Journal of Surgery and Surgical Research
Comparision Effectiveness of Two Different Implant Surface Decontamination Methods by Surgical Treatment of Periimplantitis: A Clinical Trial
Elif Öncü* and Bilge Can
Cite this as
Öncü E, Can B (2019) Comparision Effectiveness of Two Different Implant Surface Decontamination Methods by Surgical Treatment of Periimplantitis: A Clinical Trial. J Surg Surgical Res 5(1): 019-026. DOI: 10.17352/2455-2968.000062Objective: The formation of bacterial biofilm on implant surfaces is the primary etiologic reason for peri-implantitis. The aim of this study is to present a new formulation including erythritol powder, which is widely used in air-polishing devices, and ultrasonic scaler with polyetheretherketone-coated tips, and to compare treatment effectiveness of them by comparison with conventional plastic scaler with 0.12% chlorhegxidine decontamination.
Materials and methods: In this randomized, controlled study, 18 patients with peri-implantitis were included. A total of 40 dental implants were debrided with either ultrasonic instruments (test, n=20) or plastic scaler (control, n=20). Gingival recession depth (RD), keratinized tissue width (KTW), probing depth (PD), Gingival Index (GI) were evaluated at baseline and after 1 year. Supportive and nonsurgical periodontal therapies were firstly consulted to reduce the inflammation, before the surgical treatments of the defects. The formation of bacterial biofilm on implant surfaces was removed by standard plastic curettes, debridement made by combined 0.12% chlorhegxidinerinse or mechanical debridement made by ultrasonicpolyetheretherketone coated tips developed for implant surface and combined air-flow debridement.
Results: After 1 year healing period, PD, GI and PI levels of the patients in both group were significantly lower. Combined air-flow debridement and the mechanical debridement performed by using ultrasonic polyetheretherketone-coated tips were better at decreasing the level of periodontal pocket depth and plaque scores than plastic curettes.
Conclusion: Ultrasonic driven instruments equipped with a special tip and airflow devices using erythritol powder seem to be a viable alternative to the traditional debridement of implants in periimplant mucositis.
Abbreviations
BOP: Bleeding on Probing; RD: Gingival Recession; KTW: Keratinized Tissue Width; PD: Probing Depth; GI: Gingival Index; PI: Plaque Index
Introduction
As a result of increasing dental implant use, problems related to implant-supported dental prosthesis have considerably increased. The prevalence of peri-implantitis during the first five to ten years following the implant replacement is reported to be 20% and this rate is in rise [1-6]. Peri-implantitisis an infectious disease causing inflammation in periodontal tissues surrounding dental implants and it’s characterized by gingival bleeding, suppuration, edema, increasing periodontal pocket depth and radiographic bone loss [1-4]. The most important factor causing peri-implantitis is the formation of bacterial biofilm on dental implants and bacterial colonization [4,5].
Various factors are inherent in peri-implant disease etiology [4]. A sole factor or the combination of some factors may contribute to the disease. These factors may be cited as follows: factors related to the patient (parafunctional habits like bruxism, smoking, systemic or genetic diseases, periodontal health conditions of existing dentition, periodontitis past and oral hygiene status), the volume of attached gingiva surrounding implant, the health condition, the volume and quality of alveolar bone tissues, factors related to chosen implant systems (surface features of the implant, the length-diameter of the implant), occlusal and lateral forces (the direction, volume and frequency of the force), occurrences during implant surgery (further surgical implementations, surgical complications), the level of information and experience of the physician following surgical and prosthetic operations [6-8].
Peri-implantitis is characterized by inflammation and crestal bone loss surrounding implants, and clinical findings of the inflammation should be observed. Peri-implant clinical findings (mobility, bleeding upon probing, plaque index, probing pocket depth and measurements of attachment level), radiographic findings (implant-bone relation, the volume of alveolar bone) and biochemical tests are distinctive diagnostic methods in the maintenance of implant functions [7].
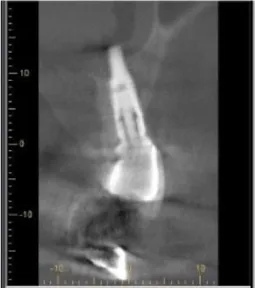
Bleeding upon probing existing around the implants that means the deterioration of tissues surrounding implants and the existence of an active disease. It’s also an initial crucial symptom to foresee attachment loss in the future [9]. In the clinical examination of periodontal health of implants, one of the widely preferred diagnostic parameters is the measurement of pocket depth and attachment level [8]. In the evaluation of peri-implant hard tissue, periapical or panaromic radiographs and dental tomographs are used. By means of radiographic scans, the level of implant-bone contact and resorption occurring in bone is determined and by comparison with earlier scans, the diagnosis may be realized [10].
In the occurrence of peri-implantitis, the primary etiologic factor is the formation of bacterial biofilm on implant surfaces [2]. These bacteria inhabiting in biofilm highly resist against topical disinfectants and systemic antibiotics [3]. Therefore, the first aim of related therapies is the effective mechanical removal of the biofilm. Although many studies in the literature suggest that mechanical periodontal therapy is effective for peri-implantitis, there’s no study comparing which method is better than the other [11-15].
Soft tissue surfaces of edentulous individuals serve as a reservoir for peri-implant colonization and periodontal pathogens [16]. In implant microflora of partly toothless individuals, a high level and frequency of P.gingivalis and P.intermedia, a low level of coccoid cells and a significant level of bacillus and spirochetes are identified [16,17]. In microflora of peri-implantitissite, a high level of bacillus, spirochetes and fusiforms are diagnosed and it is reported that coccoid cells compose only 50% microflora [17,18]. Since peri-implantitis is a disease with a high level of microbial activity, in many studies antibiotics are offered to be prescribed, however, research studies show that antibiotics are not potent enough to treat peri-implantitis and mechanical treatment is also required [19]. In peri-implantitis therapy, the etiologic factor should be removed. Thus, mechanical debridement of calculus and bacterial plaque is compulsory. Owing to the screwed character of implants, a mechanical debridement is not so easy [18]. Considering that metal currettes or tips will adversely affect implant surface, custom design carbon fiber, titanium or plastic curettes or ultrasonic instruments developed for implant surface should be used in implant therapy. In addition to these methods, air-abrasive debridement have been widely used to support the therapy recently [20-23]. In addition to mechanical treatment and decontamination are also preferred in peri-implantitis therapy [23]. For decontamination, various materials (chlorhegxidine rinse, citric acid solution, tetracycline solution, iodin irrigation, saline irrigation etc.) and various lasers (Diod, Er:YAG and Nd. YAG) are used [24-28].
Depending on clinical and radiographic examinations, the patients included in this study who developed peri-implant infections in the region of the implants because of dental plaque. The aim of the study is to compare clinical findings of methods of mechanical removal of bacterial biofilm made by standard plastic curettes, debridement made by combined 0.12% chlorhegxidinerinse and mechanical debridement made by ultrasonic polyetheretherketone coated tips developed for implant surface and combined air-flow with erythritol powder debridement.
Materials and Methods
Subject selection
18 systemically healthy patients, who applied to the Department of Periodontology, Necmettin Erbakan University and whose at least 1 dental implant was diagnosed by peri-implantitis and who has never taken peri-implantitis therapy, were included in the study. All patients were informed about the study and given informed consent form, and only volunteers were included. The research was conducted in accordance with the principles outlined in the Declaration of Helsinki. This prospective clinical study was approved by the Research Ethics Committee of Necmettin Erbakan University. This study is registered at ClinicalTrials.gov. (NCT03241953).
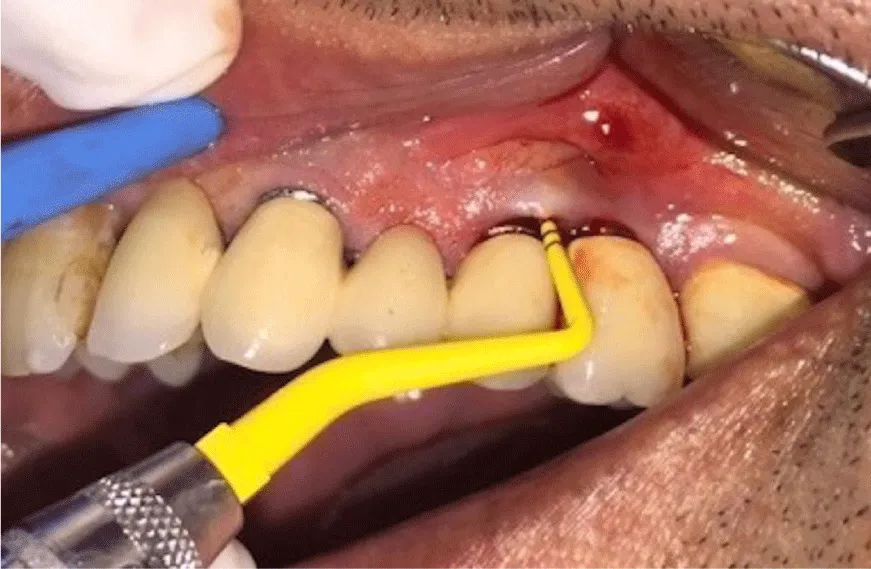
The patients, who were systemically healthy, who don’t smoke and free of parafunctional habits like bruxism, and didn’t have any kind of periodontal therapy within the previous year and had implants for at least 5 years, were included in the study. In addition, the inclusion criteria were as follows: having pocket depth over 5mm in implants diagnosed by periimplantmucositis or peri-implantitis (Figures 1,2) and having no mobility. The patients with chronic bronchitis or asthma and major systemic illnesses (i.e. diabetes mellitus, cancer, HIV, bone metabolic diseases or disorders that compromise wound healing, radiation or immunosuppressive therapy) and those who had taken antibiotics, anti-inflammatory drugs or other medication within the previous 28 days were excluded in the study.
18 patients (mean age 52 years) diagnosed with periimplant mucositis or peri-implantitis were included in this randomized and controlled clinical study. Randomization was performed with coin. The evaluation was blinded for treatment modality. A total of forty dental implants were debrided with either standard plastic curettes, debridement made by combined 0.12% chlorhegxidine rinse(control, n=20) or mechanical debridement made by ultrasonic polyetheretherketone coated tips developed for implant surface and combined air-flow with erythritol powder debridement (test, n=20).
Clinical measurements
On six sites of all implants, the following clinical parameters were recorded: Plaque Index (PlI; Silness&Lo€e 1964), PD, Bleeding on Probing (BOP) and Gingival Recession (RD).
Keratinized tissue width (KTW) was measured at buccal midpoint of implants. Bone loss volume was recorded on periapical radiographs by measuring the distance from bone implant abutment placement to alveolar bone level. All measurements were taken at the beginning.
Phase IPeriodontal therapy
Upon the recordings, all patients were given Phase 1 periodontal therapy and informed about hygiene control. Prior to surgical operation, professional supragingival and subgingival debridement was performed. The patients with a good level of oral hygiene were included in the study. Oral hygiene controls were performed in the first, third and sixth months prior to and after operations. Occlusion controls of all implant supported dental prosthesis were performed, and if present, extreme contacts were removed.
Phase II Periodontal therapy
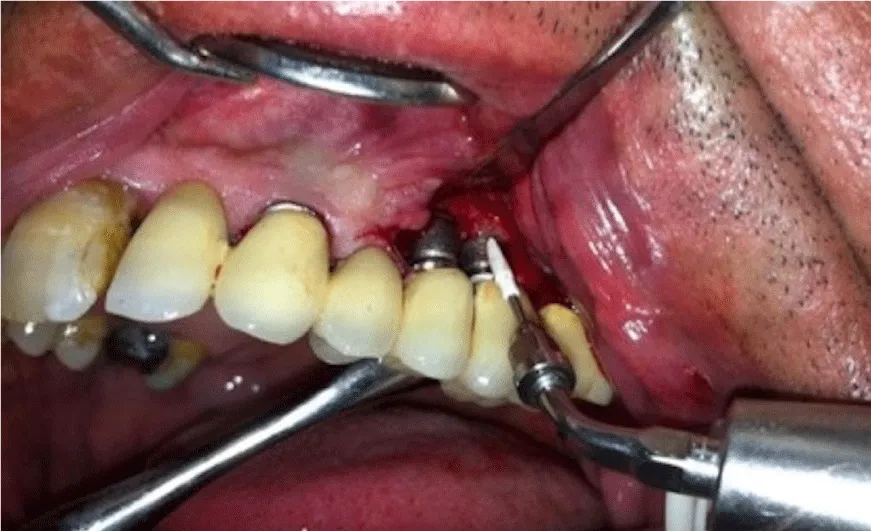
4 weeks after the initial periodontal treatments, for the treatment of the sites with pocket depth deeper than 6mm, flap operation was performed to achieve a direct reach to implant surfaces. All surgical procedures were carried out with local infiltration anesthesia (Ultracaine D-S, Hoechst). Followed Around affected implants, intrasulcular incisions were performed and mucoperiostal flaps with full thickness were raised both buccally and palatally. Implant surface decontamination was performed using with either plastic curettes or ultrasonic scaler (Figure 3); In control group, plastic curettes (Hue-Friedy Co., Chicago, IL, USA) were used for debridement and implant surfaces were decontaminated by 0.12% chlorhegxidine solution. In test group, sub-gingival debriment with ultrasonic polyetheretherketone coated tips was for nearly 20s per site (EMS Master Piezon LED, implant care system, Nyon, Switzerland). A special design disposable thermoplastic elastomer nozzle (Perio-flow Nozzle EMS Electro Medical Systems, Nyon, Sweden.), which horizontally gives out the erythritol powder, was utilized [29,30].
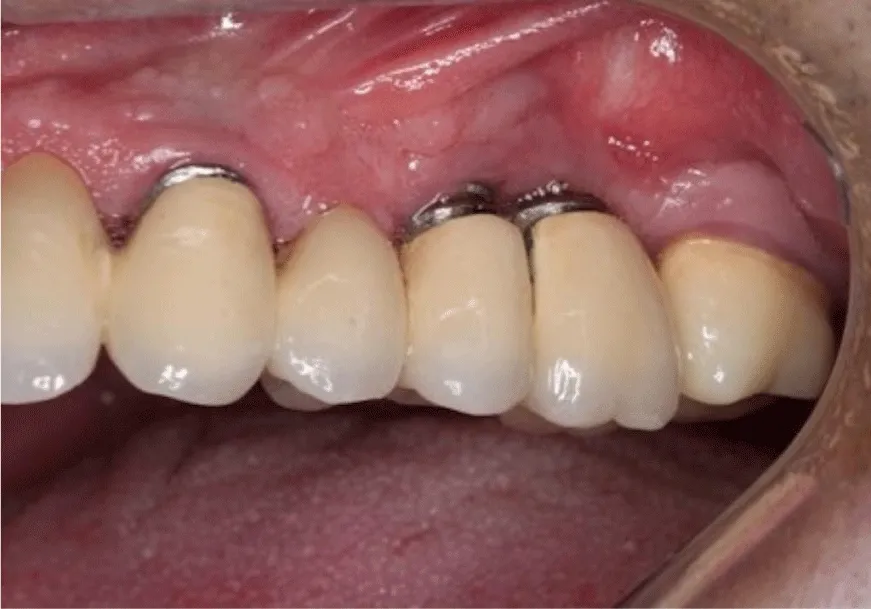
After the debriding of implant surfaces the flap was sutured by 4-0 vicryl. The sutures were removed 10 days after the operation and post-operative controls were performed. The patients were invited to the follow ups in the first, third and sixth month after the operation. Clinical and radiographic measurements were repeated every six months (Figure 4).
Statistical analysis
A power analysis was done to determine the proper number of subject. While evaluating the findings of the study, IBM SPSS Statistics 22 (IBM SPSS, Turkey) software was used and the conformity of normally distributed parameters were checked by Shapiro Wilks test. In the comparison of quantitative data, Mann Whitney U test was used to compare non-normally distributed parameters of both groups. For non-normally distributed parameters of each group Wilcoxon Sign test was used. The relations among parameters were evaluated by using Sperman’s Rho correlation analysis. The significance was evaluated at p<0.05 level.
Results
In both groups, no complication was observed after the operations and the level of recovery was satisfying.
The mean initial periodontal pocket depth of the groups was not significantly different (p>0.05) (Table 1). In control group, the mean periodontal pocket depth in the sixth month, was statistically significant and higher than the mean pocket depth of the test group (p: 0.001: p<0.05) (Table 1). The decreasing volume of pocket depth in the test group in the sixth month was significantly higher than the control group (p: 0.001: p<0.05) (Table 1).
In control group, the decrease in the mean periodontal pocket depth in the sixth month is statistically significant thanthe initial periodontal pocket depth (p: 0.001: p<0.05). In test group, the decrease in the mean periodontal pocket depth in the sixth month is statistically significant thanthe initial periodontal pocket depth (p: 0.001: p<0.05) (Table 1).
No statistically significant finding was observed in the mean initial gingival index of both groups (p>0.05) (Table 2) and no statistically significant finding was observed in the mean gingival index in the sixth month (p>0.05) (Table 2). In control group, the mean gingival index scores in the sixth month, was statistically significant and higher than the test group (p: 0.001: p<0.05) (Table 1).
There’s no significant difference between the initial and the sixth month gingival index levels of both groups (p>0.05) (Table 2). In control group, the decrease in the mean gingival index in the sixth month compared to the initial level is statistically significant (p: 0.001: p<0.05) (Table 2). In test group, the decrease in the mean gingival index in the sixth month compared to the initial level is statistically significant (p: 0.001: p<0.05) (Table 2).
No statistically significant difference was observed in initial KTW measurements of both groups (p>0.05) (Table 3). When the findings of the patients of both groups were studied, a reversed, measured by 89.3% and statistically significant relation was found between the initial pocket depth and initial KTW levels (p: 0.001: p<0.05).
No statistically significant difference was observed in the mean initial gingival recession levels of both groups (p>0.05) (Table 4). No statistically significant difference was observed in the mean gingival recession levels in the sixth month of both groups (p>0.05) (Table 4). No statistically significant difference was observed in the mean gingival recession levels in the sixth month of both groups compared to the mean initial level (p>0.05) (Table 4).
In control group, the increase in the mean gingival recession levels in the sixth month compared to the mean initial level is statistically significant (p: 0.007: p>0.05) (Table 4).
In test group, the increase in the mean gingival recession levels in the sixth month compared to the mean initial level is statistically significant (p: 0.023: p>0.05) (Table 4).
There is no statistically significant difference in the mean initial plaque index levels of both groups (p>0.05) (Table 5). There is no statistically significant difference in the mean plaque index levels in the sixth month (p>0.05) (Table 5). No statistically significant difference was observed in the mean plaque index levels in the sixth month of both groups compared to the mean initial level (p>0.05) (Table 5). There is statistically significant difference for the plaque index scores in the test group in sixth month, it was significantly lower than the control group (p: 0.001: p<0.05).
There is no statistically significant difference in the mean initial and in the sixth month bone levels of both groups (p>0.05). There was no change found in bone levels between pre- treatment and post-treatment.
Discussion
One of the mostly diagnosed complications in dental implants is peri-implant diseases [1-5]. According to the studies and systematic compilations, the patients with chronic periodontitis background have a higher risk of having peri-implantitis [31]. In accordance with the literature, it was also found that all patients included in our study had chronic periodontitis. It was reported that peri-implantitis develops as a result of the ongoing oral hygiene habits of the patients with periodontitis as it causes plaque accumulation on the implants [28]. One of the reason for periimplant mucositis and peri-implantisis is the bacterial colonization occurring on the implant surface, therefore, for a successful therapy, bacterial population should be declined and bacterial biofilm should be debrided [24].
Owing to the rough and screwed structure of implant surface, an entire debriding process of the implant surface is relatively difficult. Though various scientific studies focus on peri-implantitis therapy, there’s no concrete evidence regarding which method is the best option.
The main point in peri-implantitis treatment is the entire removal of bacterial biofilm. In many studies, it’s suggested that for a successful peri-implantitis therapy, open surgical treatment [27,28,32,33], with various decontamination methods like air powder flow, saline wash, citric acid, laser, hydrogen peroxide, and electrochemical decontamination were used, however, no concrete evidence regarding which of them could be the best method was mentioned [16,31,34,35,]. In this study, we compared and evaluated the clinical findings of methods of mechanical removal made by standard plastic curettes, debridement made by combined serum, mechanical debridement made by ultrasonic polyetheretherketone coated tips developed for implant surface and combined air-flow debridement, which are used in implant sites diagnosed by peri-implantitis. Considering the results of the study, it was concluded that air-flow decontamination combined by ultrasonik polyetheretherketone coated tips is more effective than traditional methods for a better implant surface debridement.
Inadequate oral hygiene habits of the patients with periodontitis plays a critical role in peri-implantitis development, however, periodontal pockets and gingival sulcus serving as a reservoir poses a risk for peri-implantitis [36,37]. In our study, when the periodontitis background of all patients is considered, both plaque accumulation around implants and peri-implant soft tissues may have serves as a reservoir. Nevertheless, this could be an assumption only as we didn’t perform any microbiological analysis.
The determination of bleeding upon probing is the first symptom of peri-implant diseases. The severity and development of the disease may be determined through bleeding upon probing [5]. Bleeding upon probing may also mean active tissue depletion in peri-implant tissues [33]. In this study, according to initial gingival index levels, bleeding upon probing was observed in all peri-implantitis sites and at the end of the study a significant decreased in bleeding scores was observed in both groups but in control group, the mean gingival index scores in the sixth month, was statistically significant and higher than the mean pocket depth of the test group. The increase in pocket depth level is one of the precise symptoms of peri-implantitis. In our study, nonhealing implants, despite phase I periodontal treatments, with periodontal pocket depth level over 6 mm were included. At the end of the study, for the both groups, a significant decreased was observed in pocket depth. However, the volume of the decreased was found significantly higher in the test group [38].
In many studies, smoking is suggested to be one of the vital risk factors triggering the development of peri-implantitis [34,39]. The fact that the total exposure time, frequency and volume of smoking may affect the severity of peri-implantitis was reported in some studies [39]. As smoking may affect the healing results of the groups, the patients addicted to smoking were not included in our study.
One another reason for peri-implantitis is extreme occlusal forces and badly planned prosthesis [40]. In our study, no defected extreme occlusal forces causing peri-implantitis were observed. However, as they may pose a risk for healing period, we took out implant supported prosthesis from occlusal contact in our study.
In many studies it is reported that in peri-implantitis cases, which lack a clear bone wall around the implant, have no intraosseous pocket deformation and have horizontal bone loss, bone regenerations procedures slightly work, and thus, only implant surfaces should be debrided and soft tissues should be repositioned in order to make the patient follow oral hygiene procedures [32]. As the patients included in our study didn’t have suitable indications for bone regeneration, we didn’t perform any graft or membrane operations on the peri-implantitis site.
Periodontal curettes(Plastic or titanium curettes, carbon-fiber curettes, tefloncurettes, ultrasonic devices)are made of different materials as they are recommended to be used in different operations, however, they all have been produced for use specifically to debride implant surfaces [11,41]. Plastic curettes are the most fragile of all curette types and have restricted capacity for debridement operations.40 However, plastic curettes are the mostly preferred instruments for implant surface debridement.
Ultrasonic driven instruments with polyetheretherketone-coated tips are also used to debride the implant surface. The tip is a modified and made of a high-tech plasticmaterial and has a stainless steel core. It’s really advantageous to debride the implant surface easily and is also comfortable for the patients. The device is for the debridement of plaque and calculus from all around the implant neck and the abutment to achieve a clean and smooth surface [13].
Many studies have claimed that air-abrasive systems are useful for implant surface decontamination [14]. Standard powdered air-abrasive systems rely on air-spray of sodium bicarbonate. They are used for polishing and for removing tooth stains, however, owing to their high abrasiveness, they cannot be used for implant operations as they may damage hard and soft tissue [14]. Apowered air-abrasive system using low-abrasive amino-acidglycine powder has been demonstrated as an effective method for the removal of the bacterial biofilm from the root surface, without damaging hard and soft tissues [15] and it has been recommended for debriding implantsurfaces [29]. Following glycine powder, erythritol powder which has smaller particles and is less abrasive has been developed. As it is less abrasive and has really small particles that do not damage implant surface, as an air-abrasive method in our study, we decided to use air-flow with erythritol powder for the debridement of the implant surface.
Since plastic curettes are mostly preferred in clinics treatment, we planned to use them for our control group. We compared the results of the treatments using the newest technology ultrasonic driven devices including polyetheretherketone-coated tips with the air-flow with erythritol powder combination. The results of the test group were also found successful at the end of the study. However, the fact that we included a very low number of total dental implants in the study and we didn’t perform any microbiological analyses were the limitations of our study. We believe that studies in the future may focus on the issue as more information is required.
Conclusion
It was found in the study that the combined air-flow debridement and the mechanical debridement performed by using ultrasonic polyetheretherketone-coated tips developed for implant surface debridement in peri-implantitis therapy were better at decreasing the level of periodontal pocket depth than plastic curettes. As these methods are more comfortable for clinicians and patients, they are considered to be a viable alternative to standard plastic curettes and 0.12% chlorhegxidine combination.
Compliance with Ethical Standards: The research was conducted in accordance with the principles outlined in the Declaration of Helsinki. This prospective clinical study was approved by the Research Ethics Committee of Necmettin Erbakan University.
Conflict of interest: We have no conflict of interest. We have seen and agree with the contents of the manuscript and there is no financial interest to report. We declare that he has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
- Paster BJ. Dewhirst FE (2009) Molecular microbial diagnosis. J Periodontol 51: 38–44. Link: https://tinyurl.com/yy5n9hdt
- Quirynen M, Vogels R, Pauwels M, Haffajee AD, Socransky SS, et al. (2005) Initial subgingival colonization of pristine pockets. J Dent Res 84: 340-344. Link: https://tinyurl.com/yyjesune
- Teles RP, Haffajee AD, Socransky SS (2006) Microbiological goals of periodontal therapy. J Periodontol 42: 180-218. Link: https://tinyurl.com/y6szanyb
- Zitzmann NU, Berglundh T (2008) Definition and prevalence of periimplant diseases. J Clin Periodontol 35: 286-291. Link: https://tinyurl.com/yxflmx52
- Hultin M, Gustafsson A, Hallström H, Johansson LA, Ekfeldt A, et al. (2002) Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res 13: 349-358. Link: https://tinyurl.com/yy5vuxge
- Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C (1992) Experimental breakdown of periimplant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3: 9-16. Link: https://tinyurl.com/y383d3jg
- Lang NP, Adler R, Joss A, Nyman S (1990) Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol 17: 714-721. Link: https://tinyurl.com/y2d9k5fy
- Hammerle CH, Glauser R (2004) Clinical evaluation of dental implant treatment. Periodontol 2000. 34: 230-239. Link: https://tinyurl.com/y3owebqj
- Lekholm U (1986) Marginal tissue reactions at osseointegrated titanium fixtures (II). A cross-sectional retrospective study. Int J Oral Maxillofac Surg 15: 53-61. Link: https://tinyurl.com/y6fgv7bb
- İplikçioğlu H, Akça K, Çehreli MC (2002) The use of computerized tomography for diagnosis and treatment planning in implant dentistry. J Oral Implantol 28: 29-36. Link: https://tinyurl.com/yyuob7pv
- Maximo MB, de Mendonca AC, Renata Santos V, Figueiredo LC, Feres M, et al. (2009) Short-term clinical and microbio logical evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res 20: 99-108. Link: https://tinyurl.com/y5vp55fb
- Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM (2002) Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol 73: 1118-1125. Link: https://tinyurl.com/y4g76xgg
- Thone-Muhling M, Swierkot K, Nonnenmacher C, Mutters R, Flores-de-Jacoby L, et al. (2010) Comparison of two fullmouth approaches in the treatment of peri-implant mucositis:a pilot study. Clin Oral Implants Res 21: 504-512. Link: https://tinyurl.com/y3uqxm97
- Kontturi-Narhi V, Markkanen S, Markkanen H (1990) Effects of airpolishing on dental plaque removal and hard tissues as evaluated by scanning electron microscopy. J Periodontol 61: 334-338. Link: https://tinyurl.com/y65ru4pw
- Petersilka GJ, Steinmann D, Haberlein I, Heinecke A, Flemmig TF (2003) Subgingival plaque removal in buccal and lingual sites using a novel low abrasive air-polishing powder. J ClinPeriodontol 30: 328-333. Link: https://tinyurl.com/y2bghfhj
- Apse P, Ellen RP, Overall CM (1989) Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: a comparison of sites in edentulous and partially edentulous patients. J Periodontal Res 24: 96-105. Link: https://tinyurl.com/y6rz88ak
- Papaioannou W, Quirynen M, Nys M (1995) The effect of periodontal parameters on the subgingival microbiota around implants. Clin Oral Implants Res 6: 197-204. Link: https://tinyurl.com/yxtglzgt
- Berglundh T, Lang NP Lindhe J (2008) Treatment of Peri-implant Lesions. In: Lindhe J, Lang NP, Karring T.(eds), Clinical Periodontology and Implant Dentistry, 5th. Ed., Blackwell Publishing, Munksgaard 11: 875-881.
- Berglundh T, Krok L, Liljenberg B (1998) The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease, A prospective, controlled clinical trial. J ClinPeriodontol 25: 354-362. Link: https://tinyurl.com/y4p684oq
- Matarasso S, Quaremba G, Coraggio F, Vaia e, Cafiero C, et, al. (1996) Of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res 7: 64-72. Link: https://tinyurl.com/y5hyxsmo
- Porras R, Anderson GB, Caffesse RG, Narendran S, Trejo PM (2002) Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol 73: 1118-1125. Link: https://tinyurl.com/y4g76xgg
- Trejo PM, Bonaventura G, Weng D, Caffesse RG, Bragger U, et al. (2006) Effect of mechanical and antiseptic therapy on peri-implant mucositis: an experimental study in monkeys. Clin Oral Implants Res 17: 294-304. Link: https://tinyurl.com/yyusrsfx
- Giannini R, Vassalli M, Chellini F, Polidori L, Dei R, et al. (2006) Neodymium:yttrium aluminum garnet laser irradiation with low pulse energy: a potential tool for the treatment of peri-implant disease. Clin Oral Implants Res 17: 638-643. Link: https://tinyurl.com/y6agml3j
- Mombelli A, Müller N, Cionca N (2012) The epidemiology of periimplantitis. Clin Oral Implants Res 23: 67–76. Link: https://tinyurl.com/y65snqub
- Mombelli A, Lang NP (1998) The diagnosis and treatment of peri-implantitis. Periodontol 2000. 17: 63–76. Link: https://tinyurl.com/y4bfp72y
- Lang NP, Wilson TG, Corbet EF (2000) Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin Oral Implants Res 11: 146-155. Link: https://tinyurl.com/y6kaoeh6
- Rinke S, Ohl S, Ziebolz D, Lange K, Eickholz P (2011) Prevalence of peri-implant disease in partially edentulous patients: A practice-based cross-sectional study. Clin Oral Implants Res 22: 826-833. Link: https://tinyurl.com/yx97hw9n
- Esposito M, Grusovin MG, Worthington HV (2012) Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur J Oral Implantol 5: S21-S41. Link: https://tinyurl.com/y5gdau9e
- Sahm N, Becker J, Santel T, Schwarz F (2011) Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J ClinPeriodontol 38: 872–878. Link: https://tinyurl.com/y6fm2jzf
- Schar D, Ramseier CA, Eick S, Arweiler NB, Sculean A, et al. (2013) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: sixmonth outcomes of a prospective randomized clinical trial. Clin Oral Implants Res 24: 104-110. Link: https://tinyurl.com/y5nyq8zg
- Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Brägger U, Hämmerle CH, et al. (2003) Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10 year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res 14: 329-339. Link: https://tinyurl.com/yxrvjh6t
- Figuero E, Graziani F, Sanz I, Herrera D, Sanz M (2014) Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 66: 255-273. Link: https://tinyurl.com/y4tz4tdw
- Nogueira-Filho G, Iacopino AM, Tenenbaum HC (2011) Prognosis in implant dentistry: a system for classifying the degree of peri-implant mucosal inflammation. J Can Dent Assoc. 77: b8. Link: https://tinyurl.com/yymzaya3
- Baqain ZH, Moqbel WY, Sawair FA (2012) Early dental implantfailure:risk factors. Br J Oral Maxillofac Surg 50: 239-243. Link: https://tinyurl.com/yyjdktlq
- Bain CA, Moy PK (1993) The association between the failure of dental implants and cigarette smoking. Int J Oral Maxillofac Implants 8: 609-615. Link: https://tinyurl.com/y6g4jbzn
- Lee K, Tanner A, Maiden M, Weber H (1999) Pre‐and post‐ implantation microbiota of the tongue, teeth, and newly‐ placed implants. J Clin Periodontol 26: 822-832. Link: https://tinyurl.com/y5s57n4p
- Mombelli A (2002) Microbiology and antimicrobial therapy of peri‐implantitis. Periodontol 2000. 28: 177-189. Link: https://tinyurl.com/y5293jbg
- Serino G, Turri A, Lang NP (2013) Probing at implants with periimplantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res 24: 91-95. Link: https://tinyurl.com/yyxeuz9p
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. et al. (2015) A systematicreviewand meta-analysis. Clin Oral Implants Res 26: 62-67. Link: https://tinyurl.com/y5s8ar7c
- Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, et al. (2008) A systematic review of the 5‐year survival and complication rates of implant‐supported single crowns. Clin Oral Implants Res 19: 119-130. Link: https://tinyurl.com/y47wzkc4
- Heitz-Mayfield LJ, Salvi GE, Botticelli D, Mombelli A, Faddy M, et al. (2011) Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res 22: 237-241. Link: https://tinyurl.com/y5a5gzdu
- Meffert R (1996) Periodontitis vs. peri-implantitis: the same disease? The same treatment? Crit Rev Oral Biol Med 7: 278-291. Link: https://tinyurl.com/y5w2qeku
- Schenk G, Flemmig TF, Betz T, Reuther J, Klaiber B (1997) Controlled local delivery of tetracycline HCl in the treatment of periimplant mucosal hyperplasia and mucositis. A controlled case series. Clin Oral Implants Res 8: 427-433. Link: https://tinyurl.com/y36k5xn8
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
