Journal of Surgery and Surgical Research
Functional Outcome and Life Quality after Unilateral Fixed Proximally-Based Gluteoplasty for End-Stage Fecal Incontinence
Mahmoud Abdelnaby1, Mohamed El-Said2, Sameh Hany Emile1* and Ahmed Abdel Mawla1
2Assistant lecturer of General surgery, General Surgery department, Mansoura faculty of medicine, Egypt
Cite this as
Abdelnaby M, El-Said M, Emile SH, Mawla AA (2017) Functional Outcome and Life Quality after Unilateral Fixed Proximally-Based Gluteoplasty for End-Stage Fecal Incontinence. J Surg Surgical Res 3(1): 010-014. DOI: 10.17352/2455-2968.000036Introduction: Many patients with fecal incontinence (FI) have no treatment option except diversion. Gluteoplasty can be the ultimate resort for these patients despite unsatisfactory long term functional results. Different gluteoplasty techniques were described. The aim of this study was to evaluate the impact of fixing the proximally-based gluteus muscle flap to the contralateral ischial tuberosity on functional outcome in patients with end-stage FI.
Patients and methods: Prospectively collected data of 17 patients with end-stage FI managed with unilateral proximally-based fixed gluteoplasty were retrospectively reviewed. Endoanal ultrasound and electromyography were performed before surgery to assess the condition of anal sphincter and gluteus maximus muscles. Patients were evaluated preoperatively and six months postoperatively by Wexner continence score and fecal incontinence quality of life questionnaire.
Results: All patients showed significant improvement in the continence state and quality of life after surgery. Patients with an adequate external anal sphincter (EAS) remnant (> 50%) were completely continent or complained only of minor FI while those with larger EAS defects were continent only to solid stool, however showed significant improvement in life quality.
Conclusion: Proximally-based fixed gluteoplasty can improve life quality and restore complete continence in patients with an adequate EAS remnant and partial continence in patients with larger EAS defects.
Introduction
Fecal incontinence (FI) is a distressing condition that has a great impact on patients’ quality of life [1]. Sometimes all treatment options fail to restore an acceptable continence level and the ultimate solution for the patient will be creation of permanent stoma [2]. Given the negative influence of stoma on patients’ life quality and daily activities, several other options were devised as alternatives to stoma formation. These options include gracilis muscle transposition [3], gluteus muscle transposition [4], artificial bowel sphincter (ABS) [5], and antegrade colonic irrigation [6], among other options.
Chetwood first described gluteoplasty for the treatment of FI in 1902; however the procedure did not gain much popularity until thirty years later when it was revived again [4,7]. Different techniques were advocated for performing gluteoplasty. Such techniques involved using unilateral or bilateral muscle flaps, using proximally- or distally-based muscle flaps, and creation of free floating flaps or fixing them to the contralateral ischial tuberosity [7].
Proximally-based gluteus muscle flaps were recommended over distally-based ones as they can extend for longer distance enabling its tension-free wrap around the anal canal easily [8]. Guelinckx et al. [9] fixed a unilateral proximally-based gluteus muscle flap to the contralateral ischial tuberosity to restore the resting length of the muscle flap and considered this to be a prerequisite for good functional outcome.
The aim of the current study was to evaluate the functional outcome of patients with end-stage FI after proximally-based gluteoplasty performed with fixation of the gluteal muscle flap to the contralateral ischial tuberosity. The functional improvement was assessed by the improvement in Wexner continence score and the improvement in life quality of the patients.
Patients and Methods
Study design and setting
We retrospectively evaluated the functional results of 17 patients with end-stage FI treated with unilateral fixed proximally-based gluteoplasty between April 2015 and July 2016. Patients were assessed, operated on, and followed in private hospitals in Mansoura city, Egypt. Ethical approval for the study was obtained from the institutional review board of Mansoura Faculty of Medicine (code: R/16.04.72). The study was registered in www.researchregistry.com with the unique identifying number (UIN): researchregistry2182.
Eligibility criteria
We included patients with major end-stage FI due to External anal sphincter (EAS) failure. Major FI was defined as incontinence to diarrhea or solid stool and end-stage FI was defined as the worst degree of FI in which the only remaining option for treatment, other than muscle transfer, is diversion.
Preoperative assessment
Detailed history about the type and duration of complaint and other associated symptoms was taken from the patients. Patients were asked about previous medical or surgical treatments received for the current complaint and the degree of FI was assessed using Wexner continence score [10], with special emphasis made on symptoms as urgency and the sense of rectal distension. The impact of FI on patients’ quality of life was evaluated using the fecal incontinence quality of life (FIQL) score [11].
Patients were examined locally in the left lateral position by digital rectal examination and proctoscopy, and were assessed by endoanal ultrasonography (EAUS) to evaluate the integrity of the EAS, and by electromyography (EMG) for assessment of the contractile activity of both gluteus maximus muscles.
Surgical technique
Informed consent about the nature and potential benefits and complications of the procedure was taken from all patients preoperatively. Mechanical bowel preparation was performed over two days before the operation using 4L of oral isotonic polyethylene glycol (PEG) solution with restriction of oral intake to clear liquids only.
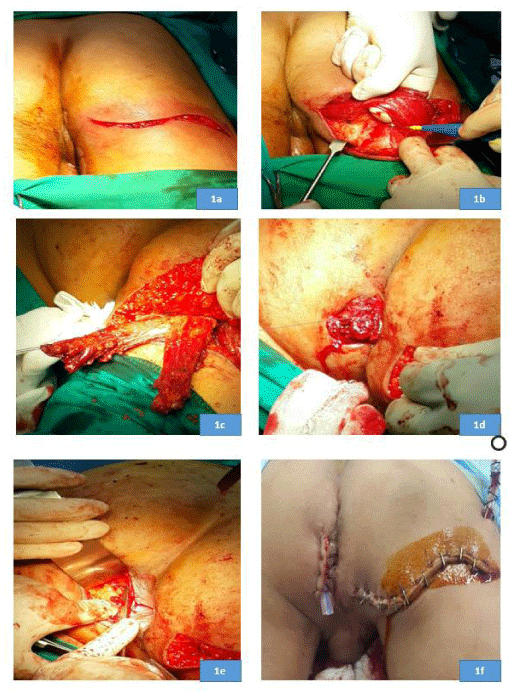
After induction of general anesthesia and administration of prophylactic antibiotics (1gm of cefotaxime and 500mg of metronidazole), patients were positioned in the prone jack-knife position. An S-shaped incision (Figure 1a) was made near the infra-gluteal fold to access the gluteus maximus muscle. The lower third of the muscle was detached from the posterior gluteal tubercle of the femur, with a strip of tendon and periosteum if possible, for later fixation to the ischial tuberosity (Figure 1b). Next, the muscle flap was dissected from the lateral to medial side until the inferior gluteal nerve and vessels were clearly identified, then the muscle flap was divided along the line of its fibers into two strips (Figure 1c).
During harvesting the muscle flap we made sure that the sciatic nerve was not exposed to avoid direct pressure over it during setting; in case the nerve was exposed, a part of the harvested flap was separated to cover it. Afterwards, an incision was made on the other side of the anus exposing the contralateral ischial tuberosity; then two tunnels were made in front of and behind the anal canal. The two muscle strips were transposed around the anal canal (Figure 1d) and sutured to the contralateral ischial tuberosity with a modified Kessler tendon repair (Figure 1e). Sutures were taken between the two muscle strips on both sides of the anus to make the muscle wrap adequately tight to generate a resting tone in the anal canal. The gluteal incision was closed over a suction drain and the para-anal incision was closed over a rubber or silicon drain (Figure 1f).
Postoperatively, patients were maintained on a low-residue diet. Oral antibiotics were continued for 5 days after surgery. Sutures were removed upon complete healing of the wound, within 14 days postoperatively, and the drains were removed after 10 days. Patients were instructed not to sit or climb stairs for three weeks and were advised to do a 15 minutes of Kegel exercise every day after complete wound healing.
Follow-up and outcomes assessed
Patients were followed every week for one month, then on monthly basis for at least six months. At every visit wound healing was assessed by the operating surgeon. At 6 months postoperatively, the continence state was assessed using Wexner score and quality of life was evaluated using FIQL. Patients were asked to grade their degree of satisfaction with the operation as 1: satisfied, 2: partially satisfied, and 3: not satisfied.
The primary outcome of the study was the improvement in Wexner score and FIQL at six months of follow-up, secondary endpoints included operation time, hospital stay, and postoperative complications.
Statistical analysis
Data were analyzed using SPSS (Statistical Package for Social Science) version 21 (IBM corp., Bristol, UK). The description of data was done in form of mean ± SD for continuous data and percent for categorical data. Analysis of statistical significance between different groups was done using Student t-test for continuous variables. P value < 0.05 was considered significant.
Results
Patients’ characteristics
Seventeen patients with end-stage FI were studied. Patients were nine males and eight females with mean age of 19.1± 12.1 years, ranging between five and 42 years. Causes of end-stage FI were surgery for anorectal malformations (ARMs) (n=7), surgery for congenital megacolon (n=4), surgery for anal fistula (n=2), obstetric trauma (n=2), hemorrhoidectomy (n=1) and rectal prolapse (n=1). Overlapping anal sphincter repair (OASR) was tried in five patients who developed FI after anal fistula surgery, hemorrhoidectomy or vaginal delivery and failed.
The mean extent of EAS defect measured by EAUS represented 54.7 ±43.1 (0-100) % of the EAS circumference. Patients with FI after previous surgery for ARM were found to have clinically absent EAS and the EAS defect was considered to be 100%. Patients with FI after surgery for congenital megacolon or due to rectal prolapse had an intact, yet markedly degenerated EAS, thus the sphincter defect was considered to be 0%. Other patients had an EAS defect of 40% or 50% of the anal circumference.
Outcome of surgery
The mean preoperative Wexner continence score was 17.8 ± 2.1 which decreased significantly to 4.06 ± 4.2 at six months postoperatively. All parameters of FIQL significantly increased postoperatively (Table 1). A subgroup analysis of patients who developed FI after previous surgery for ARMs and had almost absent EAS was undertaken and showed a similar improvement in the continence state and quality of life after operation (Table 2). Eleven (64.7%) patients reported complete satisfaction, four (23.6%) were partially satisfied, while two (11.7%) were not satisfied with the outcome of their operation.
The degree of improvement in the continence level varied according to the preoperative size of the EAS defect. Ten patients had a defect involving less than 50% of the EAS, eight of which became completely continent after gluteoplasty; while two complained of minor FI (Wexner score less than 5). In seven patients with history of surgical correction of ARM, the majority of the EAS was deficient, those patients showed partial improvement and were continent to solid stool only.
The mean operation time was 61.7± 8.3 (range, 50-75) minutes, the mean hospital stay was 4.5± 0.7 (range, 3-5) days, complications occurred in four (23.5%) patients; disruption of the perianal wound occurred in two patients and was managed conservatively with daily dressing until complete healing, one patient developed infected wound collection and was treated with drainage and antibiotic therapy, one female patient complained of numbness sensation in the leg and was managed with vitamin B12 injection. The median follow-up period was 11 (range, 6-21) months, 88% of the patients were followed for more than 6 months.
Discussion
The use of gluteus maximus muscle flap is one of the oldest methods devised for the treatment of FI. Advantages of the gluteus maximus muscle include its good vascularity being supplied by the inferior gluteal artery, offering a large and strong bulk to help buttress the anal canal, acting as a significant adjunct to the EAS being activated during walking, yet without impairing gait or pelvic stability [12].
The colorectal surgery unit in Mansoura University Hospitals (MUH) has a vast experience in gluteus muscle transposition with encouraging results. Experience started with the unilateral distally-based flap [13], then it was augmented with fascia lata graft to increase flap length and prevent disruption of the sutures holding the flaps around the anal canal [14], finally a proximally-based flap was used as it was deemed better than the distally-based flap since it can be lengthened without much tension [8,15].
It was postulated that the gluteus muscle flap transplanted around the anal canal never regains its ideal resting length which may explain the poor functional results of gluteoplasty for FI. There is an ideal resting length of any skeletal muscle at which there is a maximal overlap between the actin and myosin filaments so that on stimulation the strongest contraction occurs [9]. Based on this concept we opt to fixate the gluteus muscle flap, after it has been wrapped around the anal canal, to the contralateral ischial tuberosity aiming to restore its ideal resting length. Furthermore, such fixation to the contralateral ischial tuberosity can stretch the transplanted muscle flap increasing the resting anal pressure generated by the transplanted muscle.
Few series described the outcome of fixed gluteoplasty. Bruininge et al. [16] published a case report describing bilateral proximally-based gluteoplasty with both muscle flaps being fixated to the ischial tuberosities on both sides. On other hand, Orgel and Kucan [17], used a unilateral proximally-based gluteoplasty with anchoring of the muscle flap to the contralateral ischial tuberosity in three patients. Guelinckx et al. [9] employed the same technique of Orgel and Kucan in 11 patients, yet the authors implanted electrodes and pulse generators in most of the patients studied, thus the effect of fixating the gluteus flap on functional outcome might be confused with the effect of electric stimulation and hence cannot be ascertained.
These earlier attempts involved very few patients which hindered reaching meaningful conclusions. In addition, no assessment of the quality of life of patients has been made in the previous trials. The present study represents the largest series evaluating the effect of fixating the gluteus muscle flap to the contralateral ischial tuberosity on the functional outcome and life quality of patients with end-stage FI. Overall, we found unilateral fixed proximally-based gluteoplasty achieve significant improvement in the continence state and quality of life with complete satisfaction reported by around two-thirds of the patients.
The preoperative size of the EAS defect was a prognosticator for the postoperative outcome. Patients with defects involving less than half the EAS circumference restored complete continence or complained only of minor FI or soiling in line with previous study [18], that correlated EAS defect size with severity of clinical symptoms. This may suggest a new effective treatment alternative to OASR in patients with an EAS defect involving more than one-third but less than one-half of the anal circumference who may suffer early disruption of sphincter repair and failure after OASR [19].
On the other hand, patients with larger EAS defects showed less improvement, yet it still was statistically significant. Our series included seven patients with ARM which is frequently associated with congenitally underdeveloped EAS, after gluteoplasty the continence state and quality of life of these patients improved significantly and five of them were partially satisfied with the outcome of the operation. Despite not achieving an acceptable continence state, the sense of rectal distension was regained in all of these patients and simple strategies as disposable enema before going out could compensate for their poor continence state improving their quality of life significantly. Therefore, fixed gluteoplasty can be considered a valid option for patients with end-stage FI who do not have other choices except permanent diversion.
It was reported that some patients lack a rectal reservoir or the sense of rectal distension and when a Duhamel operation or lining the anal canal with skin is performed with gluteoplasty, these patients achieve good continence [20]. Although these procedures were not tried in the present report, we think that adding them to gluteoplasty for patients with no adequate EAS remnant may improve the functional results, nevertheless no firm conclusions can be made on the effectiveness of these procedures awaiting prospective trials investigating their clinical utility.
It is important to note that the beneficial effect of gluteoplasty takes around 3 - 6 months to become evident. This was noted by other authors [4] and was explained by denervation and re-innervation of the transplanted muscle flap. We suggest that this re-innervation can involve both the inferior rectal and the pudendal nerve if there is a good remnant of the EAS with good contact between it and the transplanted muscle flap and this can explain the excellent results obtained in patients with an adequate remnant of EAS.
One of the common problems encountered after gluteoplasty is the wound-related morbidity which was recorded in three of our patients. Since gluteoplasty typically involves performing two incisions in proximity to the anal verge; the incidence of wound infection and disruption can predictably by high. Perhaps using negative pressure wound therapy could manage to reduce the incidence of wound-related complications after gluteoplasty similar to its role in management of pilonidal sinus disease [21].
In addition to gluteoplasty, other options were endorsed for the treatment of end-stage FI. These alternatives include stimulated gracilopasty, ABS, Magnetic anal sphincter, and sacral nerve stimulation (SNS). Gracilis muscle transposition was first used for the treatment of neurogenic FI in children back in 1952 [22]. Although it was first considered an optimal treatment choice due to its proximity to the anal canal, easy mobilization, and proximal blood supply, and innervation; the improvement in continence state was temporary due to the rapid fatigability of the gracilis muscle [12]. To avoid the rapid fatigue of the gracilis muscle, electrical stimulation was added to convert the gracilis muscle to slow twitch muscle allowing it to function as a neo-sphincter. The largest series by the Dynamic Graciloplasty Therapy Group reported a short-term success rate of 63% that declined at 18 months to 57% for patients who did not have a protective stoma with significant improvement in life quality [23].
ABS is a modification of the highly successful artificial urinary sphincter for urinary incontinence. The largest multicenter cohort study involving 112 sphincters who received ABS implantation reporting a significant improvement in the continence state and life quality. Major incontinence to liquid and solid stool became occasional minor soiling. However, complication of ABS implantation warranted surgical revision in 28 of 38 patients and 36% of patients required their device to be explanted with an overall failure rate of 30% [24].
SNS increases sympathetic and parasympathetic innervation of the anal sphincters resulting in direct voluntary contraction of the EAS and tonic stabilization of the internal sphincter [12]. It has been reported that SNS is superior to anal sphincter repair in the treatment of FI caused by sphincter defects [25]. In a single-center study [26], 127 patients received implantation of pulse generator and were followed for a mean period of 2.7 years, significant improvement in the St Mark continence score and FIQL were observed. Complications included 17 lead dislodgements, seven superficial infections, five infections requiring surgery and five repositioning of a rotated pulse generator. Although the initial reports on using SNS for FI is encouraging, the cost of the implantable pulse generator might be prohibitive for resource-limited countries.
Limitations of the current study include its retrospective nature, short follow-up, and small number of patients included. In order to substantiate the promising initial results reported in this study, prospective trials on a larger number of patients with follow-up exceeding two years are required.
Conclusion
The initial functional outcomes of unilateral proximally-based fixed gluteoplasty for end-stage FI are excellent provided that an adequate remnant of the EAS is present, otherwise the functional results are less than satisfactory, yet associated with significant improvement in patient’s quality of life if simple compensatory actions are added.
- Wald A (2007) Clinical practice. Fecal incontinence in adults. N Engl J Med 356: 1648-1655. Link: https://goo.gl/Dwm7Zo
- Norton C, Burch J, Kamm M (2005) Patients’ views of a colostomy for fecal incontinence. Dis Colon Rectum 48: 1062-1069. Link: https://goo.gl/rvVheb
- Corman M (1979) Management of faecal incontinence by gracilis muscle transposition. Dis Colon Rectum 22: 290-292. Link: https://goo.gl/BGvYnu
- Devesa J, Vicente E, Enriquez J, Nufio J, Bucheli P, et al. (1992) Total fecal incontinence–a new method of gluteus maximus transportation: preliminary results and report of previous experience with similar procedures. Dis Colon Rectum 35: 339-349. Link: https://goo.gl/zj3IBs
- Vaizey C, Kamm M, Gold D, Bartram C, Halligan S, et al. (1998) Clinical, physiological, and radiological study of a new purpose designed artificial bowel sphincter. Lancet 352: 105-109. Link: https://goo.gl/mM2pjf
- Malone P, Ransley P, Kiely E (1990) Preliminary report: the antegrade continence enema. Lancet 336: 1217-1218. Link: https://goo.gl/LjLV0Q
- Hentz V (1982) Construction of a rectal sphincter using the origin of the gluteus maximus muscle. Plast Reconstr Surg 70: 82-85. Link: https://goo.gl/T1mn4D
- Pak-Art R, Silapunt P, Bunaprasert T, Tansatit T, Vajrabukka T (2002) Prospective, randomized, controlled trial of proximally based vs. distally based gluteus maximus flap for anal incontinence in cadavers. Dis Colon Rectum 45: 1100-1103. Link: https://goo.gl/uergts
- Guelinckx P, Sensil N, Gruwez J (1996) Anal sphincter reconstruction with the gluteus maximus muscle anatomic and physiologic considerations concerning conventional and dynamic gluteoplasty. Plastic and reconsteuctive surgery 98: 293-302. Link: https://goo.gl/4k21aX
- Jorge JM, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36: 77–97. Link: https://goo.gl/WbxflR
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, et al. (2000) Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 43: 9-16. Link: https://goo.gl/MbbI79
- Margolin DA (2008) New Options for the Treatment of Fecal Incontinence. Ochsner J 8: 18-24. Link: https://goo.gl/9vHL8B
- Farid M, Farag A (2000) The use of unilateral gluteus maximus muscle for the management of fecal incontinence following anorectal surgery. Tech Coloproctol 4: 7-12. Link: https://goo.gl/CfuC1O
- Farid M, Moneim H, Mahdy T, Omar W (2003) Augmented unilateral gluteoplasty with fascia lata graft in fecal incontinence. Tech Coloproctol 7: 23-28. Link: https://goo.gl/Ypvjz4
- Farid M, Ghazy H, Monem H, Omar W, El-Nakeeb A, et al. (2008). Proximally based versus distally based gluteus muscle flap in treatment of end stage fecal incontinence. Egyptian Journal of Surgery 27: 200-207. Link: https://goo.gl/uUk5jT
- Bruining H, Bos K, Colthoff E, Tolhurst D (1981) Creation of an anal sphincter mechanism by bilateral proximally based gluteal muscle transposition. Plast Reconstr Surg 67: 70-73. Link: https://goo.gl/rMRDxS
- Orgel M, Kucan J (1985) A double-split gluteus maximus muscle flap for reconstruction of the rectal sphincter. Plast Reconstr Surg 75: 62-67. Link: https://goo.gl/pKhXgK
- Emile SH, Youssef M, Elfeki H, Thabet W, Elgendy H, et al. (2016) Effect of age, patient's sex, and type of trauma on the correlation between size of sphincter defect and anal pressures in posttraumatic fecal incontinence. Surgery 160: 1318-1325. Link: https://goo.gl/v2t5dy
- Khafagy W, El-Said M, Thabet M, Aref S, Omar W, et al. (2017) Evaluation of anatomical and functional results of overlapping anal sphincter repair with or without the injection of bone marrow aspirate concentrate: a case–control study. Colorectal Disease 19: 66-74. Link: https://goo.gl/HBa2MQ
- Meehan J, Hardin W, Georgeson K (1997) Gluteus maximus augmentation for the treatment of fecal incontinence. J Pediatr Surg 32: 1045-1047. Link: https://goo.gl/rYR59m
- Isik A, Idiz O, Firat D. (2016) Novel approaches in pilonidal sinus treatment. Prague Medical Report 117: 145–152. Link: https://goo.gl/t3k7xS
- Pickrell KL, Broadbent TR, Masters FW, James T. Metzger (1952) Construction of a rectal sphincter and restoration of anal continence by transplanting the gracilis muscle: a report of four cases in children. Ann Surg 135: 853–862. Link: https://goo.gl/gVGDi3
- Matzel KE, Madoff RD, LaFontaine LJ, Baeten CG, Buie WD, et al. (2001) Complications of dynamic graciloplasty: incidence, management, and impact on outcome. Dis Colon Rectum 44: 1427–1435. Link: https://goo.gl/Nu8cu6
- Wong WD, Congliosi SM, Spencer MP, Corman ML, Tan P, et al. (2002) The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum 45: 1139-1153. Link: https://goo.gl/El3CG9
- Rodrigues FG, Chadi SA, Cracco AJ, Sands DR, Zutshi M, et al. (2016) Faecal incontinence in patients with a sphincter defect: comparison of sphincteroplasty and sacral nervestimulation. Colorectal Dis. Link: https://goo.gl/wlu2F4
- Patton V, Abraham E, Lubowski DZ. (2016) Sacral nerve stimulation for faecal incontinence: medium-term follow-up from a single institution. ANZ J Surg. Link: https://goo.gl/Xoejnk
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
