Journal of Surgery and Surgical Research
Metabolism, inflammation and postoperative time are the key to early diagnosis of anastomotic leak
Daniel T Jansson1, Ioannis Oikonomakis2, Ida E U Hall Strand3, Adrian D Meehan4 and Kjell S Jansson2*
2Department of Surgery, Örebro University Hospital, Södra Grev Rosengatan, 701 85 Örebro, Sweden
3Department of Medicine, Skaraborg Hospital, Lidköping, Sweden
4Department of Geriatric, Örebro University Hospital, Örebro University, Örebro, Sweden
Cite this as
Jansson DT, Oikonomakis I, Hall Strand IEU, Meehan AD, Jansson KS (2019) Metabolism, inflammation and postoperative time are the key to early diagnosis of anastomotic leak. J Surg Surgical Res 5(2): 078-085. DOI: 10.17352/2455-2968.000078Objective: The aim of the study was to find laboratory samples for early diagnosis of anastomotic leak.
Summary background data: Anastomotic leakage after rectal cancer surgery is a severe complication with high mortality. Outcome is highly dependent on early diagnosis.
Methods: 29 patients were investigated postoperatively after having undergone low anterior resection due to cancer recti. Patient outcomes were divided into three groups: Anastomotic leak in 7 patients, other complications in 9 patients and 13 patients who were free of complications. Patients were monitored every 6th hour with blood and intraperitoneal samples in order to identify laboratory markers for early detection of anastomotic leakage. An anastomotic leak index was created, a scoring system where points count for values higher than reference values of CRP and interleukin 6 in blood and intraperitoneal lactate, lactate/pyruvate ratio and interleukin 6 were measured at 18, 24, 42 and 48 hours postoperatively.
Results: Significant differences between groups were found regarding CRP, Interleukin 6, fibrinogen and D-dimer in blood. Intraperitoneal differences were found not only in lactate and lactate/pyruvate ratio measured by microdialysis, significant differences in interleukin 6, interleukin 10 and tumour necrosis factor-α could also be demonstrated between the groups. The anastomotic leak index had a sensitivity and specificity of 86% (p=0.0007).
Conclusions: The most important factor was time after operation. No laboratory parameter in itself could predict an anastomotic leak but the anastomotic leak index was a useful tool in the monitoring and assessment of clinical outcome.
Mini abstract: Patients with anastomotic leak after rectal surgery were monitored with higher intraperitoneal cytokines and lactate/pyruvate ratio. The results suggest intraperitoneal microdialysis combined with blood samples of CRP and IL 6 as a feasible method for early diagnosis of anastomotic leak.
Introduction
Anastomotic leakage after colorectal surgery is a common and feared complication. The incidence of anastomotic leakage in Sweden 2015 reported by the Regional Cancer Center (RCC) was 7%-12% (depending for instance on hospital) and postoperative 30 days mortality was 1%-6% (depending for instance on the age of the patient). Surgical results have improved in recent years primarily because of the early diagnosis of anastomotic leakage but the diagnosis of anastomotic leakage remains difficult and is often discovered at a late stage, not seldom at reoperation. Early in the postoperative phase, Computer Tomography (CT) with an anal enema is often utilized for leakage detection. CRP can be useful in diagnosis but only first after day four or five postoperatively [1,2].
Intraperitoneal Microdialysis (IPM) has been introduced as a promising method for the prediction of surgical complications after gastrointestinal surgery [1,3-9]. Results of Intraperitoneal Microdialysis (IPM) have been presented in both animal and human studies [10-12]. Early metabolic changes may arise prior to various other postoperative complications [3,5,13], such as abdominal compartment syndrome [1,14,15]. Subcutaneous and intraperitoneal locations of measurements have previously been compared [16]. Biochemical changes have been shown to occur intraperitoneally prior to complications [3,13,17], suggesting that major surgical complications are preceded by splanchnic hypoxia/ischemia, changes which are possible to be detected by IPM.
Splanchnic ischemia and proinflammatory cytokine activation have been described as early events in the gradual development of shock and organ failure, suggesting the gastrointestinal tract to be the major source of the postoperative inflammatory response [18-20]. Studies have indicated that primary intestinal ischemia initiates and accelerates intraperitoneal inflammation, thus leading to serious complications in the postoperative recovery [21-23].
Intraperitoneal cytokines IL 6 and IL 10 have been reported to predict anastomotic leakage in the first postoperative day, while blood IL6 was higher, it was nevertheless not predictive of anastomotic leakage [19]. In a systematic review and meta-analysis of seven articles it was found that peritoneal IL 6 and TNF-α are significantly associated with colorectal anastomotic leakage and their monitoring might lead to early detection of leakage [24].
The aim of the study was to identify feasible biomarkers for the early diagnosis of anastomotic leakage after rectal surgery.
Materials and Methods
Patients
29 patients were investigated postoperatively after open low anterior resection due to cancer recti. These patents were divided into three groups according to outcome: Anastomotic leakage (leak), free of complications (free) and other complications (other).
In the leak group there were 7 patents (5 men, 2 women) median age 71 years (range 51-89), median anastomotic level 5 cm (range 3-5), and all patients received preoperative radiation 5x5 Gy. Peroperatively, 4 patients were also operated with a loop ileostomy, 1 patient transversostomy and 2 patients did not receive any divergent stoma (Table 1).
In the free group, 13 patients (4 men, 9 women) with a median age 69 years (range 53-86), median anastomotic level 5.25cm (range 3-9). Nine patients received 5x5 Gy preoperative radiation and 1 patient was given 2x25 Gy, while the remaining 3 patients did not receive any preoperative radiation treatment. Peroperatively, 6 patients were also operated with a loop ileostomy, 2 patients with transversostomy and 5 patients did not receive any divergent stoma (Table 1).
The “other” complications group included 9 patients (1 man 8 women) with a median age of 67 years (range 29-80), and a median anastomotic level 5cm (range 4-10). All 9 patients were given 5x5 Gy preoperative radiation treatment. Peroperatively, 4 patients were also operated with a loop ileostomy and 5 patients did not receive any divergent stoma (Table 1).
Samples
Intravenous blood samples were collected preoperatively and every 6th hour postoperatively. The blood samples included were C-reactive protein (CRP), D-Dimer, Fibrinogen, Plasminogen activator inhibitor-1 (PAI-1), Protein C (Prot C), prothrombin complex (PK), creatinine, albumin, complement factor 3d, Interleukin 6 (IL 6), Interleukin10 (IL10), Tumour Necrosis Factor α (TNF-α).
Intraperitoneal fluid was collected by an intraperitoneal drainage, inserted peroperatively. The first sample was collected six hours postoperatively and collection was continued every 6th hour. The collected samples included IL 6, IL 10 and TNF-α.
All blood samples and intraperitoneal cytokines were analyzed at the Clinical Department of Laboratory Medicine at the University Hospital of Örebro.
Intraperitoneal lactate and pyruvate were collected and analyzed using microdialysis equipment (M-dialysis AB, Stockholm, Sweden), thereby allowing the lactate/pyruvate ratio to be determined. Before closing the abdomen, a microdialysis catheter M-dialysis 62 was introduced intraperitoneally through a small incision in the abdominal wall with an M-dialysis needle and placed free-floating in the intraperitoneal cavity. The catheter was fixed to the skin with a suture to minimize the risk of unintentional extraction. Samples were continuously collected from the intraperitoneal fluid every second hour from 2-60 hours postoperatively in microvials from the microdialysis catheters and immediate analysis was performed in the analyzer.
Microdialysis
The microdialysis catheter is a 0.9 mm thin, double lumen concentric plastic tube with a 30 mm semi-permeable tubular membrane (cut off at 20.000 Dalton) at its distal end. An M-dialysis 62 gastrointestinal catheter with 210 mm shaft and 30 mm membrane was used in the peritoneal cavity. Physiologic perfusion fluid T1 was pumped at a rate of 0.3 µl/min from an M-dialysis 106 microdialysis pump through the outer tube of the catheter and flowed underneath the membrane, where the exchange between the intraperitoneal fluid and the perfusion fluid took place. At the tip the fluid entered the inner tube through a small hole, flowed backwards and was finally collected in a microvial. The perfusate equilibrates with molecules in the intraperitoneal fluid. In this way, microdialysis monitors substances supplied from the blood as well as substances originating from cell metabolism. A microvial with the microdialysis sample takes 7 minutes to be analyzed for glucose, pyruvate, lactate and glycerol. The lactate/pyruvate ratio was calculated in the analyzer.
Cytokines
The cytokines, TNF-α, IL 6 and IL 10 were analyzed from intraperitoneal fluid, which was collected from an 18 French pelvic drain, and intravenous blood every 6th hour until 48th postoperative hours. Samples were determined using an enzyme-labelled chemiluminiscent sequential immunometric assay upon an Immulite® instrument (DPC, Los Angeles, California, USA) according to the manufacturer’s instructions.
Statistical analysis
In this study, metabolic samples from microdialysis, intraperitoneal lactate and pyruvate were collected every 2nd hour. Due to the large amount of data, we present the median value and interquartile range from 2nd to 6th hour and soon.
Due to large variations over time in the leakage group, statistical analysis was conducted using Kruskal-Wallis test (leak, free and other) and the Wilcoxon rank sum test (leak and free) for variables ascertained every 6th hour in every analysis. P values <0.05 were regarded as significant. All statistical analyses were performed in Statistix 8.
The study was approved by the local Ethics Committee and, after informed consent, patients were included in the study.
Results
Blood cytokines (Table 2)
TNF-α (tumor necrosis factor α): Anastomotic leak group started preoperatively at 5.6, the free group at 6.7 and other complications group at 6.0 ng/L. Initially increasing levels peaked at 42 hours at 10.9, 7.3 and 10.4 respectively, and no significant differences between the groups were seen.
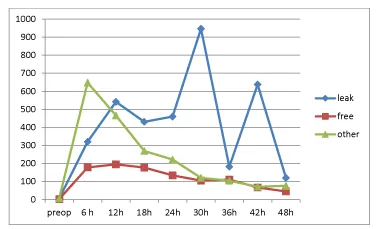
IL 6 (interleukin 6)(Figure 1): Preoperative low values (5.8, 2.4, 6.9ng/L) increased quickly and an irregular pattern was observed, whereby the leak group peaked after 30 hours (947 ng/l), the free group peaked after 12 hours (195ng/l) and other complication group peaked after 6 hours (648ng/l). Significant differences in Wilcoxon rank sum test comparing leak and free groups were seen after 12, 18, 24, 30 and 42 hours respectively. Significant differences comparing all three groups in Kruskal Wallis test was noted after 12, 18, 24 and 30 hours.
IL 10 (interleukin 10): The low levels before operation (4.0, 4.0, and 4.0ng/L) increased rapidly, where all three groups peaked after the 6th postoperative hour (27.4, 15, and 16.9ng/l), with parallel declines thereafter. No significant differences between groups could be detected.
Various/others blood samples (Table 2)
Fibrinogen: Preoperative values in the three groups started at 4.0, 3.3 and 4.2g/L respectively. Values increased in all three groups to 6.0, 5.6 and 6.8g/l after 54 hours. The increase was faster in the leakage group, leading to significant differences at 12, 24 and 30 hours when using the Wilcoxon rank sum test comparing leak and free groups, whereas, the Kruskal Wallis test showed significant differences after 12 and 30 hours when comparing all three groups.
PAI (Plasminogen Inhibitor Activator): Preoperative values in the leak, free and other groups were 4.6, 13.0 and 4.6ng/L. Maximum values were observed after 6 hours (28.3, 22.7, 13.2ng/l). Values decreased up to 54 hours and no significant differences between groups were seen at any time.
TPA (Tissue Plasminogen Activator): Preoperative values were 8.1, 12.0 and 10.7 ng/ml respectively. All three groups showed parallel values and peaked after 24 hours (leak 15.6, free 17.1 and other 18.0 ng/ml), and stable values prevailed in the remainder of the observation period. No significant differences were detected between the groups.
Protein C: Preoperative levels (1.1, 1.1 and 1.1 U/ml) slowly decreased in parallel in all groups and ended at 0.71, 0.92 and 0.78 U/ml. No significant differences were found.
D-Dimer: Initial levels of 0.7, 0.6 and 0.4 mg/L, increased rapidly, peaking in the leakage group after 18 hours at a value of 5.1 mg/l, with 2.2 mg/l and 3.3 mg/l observed in the remaining two groups. Significant differences between groups were found in both tests after 18 and 36 hours.
PK (Prothrombin Complex): Preoperative levels in all groups was 1.0 (INR). Values increased 24 hours after surgery (1.4, 1.3, and 1.4 respectively) and slight decreases were noted in all groups during the remainder of the observation period. Despite initial increases, no significant difference could be detected between the groups.
Creatinine: Almost equal values were observed preoperatively (70.1, 71.6, and 78.5µmol/L), and while levels increased during the first 24 hours (86.2, 86.8 and 87.2 µmol/L), they declined similarly thereafter. No significant differences were seen.
Albumin: Preoperative values were 33.1, 34.5 and 36.6 g/L respectively. Values decreased during the first 18 hours (24.0, 24.6, and 25.2g/l). In the free and other groups, slight increases were noted, while values in the leak group continued to decrease to 22.4 g/l at the end of the study period, but this difference was not significant at any time.
C3d (complement factor C3d): Very small differences in preoperative values between the groups as well as postoperatively, varying between 6.0 and 6.4.
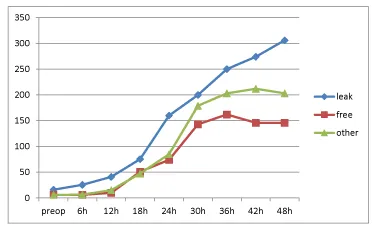
CRP (C Reactive Protein) (Figure 2): A small difference, but not significant, was noted preoperatively (16, 6, and 6mg/L in the respective groups). Levels increased in all groups but a faster and higher increase was seen in the leakage group. After 12 hours, levels were 41, 10 and 15 mg/L, after 24 hours levels were 160, 74 and 85mg/l, after 36 hours 250, 162 and 203mg/L. After 48 hours levels had increased to 306mg/L in the leakage group, 146mg/L in the free group and 203mg/L in the other complication group. Significant differences were noted in the Wilcoxon rank sum test between leak and free groups after 12, 24, 30, 36, 42 and 48 hours. Significant differences were found while comparing all three groups with the Kruskal Wallis test at 12, 24, 42 and 48 hours.
Intraperitoneal cytokines (Table 2)
Extremely high levels were seen compared to blood cytokine levels.
TNF-α: Highest levels were seen at 6 hours: 591, 240 and 294ng/L respectively. Decreasing values were mostly seen in all groups until 42 hours followed by an increase after 48 hours. Significant differences between leak and free groups were seen in the Wilcoxon rank sum test after 12 and 18 hours, while in the Kruskal Wallis test revealed differences between all groups after 18 hours.
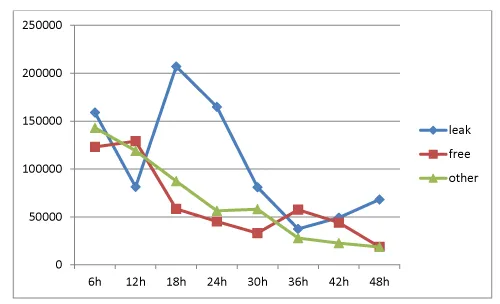
IL 6: (Figure 3) In the anastomotic leak group, levels peaked after 18 hours, 207 000ng/L. Free group peaked after 12 hours at 129 000 and other complication group peaked after 6 hours at 143 000. Differences between groups were significant in both statistical analyses after 18, 24 and 48 hours.
IL 10: Peaks were seen both in the leak group (860ng/L) and the other complication group after 6 hours (290ng/L), while the free group peaked after 12 hours (398ng/L). Kruskal Wallis test, comparing all three groups, showed statistical significance after 18 and 24 hours.
Intraperitoneal metabolism (Table 2)
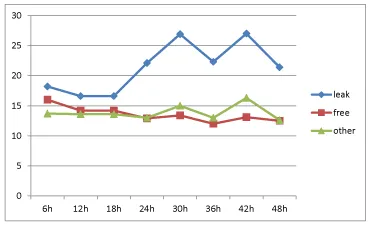
Lactate: (Figure 4) Starting values 6 hours postoperatively were 3.6, 2.6, and 2.4 mm respectively. A variation in groups was seen over time. Leak group varied between 2.8-4.8, free group varied between 1.8-2.4, while the other group varied between 2.0-3.2. Significant differences were identified in the Wilcoxon rank sum test comparing leak and free groups after 24 and 36-48 hours. Kruskal Wallis test, comparing all three groups, demonstrated significant differences at 18 and 36-48 hours.
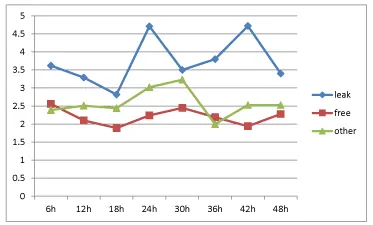
Lactate/pyruvate ratio: (Figure 5) The ratio in the leak group began at 18 after 6 hours, increased to 27 after 42 hours, and thereafter a decrease was noted. The free group started at 16, and decreased values were noted until the 36th postoperative hour. The other complication group showed stable decreasing values between 16.3 and 11.8. Statistically significant levels were found comparing leak and free groups after 6, 12 and 24-48 hours. When comparing all three groups, significant differences were found after 6 and 24-48 hours.
Anastomotic leak index: After 18, 24, 42 and 48 hours postoperatively, four measurements were calculated using five metabolites (the fifth metabolite, IL6, was measured in both blood and intraperitoneal fluid) (Table 3). The Anastomotic Leak Index was constituted by five metabolites, namely, serum CRP and IL 6, intraperitoneal IL 6, lactate and the lactate/pyruvate ratio. The cytokine IL 6 was only registered after 18 and 24 hours in both blood and intraperitoneally while CRP, lactate and the lactate/pyruvate ratio were registered at all four time-periods. Abnormal CRP values were observed higher than 70 after 18 hours, higher than 150 after 24 hours and higher than 250 after 42 and 48 hours. Blood IL 6 was higher than 400ng/L at both 18 and 24 hours while abnormal levels of intraperitoneal IL 6 should reach levels higher than 150000 after 18 hours and higher than 130000 after 24 hours. Intraperitoneal lactate should be higher than 3.5 after 18 hours and higher than 4.0 after 24, 42 and 48 hours to be considered as abnormal. An abnormal lactate/pyruvate ratio over 20 was registered at all 4 time-periods.
Sixteen separate registrations of the metabolites were recorded. For every abnormal value (higher than reference) one point was added, potentially giving a total maximal index score of sixteen. A score of 5 or more for a patient gave a sensitivity and specificity for anastomotic leak of 0.86 in this study. Reference values are presented in Table 3.
When scoring the 29 patients in this study, the leak group had a median score of 11 (Q1=5, Q3= 15), while free and other group had a median score of 1 (Free: Q1= 0, Q3=2, other: Q1= 0, Q3=4). Using the Wilcoxon rank sum test when comparing leak-free and leak-free + other groups according to the Anastomotic Leak Index, clear statistical significance could be gained (p=0.0007), similarly when comparing the leak-free-other in the Kruskal Wallis test scoring system (p=0.0007).
Discussion
This study analyzed several metabolic, inflammatory and organ dysfunctionalities every 6th hour in related samples in three groups after anterior resection due to cancer recti. Three groups have been studied: anastomotic leak group (leak), complication free group (free) and other complications group (other). The study showed that time after operation, together with specific metabolic and inflammatory biomarkers are the most important indicators for the early detection of anastomotic leak.
Cytokines showed similar reactive patterns, being more pronounced intraperitoneally, and generally showing high response rates directly postoperatively, with a subsequent rapid decline. The most suitable cytokine for early diagnosis of anastomotic leakage seems to be IL6 in blood and intraperitoneal fluid, but TNF-α and IL 10 intraperitoneally also seem to have some prognostic advantages in the early diagnosis of anastomotic leakage [25,26].
Intraperitoneal metabolic sampling with microdialysis provides a promising diagnostic strategy. Analyzing lactate and lactate/pyruvate ratio offers several advantages such as a stable sample due to the size of the holes in catheter membrane of 20 kDa, which do not allow the perfusion of enzyme lactate dehydrogenase (143 kDa) into the sample. In addition, continuous sampling over 20 minutes and a rapid analysis within in 7 minutes makes the method highly useful in the critical periods postoperatively. In the present study, the microdialysis catheter had been placed free-floating around the small intestines and demonstrated, as in other studies, that a lactate/pyruvate ratio over 20 is pathological [3,5,6]. Intraperitoneal lactate values in this study over 3.5 and 4 mM could be regarded as pathological in the first 48 hours and was an indicator of a surgical complication, such as anastomotic leakage.
CRP is still regarded as the golden standard for the laboratory diagnosis of anastomotic leakage. Previous studies have shown that CRP values over 150mg/L 4 days postoperatively (2) and CRP values over 100mg/L after 5 days (1) are indicative of anastomotic leakage. Rapid increases of CRP postoperatively with values over 70mg/L after 18 hours and 150mg/L after 24 hours should be considered as pathological.
In the present study, the greatest changes in fibrinogen and D-dimer were detected in the leakage group, illustrating the assumption that major surgical complications, such as anastomotic leakage early in the postoperative period, lead to an overactivity in the coagulation system. Fibrinogen is converted to fibrin – an acute phase reactant – which leads to blood clotting in the presence of thrombin. D-dimer is a fibrin degradation product and indicative of ongoing coagulation. In our study, fibrinogen increased persistently, with significant differences between groups found after 12, 24 and 30 hours. D-dimer showed a biphasic course, peaking after 18 hours and then again at 36 hours, with clear and significant differences between groups. These results illustrate the alarming early changes in the coagulation system postoperatively, particularly in patients developing anastomotic leakage.
No sample or biomarker can solely predict an early anastomotic leak. However, the creation of the anastomotic leak index, with two blood samples (CRP and IL 6) and three intraperitoneal samples (IL 6, lactate and lactate/pyruvate ratio) measured at 18, 24, 42 and 48 hours, provided a specificity and sensitivity of 86% in the prediction of an anastomotic leak. In clinical practice, 18 hours postoperatively is commonly the morning of the first postoperative day, 24 hours is the evening the same day, while 42 and 48 hours postoperatively are the morning and evening of the second day.
Cytokines increase quickly, followed by a rapid decrease, making cytokines only useful in the first 24 hours in the index. CRP is probably the most robust biomarker in the anastomotic leak index. Lactate and lactate/pyruvate ratio, however, have advantages such as reliability, where ratios over 20 for lactate/pyruvate and values over 3.5-4.0 mm for lactate during the first 48 hours are indicative of pathological processes. The microdialysis method is expeditious, allowing for more or less concurrent measurements.
The frequency of anastomotic leakage is around 10%-15%. Today, most patients with a low anterior resection receive a divergent stoma, but many of these patients are not offered the opportunity to discuss care options. Subsequently, many patients have problems with discomfort, thus leading to reduced quality of life and increased health care costs. Based on the results of this study, we suggest that a divergent stoma should not be performed as the primary surgical strategy. Rather, by implementing the use of the anastomotic leak index as an indicator for relaparoscopy or relaparotomy two days postoperatively, the operator can choose between one of several options such as resuturing, drainage, divergent loop ileostomy or permanent sigmoideostomy depending on anatomical features, degree of ischemic colon and degree of peritonitis. The current study is limited in that it only presents 29 patients followed after open surgery, whereof seven had anastomotic leakage, elucidating the need for further clinical studies in both open and laparoscopic surgery for the development and validation of the anastomotic leak index.
- Matthiessen P, Strand I, Jansson K, Törnquist C, Andersson M, et al. (2007) Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Rectum 50: 1918-1927. Link: http://bit.ly/2pUdd3S
- Reynolds IS, Boland MR, Reilly F, Deasy A, Majeed MH, et al. (2017) C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 19: 812-818. Link: http://bit.ly/2WezXaV
- Jansson K, Ungerstedt J, Jonsson T, Redler B, Andersson M, et al. (2003) Human intraperitoneal microdialysis: increased lactate/pyruvate ratio suggests early visceral ischaemia. A pilot study. Scand J Gastroenterol 38: 1007-1011. Link: http://bit.ly/32JCkVK
- Jansson K, Redler B, Truedsson L, Magnuson A, Ungerstedt U, et al. (2004) Postoperative on-line monitoring with intraperitoneal microdialysis is a sensitive clinical method for measuring increased anaerobic metabolism that correlates to the cytokine response. Scand J Gastroenterol 39: 434-439. Link: http://bit.ly/2JfZrzF
- Horer TM, Norgren L, Jansson K (2011) Intraperitoneal glycerol levels and lactate/pyruvate ratio: early markers of postoperative complications. Scand J Gastroenterol 46: 913-919. Link: http://bit.ly/2MIgMmR
- Jansson K, Wickbom M, Skoog P, Nilsson FK, Hörer T (2017) Intraperitoneal microdialysis-after hemicolectomy and rectal resections as a method for early postoperative complications detection. Internal Med Rev 4: 1-15. Link: http://bit.ly/32LVDhi
- Sabroe JE, Axelsen AR, Ellebaek MB, Dahler-Eriksen B, Qvist N (2017) Intraperitoneal lactate/pyruvate ratio and the level of glucose and glycerol concentration differ between patients surgically treated for upper and lower perforations of the gastrointestinal tract: a pilot study. BMC Res Note 10: 302. Link: http://bit.ly/33WlB1y
- Daams F, Wu Z, Cakir H, Karsten TM, Lange JF (2014) Identification of anastomotic leakage after colorectal surgery using microdialysis of the peritoneal cavity. Tech Coloproctol 18: 65-71. Link: http://bit.ly/2PiEmbq
- Ellebaek M, Qvist N, Fristrup C, Mortensen MB (2014) Mediastinal microdialysis in the diagnosis of early anastomotic leakage after resection for cancer of the esophagus and gastroesophageal junction. Am J Surg 208: 397-405. Link: http://bit.ly/2Ngjhf8
- Ungerstedt J, Nowak G, Ericzon BG, Ungerstedt U (2003) Intraperitoneal microdialysis (IPM): a new technique for monitoring intestinal ischemia studied in a porcine model. Shock 20: 91-96. Link: http://bit.ly/362DZrz
- Ungerstedt U (1991) Microdialysis--principles and applications for studies in animals and man. J Intern Med 230: 365-373. Link: http://bit.ly/2NeowvM
- Sommer T, Larsen JF (2004) Intraperitoneal and intraluminal microdialysis in the detection of experimental regional intestinal ischaemia. Br J Surg 91: 855-861. Link: http://bit.ly/2PflFFA
- Verdant CL, Chierego M, De Moor V, Chamlou R, Creteur J, et al. (2006) Prediction of postoperative complications after urgent laparotomy by intraperitoneal microdialysis: A pilot study. Ann Surg 244: 994-1002. Link: http://bit.ly/2Pg7kZx
- Skoog P, Horer T, Nilsson KF, Agren G, Norgren L, et al. (2015) Intra-abdominal hypertension--an experimental study of early effects on intra-abdominal metabolism. Ann Vasc Surg 29: 128-137. Link: http://bit.ly/2pMI6aL
- Horer TM, Skoog P, Norgren L, Magnuson A, Berggren L, et al. (2013) Intra-peritoneal microdialysis and intra-abdominal pressure after endovascular repair of ruptured aortic aneurysms. Eur J Vasc Endovasc Surg 45: 596-606. Link: http://bit.ly/2Nbq89L
- Jansson K, Jansson M, Andersson M, Magnuson A, Ungerstedt U, et al. (2005) Normal values and differences between intraperitoneal and subcutaneous microdialysis in patients after non-complicated gastrointestinal surgery. Scand J Clin Lab Invest 65: 273-281. Link: http://bit.ly/2BFOJxY
- Vermeer TA, Orsini RG, Daams F, Nieuwenhuijzen GA, Rutten HJ, et al. (2014) Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur J Surg Oncol 40: 1502-1509. Link: http://bit.ly/2BMUFoV
- Yamamoto T, Umegae S, Matsumoto K, Saniabadi AR (2011) Peritoneal cytokines as early markers of peritonitis following surgery for colorectal carcinoma: a prospective study. Cytokine 53: 239-242. Link: http://bit.ly/360j3RX
- Sammour T, Singh PP, Zargar-Shoshtari K, Su'a B, Hill AG (2016) Peritoneal Cytokine Levels Can Predict Anastomotic Leak on the First Postoperative Day. Dis Colon Rectum. 59: 551-556. Link: http://bit.ly/35XhpR4
- Landow L, Andersen LW (1994) Splanchnic ischaemia and its role in multiple organ failure. Acta Anaesthesiol Scand 38: 626-639. Link: http://bit.ly/31JfNHw
- Tamion F, Richard V, Lyoumi S, Daveau M, Bonmarchand G, et al. (1997) Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol 273: G314-G321. Link: http://bit.ly/31QZp7W
- Van Berge Henegouwen MI, Van der Poll T, Van Deventer SJ, Gouma DJ (1998) Peritoneal cytokine release after elective gastrointestinal surgery and postoperative complications. Am J Surg 175: 311-316. Link: http://bit.ly/2WdOt2T
- Grotz M, Regel G, Bastian L, Weimann A, Neuhoff K, et al. (1998) The intestine as the central organ in the development of multiple organ failure after severe trauma--pathophysiology and therapeutic approaches. Zentralbl Chir 123: 205-217. Link: http://bit.ly/343G5p8
- Sparreboom CL, Wu Z, Dereci A, Boersema SAG, Menon AG, et al. (2016) Cytokines as Early Markers of Colorectal Anastomotic Leakage: A Systematic Review and Meta-Analysis. Gastroenterology research and practice. 2016: 3786418. Link: http://bit.ly/2MLfY0z
- Bilgin IA, Hatipoglu E, Aghayeva A, Arikan AE, Incir S, et al. (2017) Predicting Value of Serum Procalcitonin, C-Reactive Protein, Drain Fluid Culture, Drain Fluid Interleukin-6, and Tumor Necrosis Factor-alpha Levels in Anastomotic Leakage after Rectal Resection. Surg Infect (Larchmt) 18: 350-356. Link: http://bit.ly/2JlO0GN
- Zawadzki M, Krzystek-Korpacka M, Gamian A, Witkiewicz W (2018) Serum cytokines in early prediction of anastomotic leakage following low anterior resection. Wideochir inne tech maloinwazyjne 13: 33-43. Link: http://bit.ly/2PoKChQ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
