Journal of Surgery and Surgical Research
Antiemetic prescribing patterns for post-operative surgical patients
Van N Tran1*, Brennan J Fitzpatrick2, Stefanie N Edwards2, Emily J Ferraro2, Federica Marafioti2, Thi Nguyen2, Alia Rafhi2, On Na Lam2 and Vincent Chan2
2School of Health and Biomedical Sciences, RMIT University, Bundoora, VIC, Australia
Cite this as
Tran VN, Fitzpatrick BJ, Edwards SN, Ferraro EJ, Marafioti F, et al. (2019) Antiemetic prescribing patterns for post-operative surgical patients. J Surg Surgical Res 5(2): 051-055. DOI: 10.17352/2455-2968.000071Background: Post-operative nausea and vomiting is a common occurrence amongst surgical patients. Anecdotal reports suggest antiemetic prescribing patterns to be an area for improvement.
Aim: To report the most commonly prescribed antiemetic agents in a major tertiary teaching hospital in Australia; and to assess medication dosage and compared to the current national and international guidelines with recommended multimodal therapies.
Methods: A retrospective analysis from patients’ electronic medical files of patients admitted under a surgical unit that underwent operative management during a four week period was conducted.
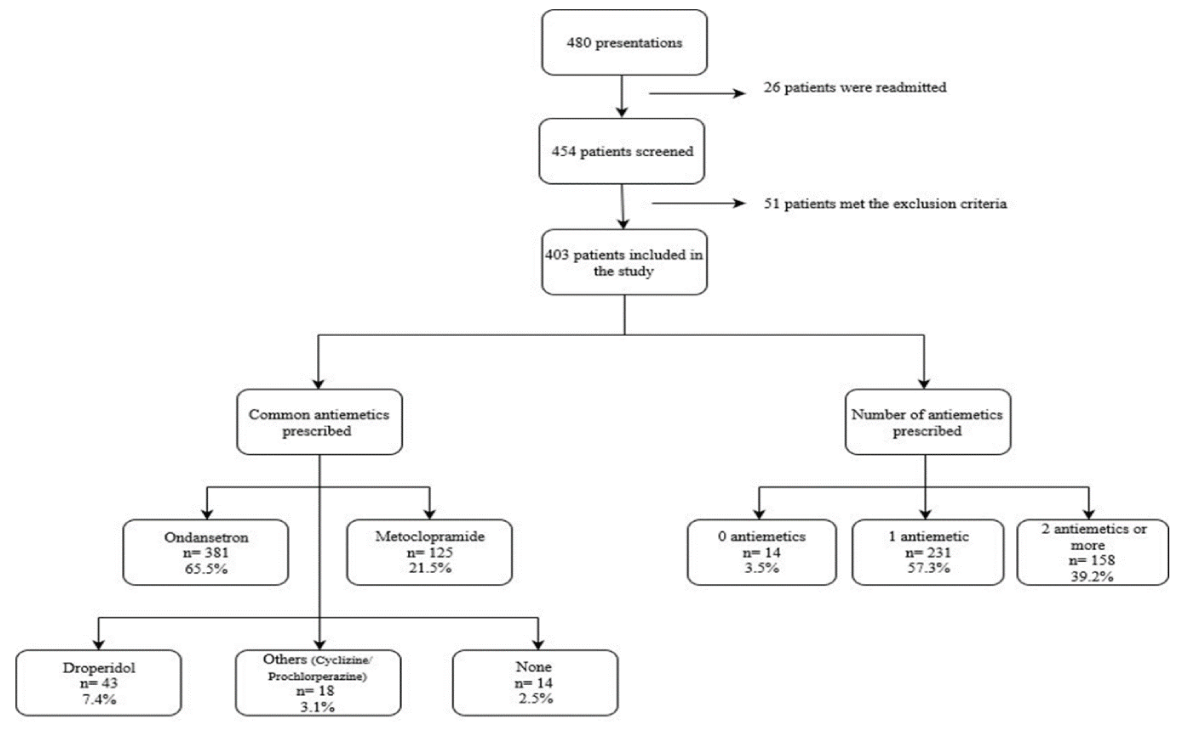
Results: A total of 480 presentations were audited during the 4-week period. Of these, 26 patients had readmissions, resulting in 454 patients screened for inclusion in this audit. A total of 51 patients met the exclusion criteria, leaving 403 patients included in this audit. Ondansetron was the most commonly prescribed antiemetic agent (65.5%), followed by metoclopramide (21.5%) and droperidol (7.4%). A single antiemetic agent was prescribed for 57.3% of patients, compared to 39.2% for multimodal therapies and 3.5% of patients did not have any antiemetic agents charted.
Conclusion: The majority of post-operative patients were prescribed ondansetron. The prescription of ondansetron as first line therapy is in line with international guidelines. An area for improvement was highlighted in the prescribing of multimodal therapy for the reduction of post-operative nausea and vomiting. Further studies and providing educational support to address discrepancies in current prescribing practices is recommended to optimise patient health outcomes.
Introduction
Post-operative nausea and vomiting (PONV) is a prevalent and distressing adverse effect and complication associated with surgery. In Australia, approximately 11 million people were hospitalised during 2016-2017, and of these one in four patients required surgical procedures [1]. This is a cause of concern, as approximately 30% of all post-operative patients and up to 80% of high risk patients will develop symptoms of nausea and/or vomiting [2]. PONV is defined as any nausea, retching or vomiting occurring during the first 24-48 hours post procedure [2]. Unresolved nausea and vomiting is often associated with a delay in recovery after surgery [3].
The onset and severity of PONV is hypothesised to be associated with: age, gender, smoking status, comorbidities, pre-surgical anxiety state, surgical procedure as well as the type and duration of anaesthesia [4]. Studies suggest complications such as electrolyte imbalance and dehydration, wound dehiscence, increased pain, obstruction of airways, aspiration and increased intracranial pressure have been associated with PONV [4]. Consequently, patients consider PONV to be a significant health burden and is the highest rated surgical outcome that they would most like to avoid [5].
The international Australian and New Zealand College of Anaesthetists (ANZCA) consensus guidelines have endorsed a multimodal prevention approach to PONV [6]. These guidelines contain revised information on identifying patient risk factors, providing rescue treatment where prophylaxis has been withheld or failed and updated data on the effects of QT prolongation. Local and national guidelines reflect the current ANZCA recommendations [6].
Anecdotal reports from clinical pharmacists, suggest inconsistent antiemetic prescribing when compared to these recommendations. The main purpose of this study was to determine the most commonly prescribed antiemetics, (including medication dose and frequency) and evaluate if multimodal therapies is commonly prescribed as per national and international guidelines. Ultimately, the aim is to achieve optimisation of quality use of medicines and improve patient satisfaction and health outcomes.
Method
Study design
This study was an inpatient retrospective audit conducted at a major tertiary teaching hospital in Australia. Data was collected by reviewing patients’ electronic medical records. The inclusion criteria were patients who were admitted to hospital for a surgical procedure during a specified 4-week period.
Inclusion criteria
All adult patients admitted to hospital under the Surgery, Perioperative, Trauma and Surgical Oncology Services division surgical units (Appendix A), who underwent a surgical procedure, were eligible for the audit if they were admitted between 1st and 31st July 2018. All patients included were retrieved from a central database.
Exclusion criteria
The patients were evaluated and inpatient ward medication charts were screened, to exclude if one or more of the following criteria were met: regularly prescribed antiemetics as an inpatient; patients under the age of 16; intensive care unit (ICU) admission; receiving chemotherapy treatment; for non-operative management; deceased during the admission. A chemotherapy agent is defined as specific chemical agents or drugs that are selectively destructive to malignant cells and tissues used for the treatment of cancer.
Outcome measures
The primary objectives for this audit was to determine the most commonly prescribed antiemetics, which includes medication dosage (dose, frequency and maximum dose) and to compare antiemetic prescribing patterns for surgical patients against local, national and international guidelines for the indication of PONV.
Data collection
Data was obtained through collecting patient information from medical records via electronic contents manager (ECM) and pathology viewer AUSCARE® [7]. This data was entered into an auditing tool, Research Electronic Data Capture (REDcap®) by two auditors. Surgical patients who were admitted into the hospital for operative management from the 1st to 31st of July 2018 were audited.
Results
Patient cohort
There were 480 presentations screened during the 4-week period. Of those presentations, 26 patients were readmitted leaving 454 patients, with 51 patients meeting the exclusion criteria.
This elimination breakdown included patients who were prescribed regular antiemetics (n=11), patients admitted to ICU (n=14), patients on chemotherapy (n=9), non-operative management (n=15) and finally deceased patients (n=2); resulting in 403 patients who were included in the study (Figure 1).
Patient characteristics
A total of 58.1% of the sample size were male. The median age was 49 years old for this cohort. Females were lighter when compared to males and males were taller when compared to females. A total of 65.0% of patients reported their smoking status to be a non-smoker, with 19.6% of patients’ status unknown and 15.4% of patients admitted to be a current smoker (Table 1).
The most common surgical unit was orthopaedics (n=111), followed by emergency general surgery (n=81), plastics (n=50), urology (n=38), trauma and transplant (n=34). The least represented surgical units included oral maxillofacial (n=29), hepatobiliary (n=20), colorectal (n=16), with only a small number of breast/oncology/endocrine (n=12) patients represented.
Primary outcome results
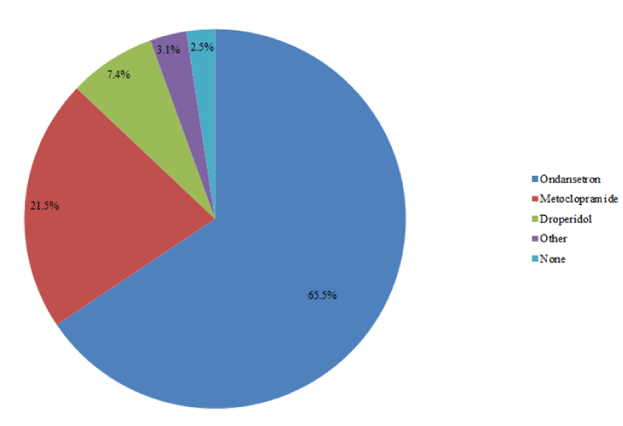
Ondansetron was found to be the most commonly prescribed (65.5%) antiemetic. Followed by, metoclopramide (21.5%) and then droperidol (7.4%). The least prescribed agents included cyclizine, prochlorperazine and domperidone, which collectively accounted for 3.1% of total prescription orders. No antiemetic agents were prescribed for 2.5% of the patient cohort (Figure 2).
Patients were prescribed 0, 1, 2 or more antiemetics for multimodal therapy. A total of 3.5% (n=14) of patients were not prescribed antiemetic agents. From all ward inpatient medication chart orders, a total of 57.3% (n=231) patients were prescribed only one antiemetic agent, with the remaining 39.2% (n=158) patients were prescribed two or more antiemetics for PONV.
Discussion
Nausea and vomiting is a post-operative complication which causes electrolyte imbalance, dehydration, increased pain as well as aspiration.(4) The purpose of the audit was to determine the most commonly prescribed antiemetic agents used. Medication dosages and completeness of antiemetic prescribing were also portrayed within the findings.
Most common antiemetics
Ondansetron is a potent, highly selective 5-Hydroxytryptamine type 3 (5-HT3) receptor antagonist and is considered to be the ‘gold standard’ antiemetic treatment [6,8]. It is recommended for both prophylaxis and curative therapy for PONV without producing significant side effects [9]. It is marketed and available for administration by PO or IV route [10]. The benefit of ondansetron as an antiemetic is the ability to be used both at induction of anaesthesia and as a treatment option post-surgery [9]. A controlled study that compared ondansetron given before induction of anaesthesia and after surgery, demonstrated that patients who received ondansetron after surgery were associated with a decrease incidence of emesis [11]. The terminal elimination half-life of ondansetron after PO dosing is 4.1 to 11.6 and 2.5 to 6.1 hours after IV dosing [10]. The high percentage of patients administered this agent may be due to its safety profile in renal and mild hepatic impairment. Patients with moderate and severe hepatic impairment should not exceed a total daily dose of 8mg [10].
A high percentage of prescribing contained the route of administration to be documented as PO/IV, of which two orders did not specify the route of administration. Ondansetron illustrates the significance of identifying the suitable route of administration. In the study by Sadawarte et al. it concluded that the effect of ondansetron PO 8mg was comparable to ondansetron IV 4mg in the setting of PONV [12]. This determines a difference in strength and effectiveness when comparing PO and IV ondansetron. The maximum daily dose for ondansetron IV is 16mg [13], whilst PO ondansetron can be used up to 24mg. If both the PO/IV route is prescribed together, it can potentially cause the maximum daily dose to be exceeded which increases side effects such as QT prolongation [12]. It was evident that prescribing PO/IV together was commonly seen throughout this audit. A lack of awareness of both the local guidelines, potential pharmacokinetic properties and time restrictions may be related to the high incidence of PO/IV prescribing. Future education regarding route of administration may improve prescribing practice and ultimately avoid negative adverse events.
Metoclopramide is a selective dopamine-2 (D2) antagonist used to prevent or relieve the symptoms of nausea and vomiting. It is a weak antiemetic with an elimination half-life of 2.5 to 5 hours [10]. Despite the fact that metoclopramide has limited effectiveness in PONV [14], it is the second agent of choice after ondansetron in this audit. Prescribing metoclopramide for the indication of general nausea and vomiting is widely accepted in practice, which may justify the high number of antiemetic orders. Metoclopramide recommended PO/IV dose in the setting of PONV is 10mg, three times daily, maximum dose of 30mg per 24 hours for no longer than five days [10]. Patients were prescribed at doses of 10mg or 20mg predominately. An alarming 12% of patients were prescribed in excess of the national recommendations. Excessive doses of metoclopramide have been associated with tardive dyskinesia, arrhythmias and neuroleptic malignant syndrome. These side effects are seen to be dose and duration-dependent [15]. The absence of a maximum dose may increase the risk of supra-therapeutic dosing and have potential for negative clinical patient outcomes.
Droperidol, a potent D2 antagonist with serotonergic and histamine antagonism, was one of the least common antiemetics prescribed in this audit despite extensive documented benefits for the treatment of PONV [6]. Droperidol is a first-generation antipsychotic with beneficial antiemetic activity. The infrequent prescribing of droperidol in this audit may be due to the attitudes and stigma associated with its primary indication [16].
Droperidol is used as an adjunct antiemetic, for further symptomatic relief of PONV [10]. Prescribing concomitantly with ondansetron for multimodal antiemetic therapy was for all droperidol orders. A clinical study by Chandrakantan et al. showed that droperidol combined with ondansetron was more effective than either drug by itself, with no additional cardiovascular risk [17]. Droperidol and metoclopramide both provide dopamine antagonistic effects. A recent analysis suggests that low dosage droperidol (≤1mg) has considerably lower side effects and adequate antiemetic effect when compared to metoclopramide (<20mg) [18]. Many side effects seen in the use of droperidol may be dose-dependent, with doses ranging from 2.5mg to a maximum of 20mg in 24-hours [10]. Only a low dose range of droperidol was prescribed in the audit. This finding was exceptional, as recommended doses of up to 1.5mg in the setting of PONV are not suggested to be associated with severe QT prolongation [19-21]. Omitted prescribing for maximum dose per 24 hours was found in a majority of orders, and this is not consistent with local guidelines as there is potential to increase the risk of unwanted adverse effects.
Multimodal therapy
A minority of patients were not prescribed antiemetics. This may be a result of the low risks associated with both the type and duration of surgery [22]. A patient reported case study by Phillips et al confirmed that every 30 minute increase in surgical time, resulted in a 60.0% increase in the baseline risk of PONV [23]. Surgeries lasting less than 60 minutes have a decreased risk of patient experiencing PONV [23].
More than half of the participants within the audit were prescribed a single antiemetic agent. Prescribing one antiemetic alone may be based on economic considerations within the tertiary hospital. Recognising a gap in knowledge, the professional judgement and medication preferences of the prescriber may be influencing factors for prescribing a single antiemetic agent [24].
A proportionally lower number of patients received two antiemetics or more. Different classes of antiemetics have different mechanisms of action. The guidelines suggest that using more than one antiemetic is not only more effective, but also reduces the likelihood of side effects [6]. Increasing the number of antiemetics administered, lowers the doses that are prescribed [6]. The 5-HT3 antagonists have better antiemetic than antinausea efficacy but are associated with headaches. These drugs can be used in combination with droperidol, which has greater antinausea efficacy and is associated with lower risk of headaches [17].
Limitations
Data was collected from an electronic medical database. The limitation of collecting data retrospectively meant there was a potential for incomplete data. Approximately 33% of patients had omitted Medication Management Plans (MMPs). MMPs are a collection tool utilised by clinical pharmacists to obtain relevant patient medication information, including regular medications, smoking status and previous medical history [25]. MMPs were accessed to ensure patients screened, met the inclusion criteria.
The type of surgery undertaken by each patient was not audited. Certain surgeries, i.e. those involving the gastrointestinal system (laparoscopy) have some evidence to suggest patients may be at a greater risk of PONV [6]. To reduce variability between surgery type, technique used, and risk of PONV; classification of surgeries by unit was used (Appendix A). Primary outcomes were measured via prescribing patterns and adherence to current hospital guidelines. The authors recognised detailed reporting of specific surgical procedures may influence the incidence of PONV and this may inevitably affect the prescribing of antiemetics administered to the patient and thus recommended this be reviewed in future research proposals [26].
An echocardiogram (ECG) is used to monitor cardiac abnormalities. Some antiemetic agents have been associated with the risk of causing QT prolongation, which has been related with fatal arrhythmias [27]. However, the authors of this audit accepted that it is difficult to conclusively equate the presence of an ECG to QT monitoring as a result of antiemetic prescribing. Therefore, as this study did not focus on ECG interpretation this limits the correlation between the management of PONV and individual patient risks.
Conclusion
Ondansetron was the most common antiemetic prescribed for PONV. This coincides with the mentioned local, national and international guidelines as first-line therapy. Single antiemetic therapy was prescribed greater than the recommended multimodal approach; further research is warranted to develop effective strategies to bridge this treatment gap. Implementing good quality use of medicines and prescribing patterns by adhering to guidelines will reduce negative health outcomes and enhance patient satisfaction.
We acknowledge the business intelligence and REDCap® as the collection program used in this audit and the Royal Melbourne Hospital pharmacy practice research committee for their contributions and assistance with the review of this report.
- Australian Institute of Health and Welfare (2018) Admitted Patient Care 2016-2017-Australian Hospital Statistics. Canberra, Australia. Link: http://bit.ly/2NU3m9Q
- Pierre S WR (2012) Nausea and vomiting after surgery. Continuing Education in Anaesthesia. Critical Care & Pain 13: 28-32. Link: http://bit.ly/32i5QCb
- Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, et al. (2012) Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 109: 742-753. Link: http://bit.ly/2XCMdGk
- Collins AS (2011) Postoperative nausea and vomiting in adults: implications for critical care. Crit Care Nurse 31: 36-45. Link: http://bit.ly/2YKy2eN
- Kranke P, Eberhart LH (2011) Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol 28: 758-765. Link: http://bit.ly/2S6qSPo
- Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, et al. (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118: 85-113. Link: http://bit.ly/2YTZCWU
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. (2009). Link: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42: 377-381. Link: http://bit.ly/2XCwLKd
- Wolf H (2000) Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand J Rheumatol Suppl 113: 37-45. Link: http://bit.ly/2XD8qz5
- Cox F (1999) Systematic review of ondansetron for the prevention and treatment of postoperative nausea and vomiting in adults. The British journal of theatre nursing : NATNews : the official journal of the National Association of Theatre Nurses 9: 556-566. Link: http://bit.ly/30lskAq
- MIMS (2018) Monthly Index of Medical Specialties: Product Information. In: online M, editor. New South Wales, Australia.
- Tang J, Wang B, White PF, Watcha MF, Qi J, et al. (1998) The effect of timing of ondansetron administration on its efficacy, cost-effectiveness, and cost-benefit as a prophylactic antiemetic in the ambulatory setting. Anesth Analg 86: 274-282. Link: http://bit.ly/2SfIN6F
- Sadawarte P, Bhure A, Deshmukh S, Parate S (2015) Comparative study of Oral Vs IV Ondansetron for reducing PONV in patients undergoing laparoscopic surgery under General anesthesia. International Journal of Pharmaceutical Science Invention 4: 01-06. Link: http://bit.ly/2XFWZqq
- Aschenbrenner D (2012) The FDA Limits Maximum IV Dose of Ondansetron. American Journal of Nursing 112: 48. Link: http://bit.ly/2G5UMhX
- Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, et al. (2002) Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth 88: 659-668. Link: http://bit.ly/2YGyjiS
- Kenney C, Hunter C, Davidson A, Jankovic J (2008) Metoclopramide, an increasingly recognized cause of tardive dyskinesia. Journal of clinical pharmacology 48: 379-384. Link: http://bit.ly/2XCNAos
- Sajatovic M, Jenkins JH (2007) Is antipsychotic medication stigmatizing for people with mental illness? International review of psychiatry (Abingdon, England) 19: 107-112. Link: http://bit.ly/30lsLL4
- Chandrakantan A, Glass PS (2011) Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth 107: i27-40. Link: http://bit.ly/2YMKpXI
- Schaub I, Lysakowski C, Elia N, Tramer MR, (2012) Low-dose droperidol (</=1 mg or </=15 mug kg-1) for the prevention of postoperative nausea and vomiting in adults: quantitative systematic review of randomised controlled trials. Eur J Anaesthesiol 29: 286-294. Link: http://bit.ly/2Js2gy5
- Calver L, Drinkwater V, Gupta R, Page CB, Isbister GK (2015) Droperidol v. haloperidol for sedation of aggressive behaviour in acute mental health: randomised controlled trial. Br J Psychiatry 206: 223-228. Link: http://bit.ly/2JrX9hi
- Lai PC, Huang YT (2018) Evidence-based review and appraisal of the use of droperidol in the emergency department. Ci ji yi xue za zhi = Tzu-chi medical journal 30: 1-4. Link: http://bit.ly/2JBUlNg
- Perkins J, Ho JD, Vilke GM, DeMers G (2015) American Academy of Emergency Medicine Position Statement: Safety of Droperidol Use in the Emergency Department. J Emerg Med 49: 91-97. Link: http://bit.ly/2xH3OgQ
- Dicus C, Arbon J, Turvey T, Blakey G, Phillips C (2011) Evaluation of Post-Operative and Post-Discharge Nausea and Vomiting in Orthognathic Surgery Patients. Journal of Oral and Maxillofacial Surgery 69: e25. Link: http://bit.ly/2YKk6l9
- Phillips C, Brookes CD, Rich J, Arbon J, Turvey TA (2015) Postoperative nausea and vomiting following orthognathic surgery. Int J Oral Maxillofac Surg 44: 745-751. Link: http://bit.ly/2S5npRn
- To TH, Agar M, Yates P, Currow DC (2014) Prescribing for nausea in palliative care: a cross-sectional national survey of Australian palliative medicine doctors. J Palliat Med 17: 1032-1036. Link: http://bit.ly/2JrK0oA
- Tong EY, Roman CP, Mitra B, Yip GS, Gibbs H, et al. (2017) Reducing medication errors in hospital discharge summaries: a randomised controlled trial. Med J Aust 206: 36-39. Link: http://bit.ly/2XAfrWs
- Rusch D, Eberhart LH, Wallenborn J, Kranke P (2010) Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Dtsch Arztebl Int 107: 733-741. Link: http://bit.ly/2LaI4D3
- Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, et al. (2008) Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology 109: 206-212. Link: http://bit.ly/2XCTQN4
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
