 hours with no side effects to report [29]. But on the other hand, due to the positive analgesic potentiating effect at such low doses, it has been postulated that it may be due to its anti-inflammatory fact by systemic absorption. Studies were designed to compare the effect of dexamethasone at high doses (8-10 mg) intravenously versus via perineural and demonstrated a saving impact of opioids in favor of the latter group of patients [30-32]. Subsequently, a meta-analysis compared these two routes of administration and estimated daily morphine savings in favor of the perineural dexamethasone group at 7.1 mg [33]. Another point of controversy is the possible neurotoxic effect of dexamethasone on neural tissue. Studies in this regard are controversial and worth their use because of the absence of harmful effects at this level, and there are even authors who give it a specific neuroprotective effect [34,35].
Finally, a Cochrane review of the highest level of evidence, published in 2017 [36], attempted to clarify this dilemma and concluded that there is a low to moderate level of evidence on the effectiveness of dexamethasone as an opioid-sparing adjuvant both intravenously and for perineurally in upper limb blocks. However, there was not enough evidence to recommend its use in lower limb blocks or children.
Dexmedetomidine: Dexmedetomidine has also been successfully used as an opioid-sparing adjuvant in the nerve block of different anatomical locations. A recent meta-analysis [37], has summarized published evidence on this drug in this clinical field. This review was made over 2007 patients included in 32 RCTs, and statistically significant differences were found in several of the variables studied; prolongation of sensory and motor block in at least 57%, and 63 % in the duration of analgesia. It also shortened the onset of analgesia by 40% and reduced postoperative morphine consumption during the first 24 hours by an average amount calculated at 10.2 mg. On the other hand, an increase in the relative risk of suffering adverse effects such as bradycardia and hypotension was detected. The estimated dose to achieve a better balance between risk and benefit was 50-60 μg.
Recently, in order to elucidate which of the two previously reviewed drugs could be chosen as the adjuvant of choice for local anesthetics in the blockade of nervous plexus, a systematic comparative review has been published between perineural dexamethasone and dexmedetomidine on the brachial plexus [38]. This direct comparison was impossible due to a lack of studies, so the authors performed an indirect meta-analysis on 3019 patients included in 49 reviews. The conclusion was that both drugs have weak quality evidence of their analgesic and motor block enhancing effect, but dexamethasone prolongs this effect about 2.5 hours longer, without the harmful hemodynamic impact of dexmedetomidine.
Opioids: Opioid pharmaceuticals have also been used as adjuvants to local anesthetics in plexus blocks with a wide variety of results. Most of them, such as morphine and fentanyl, have not been approved in clinical practice due to their sedative and depressant effects in the respiratory center. However, buprenorphine, a mu-opioid partial agonist and kappa receptor antagonist, without respiratory depressant effect and with an anti-hyperalgesic impact, has been studied demonstrating its efficacy in this field.
A recent meta-analysis of 685 patients included in 13 RCTs [39], showed a statistically significant difference in favor of perineural administration of buprenorphine versus an intravenous injection, estimated at 8 hours. However, the relative risk of producing PONV was higher in the buprenorphine group. Perineural administration was more effective in prolonging postoperative analgesia than the group that received intramuscular buprenorphine, in an estimated time of 6.4 hours. Therefore, perineural administration is more effective than systemic administration with a similar risk of PONV. Clinical doses used in clinical trials ranged from 0.1 to 0.3 mg.
Conclusions
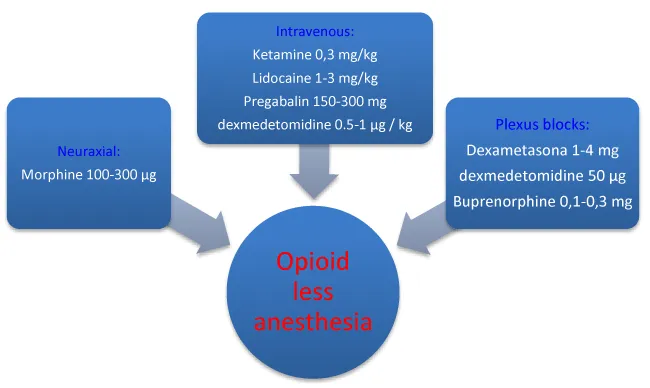
As a final reflection, we could conclude that a new field of research and clinical application of new opioid-saving pharmacological adjuvants is opened in the perioperative period within the multimodal programs of post-surgical recovery (ERAS). However, to include these drugs in our daily practice, we must base ourselves on clinical evidence standards and make rational use of them. Some studies support the use of pregabalin (oral), lidocaine, dexmedetomidine, esmolol, and ketamine as adjuvants intravenously in the form of boluses or continuous infusion, and its usefulness against placebo, but there is no evidence of the combination of them in the form of combined multi-pharmacological therapy. Its use should be limited to surgeries in which the benefit in the opioid-sparing effect exceeds that of producing severe adverse effects. Moreover, the administration of perineural dexamethasone in brachial plexus blocks has shown great efficacy and good tolerability. Dexmedetomidine and buprenorphine, on the other hand, have demonstrated good efficacy in this field with a higher incidence of adverse effects, so we must assess their use in each particular case. Within neuraxial techniques, intrathecal administration of preoperative morphine has shown the highest efficacy in terms of saving postoperative morphine, but we must maintain strict control of our patients to avoid the feared late respiratory depression. The doses usually used in clinical practice are summarized in figure 1.
- Califf RM, Woodcock J, Ostroff S (2016) A proactive response to prescription opioid abuse. N Engl J Med 374: 1480-1485. Link: http://bit.ly/2lywyG0
- Lipman AG (2016) The opioid abuse blame game. J Pain Palliat Care Pharmacother 30: 2-3. Link: http://bit.ly/2lAQhVd
- Cata JP, Bugada D, De Andrés J (2017) Opioid less perioperative care. Minerva Anestesiol 83: 315-320. Link: http://bit.ly/2jVqtCP
- Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, et al. (2017) New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152: e170504. Link: http://bit.ly/2jVYNhb
- Zaccagnino MP, Bader AM, Sang CN, Correll DJ (2017) The perioperative surgical home: a new role for the acute pain service. Anesth Analg 125: 1394-1402. Link: http://bit.ly/2jZKrfS
- Jessen Lundorf L, Korvenius Nedergaard H, Moller AM (2016) Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev 2: CD010358. Link: http://bit.ly/2lAQJ5R
- Ge DJ, Qi B, Tang G, Li JY (2016) Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep 6: 21514. Link: http://bit.ly/2kf0Xc8
- Chan IA, Maslany JG, Gorman KJ, O’Brien JM, McKay WP (2016) Dexmedetomidine during total knee arthroplasty performed under spinal anesthesia decreases opioid use: a randomizedcontrolled trial. Can J Anaesth 63: 569-576. Link: http://bit.ly/2jZLah6
- Garg N, Panda NB, Gandhi KA, Bhagat H, Batra YK, et al. (2016) Comparison of small dose ketamine and dexmedetomidine infusion for postoperative analgesia in spine surgery - a prospective randomized double-blind placebo controlled study. J Neurosurg Anesthesiol 28: 27-31. Link: http://bit.ly/2lulkSI
- Song J, Ji Q, Sun Q, Gao T, Liu K, et al. (2016) The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy. J Neurosurg Anesthesiol 28: 14-20. Link: http://bit.ly/2kqSnXz
- Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, et al. (2018) Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 43: 456-466. Link: http://bit.ly/2kcla2d
- Fabritius ML, Geisler A, Petersen PL, Nikolajsen L, Hansen MS, et al. (2016) Gabapentin for postoperative pain management - a systematic review with metaanalyses and trial sequential analyses. Acta Anaesthesiol Scand 60: 1188-1208. Link: http://bit.ly/2lWwbFi
- Doleman B, Heinink TP, Read DJ, Faleiro RJ, Lund JN, et al. (2015) A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 70: 1186-1204. Link: http://bit.ly/2kqSClr
- Mishriky BM, Waldron NH, Habib AS (2015) Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 114: 10-31. Link: http://bit.ly/2krFwEH
- Lam DM, Choi SW, Wong SS, Irwin MG, Cheung CW (2015) Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine (Baltimore) 94: e1944. Link: http://bit.ly/2kogkil
- Eipe N, Penning J, Yazdi F, Mallick R, Turner L, et al. (2015) Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain 156: 1284-1300. Link: http://bit.ly/2krFHQn
- Fabritius ML, Strøm C, Koyuncu S, Jæger P, Petersen PL, et al. (2017) Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. Br J Anaesth 119: 775-791. Link: http://bit.ly/2keYZsh
- Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, et al. (2015) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev CD009642. Link: http://bit.ly/2jW6oMR
- Watts R, Thiruvenkatarajan V, Calvert M, Newcombe G, van Wijk RM (2017) The effect of perioperative esmolol on early postoperative pain: a systematic review and meta analysis. J Anaesthesiol Clin Pharmacol 33: 28-39. Link: http://bit.ly/2lWuYOh
- De Oliveira GS Jr, Castro-Alves LJ, Khan JH, McCarthy RJ (2013) Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 119: 178-190. Link: http://bit.ly/2keZfHL
- Bujedo BM, Santos SG, Azpiazu AU (2012) A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag 8: 177-192. Link: http://bit.ly/2lWxDYg
- Liu SS, Block BM, Wu CL (2004) Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta-analysis. Anesthesiology 101: 153-161. Link: http://bit.ly/2lAk0gZ
- Guay J, Kopp S (2016) Epidural pain relief versus systemic opioid- based pain relief for abdominal aortic surgery. Cochrane Database Sys Rev CD005059. Link: http://bit.ly/2lswpDZ
- Bonnet MP, Mignon A, Mazoit JX, Ozier Y, Marret E (2010) Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain 14: 894. e1-e9. Link: http://bit.ly/2lyyTkg
- Meylan N, Elia N, Lysakowski C, Tram.r MR (2009) Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta analysis of randomized trials. Br J Anaesth 102:156-167. Link: http://bit.ly/2jTUUt6
- Pendi A, Acosta FL, Tuchman A, Movahedi R, Sivasundaram L, et al. (2017) Intrathecal morphine in spine surgery: a meta-analysis of randomized controlled trials. Spine (Phila Pa 1976) 42: E740-E747. Link: http://bit.ly/2lVpSlo
- Kumar K, Kirksey MA, Duong S, Wu CL (2017) A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth Analg 125: 1749-160. Link: http://bit.ly/2jWDNa6
- Choi S, Rodseth R, McCartney CJ (2014) Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematicreview and meta-analysis of randomized trials. Br J Anaesth 112: 427-439. Link: http://bit.ly/2lveJYl
- Liu J, Richman KA, Grodofsky SR, Bhatt S, Huffman GR, et al. (2015) Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve block? A prospective randomized double- blinded clinical study. J Clin Anesth 27: 237-242. Link: http://bit.ly/2lAGP48
- Desmet M, Braems H, Reynvoet M, Plasschaert S, Van Cauwelaert J, et al. (2013) I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth 111: 445-452. Link: http://bit.ly/2jW2rYp
- Rahangdale R, Kendall MC, McCarthy RJ, Tureanu L, Doty R, et al. (2014) The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg 118: 1113-1119. Link: http://bit.ly/2k0U26a
- Abdallah FW, Johnson J, Chan V, Murgatroyd H, Ghafari M, et al. (2015) Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med 40: 125-132. Link: http://bit.ly/2kePQ2S
- Chong MA, Berbenetz NM, Lin C, Singh S (2017) Perineural versus intravenous dexamethasone as an adjuvant for peripheral nerve blocks: a systematic review and meta-analysis. Reg Anesth Pain Med 42: 319-326. Link: http://bit.ly/2lyzPoM
- An K, Elkassabany NM, Liu J (2015) Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hiperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One 10: e0123459. Link: http://bit.ly/2lAHnac
- Marty P, Bennis M, Legaillard B, Cavaignac E, Ferre F, et al. (2018) A new step toward evidence of in vivo perineural dexamethasone safety: an animal study. Reg Anesth Pain Med 43: 180-185. Link: http://bit.ly/2lOE3IK
- Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B (2017) Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev 11: CD011770. Link: http://bit.ly/2kcoQkx
- Vorobeichik L, Brull R, Abdallah FW (2017) Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 118: 167-181. Link: http://bit.ly/2jVuKWT
- Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW (2019) Dexamethasone is Superior to Dexmedetomidine as a Perineural Adjunct for Supraclavicular Brachial Plexus Block: Systematic Review and Indirect Meta-analysis. Anesth Analg 128: 543-554. Link: http://bit.ly/2lPrs8b
- Schnabel A, Reichl SU, Zahn PK, Pogatzki-Zahn EM, Meyer-Friebem CH (2017) Efficacy and safety of buprenorphine in peripheral nerve blocks: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol 34: 576-586. Link: http://bit.ly/2krctRs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
