Open Journal of Pain Medicine
Neuropathic pain due to chronic Idiopathic Axonal Neuropathy: Fast pain reduction after topical phenytoin cream application
Jan M Keppel Hesselink1* and David J Kopsky2
2Institute for Neuropathic Pain, Amsterdam, Netherlands
Cite this as
Keppel Hesselink JM, Kopsky DJ (2018) Neuropathic pain due to chronic Idiopathic Axonal Neuropathy: Fast pain reduction after topical phenytoin cream application. Open J Pain Med 2(1): 007-008. DOI: 10.17352/ojpm.000008Pain due to chronic idiopathic axonal polyneuropathy (CIAP) is often treated with therapies based on general neuropathic pain guidelines, which are mainly developed with randomized clinical trials having evaluated treatments for painful diabetic neuropathy (PDN) and post-herpetic neuralgia. No clinical trial studying treatments for patients with painful CIAP has been yet executed, thus only extrapolation of the value of treatments from other neuropathic pain fields is possible. Clearly, the pathogenesis and pathophysiology of CIAP will be quite different from that of PDN. Many CIAP patients complain of intolerable side effects of current analgesic medication. In order to help those patients, we developed a treatment-regime consisting of the supplement palmitoylethanolamide and topical formulations of analgesics. This combination is rarely complicated by side effects. One of our most recent topical formulation we developed contains 10% phenytoin, a broad acting voltage-gated sodium-channel blocker. Phenytoin 10% cream can also be used as a stand-alone therapy as we will discuss.
We present a case of a CIAP patient, treated successfully with phenytoin 10% cream, reporting a fast onset of pain relief. In a video, uploaded at YouTube, the patient explains the effect of phenytoin 10% cream, which makes this case report more vivid.
Introduction
Chronic idiopathic axonal polyneuropathy (CIAP) is a progressive sensory or sensorimotor polyneuropathy that affects elderly patients. Sometimes this neuropathy is referred to as cryptogenic neuropathy. These patients often suffer from peripheral neuropathic pain. Randomized clinical trials (RCTs) evaluating analgesic therapies in this population however are missing [1].
Since 2010 we regularly see these patients in our Institute for Neuropathic Pain in the Netherlands. Our mission is to treat neuropathic pain patients with effective treatments with (nearly) no side effects. One of these treatments we evaluated in the past was the supplement palmitoylethanolamide, 1200 mg/day, dosed in 3 times daily in capsules of 400 mg. This supplement often reduces CIAP pain after some weeks. [2] Furthermore we developed topical formulations with (co-)analgesics with (nearly) no side effects when used properly. The latest developed topical pain cream contains the old co-analgesic compound phenytoin. [3] Phenytoin cream can be used in combination with other therapies or as a stand-alone therapy.
In CIAP patients suffering from neuropathic pain, one of the pain characteristics mostly present is burning, suggestive for the involvement of a small fiber component. [4] Most patients explain they feel the burning pain in the skin, such as sun burn, which was for us a pointer to develop topical formulations containing analgesics. In patients suffering from pain due to small fiber neuropathy we noticed a fast onset of action of compounded analgesic cream; pain could be reduced within 20 minutes after the application of a phenytoin 10% cream [5].
Here we present a patient suffering from EMG documented CIAP and burning pain, tested for his response to phenytoin 10% cream in a single-blind fashion. Meanwhile, we use this single-blind response test always before we prescribe phenytoin cream, to rule out placebo-responders already during the first visit. [6, 7]
Case Presentation
A man of 56 years old, started to have symptoms of restless legs many years ago, treated with pramipexol 0.125 mg twice daily and temazepam 10 mg once daily. He gave his informed consent for this case-presentation, as well as for a Youtube movie, where the patient explains what he noticed after the application of the phenytoin cream: https://www.youtube.com/watch?v=9NHZ-B1iYlI ).
For one year, he experienced burning feet, cramps in leg muscles, and sensibility disturbances in both feet (walking on cotton). Pain scores on the 11-point numerical rating scale (NRS) were fluctuating between 6-9 depending on his activities. He was referred to our clinic by the neurologist for further treatment of his burning pain. On examination, the score on the screening tool DN-4 (Douleur Neuropathique 4 questions), was 5, clearly indicative for neuropathic pain.
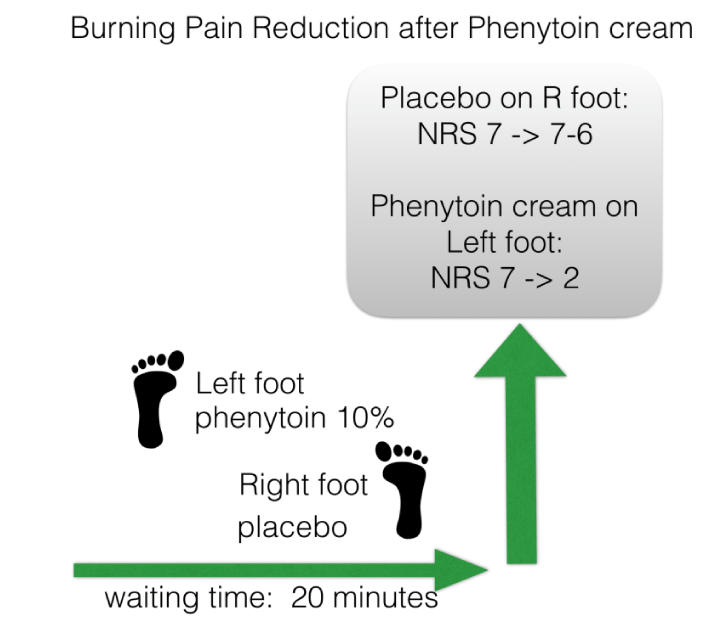
Before prescribing a topical analgesic cream, we conducted a single-blind placebo-controlled response test (SIBRET), applying placebo-cream on the right foot, and phenytoin 10% cream on the left foot (Figure 1). Our instruction was: ‘I would like to offer you to test one cream on one pain area, which I believe can help to lessen your suffering without knowing how it exactly works. The other cream I would like to offer you to test for the other area, the working mechanism is clearer, though side effects can occur. After 30 min, you will tell us whether there is a difference in pain scoring on the NRS, and based on your evaluation we know what best to prescribe you.’ [7] Within 20 minutes after application the patient clearly felt the difference: while the pain in the right foot remained more or less the same, pain in the left foot was reduced from 7 to 2 as scored on the NRS. This response led us to prescribe phenytoin 10% cream, developed together with a GMP certified pharmacist in the Netherlands.
Discussion
Peripheral neuropathic pain is a complication of many polyneuropathies. Neuropathic pain arises due to release of inflammatory molecules around the affected nerves, leading to over-activity of the sensory nerves. Since the sensory nerves reach the stratum granulosum, the upper most layer with living keratinocytes in the epidermis, active substances only need to cross the stratum corneum to influence the overactive sensory nerves. This fact stimulated us to develop topical analgesics with the purpose only reaching the epidermis, thus not reaching the blood stream, as transdermal formulations intend to do. One of our topical formulations is phenytoin 10% cream. Most polyneuropathies have a symmetrical distribution, with equal pain intensity. This second fact led us to develop SIBRET in order to identify responders. During the first visit in case of a symmetrical polyneuropathy with comparable pain intensity at both sides, we offer the patient apply on one foot placebo cream, and on the other foot phenytoin cream. For CIAP this is nearly always the case. In the case described above, the patient could correctly identify the active cream within a time-frame of 20 minutes.
Since our first introduction of the cream, we have treated hundreds of patients, most of those successfully, and we reported a series of 12 and 21 patients recently, based on SIBRET. [3, 7] In these patients, pain reduction on the phenytoin cream applied area was significantly more pronounced than at the placebo cream applied area. [3 ,7] In order to further evaluate phenytoin cream in CIAP patients we started preparing a full powered cross-over RCT in CIAP patients, together with colleagues of the Medical Academic Centre in Utrecht. This study will provide further answers of the value of phenytoin cream for pain patients due to CIAP, and will analyze the contribution of the response-test to identify responders.
Conflict of Interest
The authors patent holders of two patents related to the topical formulations of phenytoin in the treatment of pain: 1) Topical phenytoin for the use in the treatment of peripheral neuropathic pain and 2) Topical pharmaceutical composition containing phenytoin and a (co-) analgesic for the treatment of chronic pain.
- Warendorf J, Vrancken AF, van Schaik IN, Hughes RA, Notermans NC (2017) Drug therapy for chronic idiopathic axonal polyneuropathy. Cochrane Database Syst Rev 6: CD003456. Link: https://goo.gl/gkvK84
- Keppel Hesselink JM (2013) Chronic idiopathic axonal neuropathy and pain, treated with the endogenous lipid mediator palmitoylethanolamide: a case collection. Int Med Case Rep J 6: 49-53. Link: https://goo.gl/b4obJC
- Kopsky DJ, Keppel Hesselink JM (2018) Phenytoin Cream for the Treatment of Neuropathic Pain: Case Series. Pharmaceuticals (Basel) 11: E53. Link: https://goo.gl/LZ3iaS
- Keppel Hesselink JM, Notermans NC (2018) Topical phenytoin formulations for pain in small fiber neuropathy, a pathogenetic approach. Gen Int Med Clin Innov 3: 2-4. Link: https://goo.gl/AK7oMM
- Keppel Hesselink JM, Kopsky DJ (2017) Topical phenytoin cream in small fiber neuropathic pain: fast onset of perceptible pain relief. Int J Pain Relief 1: 015-019. Link: https://goo.gl/rMMQVv
- Keppel Hesselink JM (2018) Single-blind placebo-controlled response on phenytoin 10% in painful diabetic neuropathy. Gen Med Open 2: 2-3. Link: https://goo.gl/CCiYP2
- Kopsky DJ, Keppel Hesselink JM (2018) Single-blind placebo-controlled response test with phenytoin 10% cream in neuropathic pain patients. Accepted in Pharmaceuticals 11: 122. Link: https://goo.gl/PN6Gzs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
