Journal of Surgery and Surgical Research
Surgical Site Infection: The Rate and Antimicrobial Sensitivity Pattern in Electively Operated Surgical and Gynecological Patients at Kilimanjaro Christian Medical Centre, Northern Tanzania
Ayesiga M. Herman1*, Gilead Massenga2, Kondo S. Chilonga1, Rune N Philemon3, Denis Katundu1, Anzbet Lugakingira1 and Joseph Obure2
2Department of Obstetrics and Gynaecology, Kilimanjaro Christian Medical Centre, P.O Box 3010 Moshi, Tanzania
3Institute of Public Health; Kilimanjaro Christian Medical Center, P. O Box 2240 Moshi
Cite this as
Herman AM, Massenga G, Chilonga KS, Philemon RN, Katundu D (2017) Surgical Site Infection: The Rate and Antimicrobial Sensitivity Pattern in Electively Operated Surgical and Gynecological Patients at Kilimanjaro Christian Medical Centre, Northern Tanzania. J Surg Surgical Res 3(1): 001-005. DOI: 10.17352/2455-2968.000034Background: Surgical site infections are dreaded by many as they impose a greater economic costs, morbidity and mortality that in developing countries place a burden on an already burdened healthcare system. In Tanzania previously studies done in different centers reported high rates of Surgical Site Infection. This study aimed to quantify in a low income, tertiary hospital, the rate of Surgical Site infections, microorganisms implicated and their respective sensitivity pattern to local antibiotics, and associated perioperative risk factors in electively operated surgical and gynecological patients at Kilimanjaro Christian Medical Centre.
Method: 301 patients admitted for elective procedures in the surgical and gynecological units were enrolled consecutively after consenting for the study. A standardized data collection form was used to record patients’ information on perioperative risk factors. Patients were followed up in surgical outpatient clinic up to one month post discharge. Swabs from wounds showing signs of infection were taken for culture and sensitivity and processed at the laboratory as per standard operating procedures.
Results: Out of a total of 301 patients, 181 patients were from general surgical ward and 115 from gynecological wards. Females were more than males (3:1) with most, 43.9% within the age group 40 to 60 years. Overall, 21.3% of the patients developed surgical site infection. 71 organisms were isolated, S. Aureus species were the leading cause of surgical site infections making 52% of the total isolates. Most of the gram positive organisms were resistant to ampicillin, the common antibiotic used in the gynecology unit. Contaminated wounds were 10 times more likely to develop surgical site infections, and clean contaminated wounds five times when compared to clean wounds. Duration of procedure, Surgeons skills, wound class and number of people in theater showed significant association with surgical site infection, however only wound class and duration of procedure remained statistically significant after a multivariate analysis.
Conclusion: We found an SSI rate of 21.3%, slightly higher than previous studies in the same center. The wound class and duration of procedure remained significant risk factors after logistic regression analysis. As with other studies, S. Aureus was the most common causative organism for SSI isolated from our study.
Introduction
Surgical site infection (SSI) is among the most common Healthcare Associated infections (HAIs) accounting for 13% of all HAIs in hospitalized patients in the USA [1]. SSI has been found to be a major cause of morbidity and mortality resulting in a 3% mortality rate that has been reported in the USA [2]. Furthermore, SSI is increasingly used as a measure of the quality of patient care by surgeons, infection control practitioners and health planners [3].
The magnitude of surgical site infection varies from developed to developing countries as the quality of health services differs in these various settings. In developed countries such as the USA, the incidence of surgical site infections was estimated to be 1.9% where as in developing countries such as India, the incidence is estimated at 8.75 % [4,5]. In Africa surgical site infections have been estimated to have an incidence as high 27.9% [6,7].
In Tanzania past studies have reported different rates, ranging from 35% in Muhimbili National Hospital to 19.1% in KCMC [8-11]. This variability noted, compounded with limited publications, prompts for further research into the magnitude of the problem in various settings as well as noting trends in SSI over time.
Various perioperative risk factors have been associated with acquiring SSIs. They include patient related factors and surgery related factors [12]. Antibiotic therapy plays a crucial role in prevention of SSI. Therefore the choice of antibiotics in both prevention and treatment of SSI should consider the common pathogens responsible for SSIs [12].
Surgical site infections are diverse in terms of the trend of microbial agents involved. Different microbes have been listed with Staphylococcus aureus being the most common causative agent of SSI [13]. Other microbial agents which have been regularly isolated include gram negative bacilli, coagulase-negative Staphylococci, Enterococci species and Escherichia coli [14,15].
Identification of specific modifiable risk factors for SSIs can aid the development of prevention strategies, targeting risk practices especially in countries with limited resources and thus reduce morbidity, mortality and health care coast associated with SSI [16,17]. This study was done to determine the SSI rate, antibacterial sensitivity pattern and associated perioperative factors in electively operated patients in surgical and gynecological units at KCMC.
Methodology
A hospital based prospective cohort study was done between July 2013 to March 2014. The study center was KCMC, a referral hospital with 630 official beds that caters for over 15 million people in Northern Tanzania [18]. Gynecological and general surgery patients who were operated under elective basis within the study period were included. Patients who consented for the study were consecutively enrolled and followed up for 30 days post-surgery. Demographic characteristics, category of surgery and wound class, occurrence of surgical site infection, culture status, culture results on antibiotic sensitivity were recorded using standardized data collection form. Wound specimens (Swab) were collected aseptically from wound that showed signs of SSI according to CDC criteria [19]. Wound specimens were processed as per standard operative procedures, culture and antibiotic sensitivity testing was done to positive cultures against specific antibiotics commonly available at KCMC, for both gram positive and gram negative organisms.
Data analysis: SPSS version 20 was used Frequencies and percentages were used for descriptive statistics. The association between the exposure of interest (perioperative factors) and outcome (Surgical site infection) was obtained using a chi square test, and a P value of < 0.05 was considered statistically significant. The likelihood ratio was obtained by use of odds ratio. A logistic regression model was done to identify the risk factors for SSI.
Results
A total of 301 patients were enrolled in a period of 8 months. 5 patients were lost follow up, leaving 296 patients who were followed up for the outcome of interest. 181 patients were from general surgical ward and 115 from gynecological wards. Females were more than males (3:1) with the majority of patients (43.9%) within the age group of 40 to 60 years [Table 1].
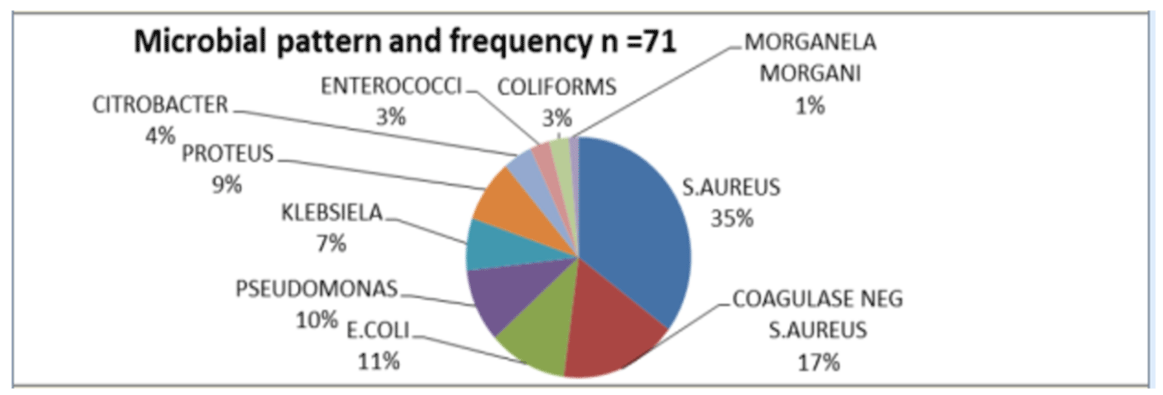
A total of 63 patients developed SSI, with an overall rate of 21.3%. 55% of all SSI occurred in gynecological patients with a rate of 30.4% (35/115) and OR 2.3 (1.36- 4.21) as compared to a rate of 15.5% in general surgical patients [Table 2]. A total of 71 organisms were isolated with some wounds showing polymicrobial infection (Figure 1). S.aureus species were the leading cause of SSI accounting for 52% of SSIs followed by E.coli 11%, Pseudomonas species 10%, Proteus species 9% and Klebsiela species 7%. Most of the gram positive organisms were resistant to ampicillin and cortrimoxazole. Ceftriaxone, ciprofloxacin and gentamycin were effective but with some organisms showing resistance [Table 3].
Clean contaminated wounds contributed more than half of the SSI. However when compared to clean wound, contaminated wounds were 10 times more likely to develop SSI followed by clean contaminated wounds (OR 10 and 5.3 respectively). Again, majority of the SSI (88.9%) occurred in patients whose operative time was beyond one hour. Patients whose operative time was more or equal to 3 hours were 2 times more likely to develop SSI as compared to those with in 1 hour [Table 2].
About 30% (n=117) of patients who got prophylactic antibiotics (ampicillin) developed SSI as compared to 15.6% (n=179) who didn’t get prophylactic antibiotics. Although it appears that prophylactic antibiotics are not protective in this study, this association is no longer apparent after accounting for other factors suggesting a confounding effect [Table 3].
SSI was less likely to occur in theater with few people (≤ 8 people), rate 17.8% (n=56) with an OR of 0.767 (0.363 -1.62) as compared to other theaters with more than 8 people (rate 22.1%; (n=240)) during the procedure. This was not statistically significant. Also Patients who were operated by consultant surgeons were less likely [OR =0.5 (0.19-1.34)] to develop infection as compared to senior and junior residents [Table 2], again showing no statistically significant association.
On controlling for confounders, wound class OR 2.278 (1.366 -3.801), P value 0.002, and duration of procedure OR 1.530 (1.001 -2.337), P value 0.049 were found to be significantly associated with SSI [Table 4].
Discussion
SSI continues to be a significant problem for surgeons worldwide. This study that looked into surgical site infection in a referral hospital in Northern Tanzania, a low income country showed an overall SSI rate of 21.3%. A previous study done in the same centre in 2003 reported an SSI rate of 19.4% [8]. Contrary to the previous study, we included gynecological surgeries, which proved to have a significantly higher (30.4%) rate of SSI compared to general surgery (15.5%) in our setting. Hence taking into account of the surgical cases only, according to these studies, the rate of SSI has not appeared to increase in ten years. Furthermore, previous studies done in 2006 and 2010 within the country reported even higher rates of 24.1% in St. Francis Designated Hospital Ifakara and 35% at Muhimbili National Hospital respectively [11,13].
We note the different SSI occurrence rates observed in the general surgical and gynecological departments. There are two main reasons that could explain the observed SSI rate difference in the two departments. The first is that most of the elective procedures in the gynecological cohort of patients in this study were clean contaminated procedures (total abdominal hysterectomy and tubal-ovarian surgeries), a factor which is known to have a higher risk of progressing into a SSI compared to clean procedures [4,12,20-22]. The second reason was the finding that the prophylactic antibiotic used is now found to be less effective due to a higher resistance trend shown by the microbes. Prophylactic antibiotics play a role in reducing SSI, therefore ensuring adequate cover of the appropriate antibiotic according to local profile is paramount [21]. In this study, ampicillin was the major antibiotic given in the gynecological patients which was found to be less effective to the microbes identified. Possibly this is one of the major contributors to the rate of SSI seen in gynecology department (on top of the increased risk due to wound class). The finding that those who were given antibiotics were more likely to develop SSI in this study can be attributed to this factor, given the ineffective antibiotics administered with the microbial resistance. The general surgery department at the time of this research did not have a clear protocol on administering antibiotics hence difficult to quantify the effect of prophylactic antibiotics for this group.
Several factors tallied with what is already known on risk factors for SSIs. We found that number of people in theatre, surgeon’s skills, duration of procedure and wound class all affected the rate of SSI [12,22]. Only the last two factors remained statistically significant in multivariate analysis. The duration of procedure was directly proportional to SSI risk and showed increasing risk of SSI with increased operative time that was statistically significant with an operation time of 2 hours or more as compared to ≤ 1 hour. This is expected and demonstrated elsewhere [22]. In a multivariate analysis, we found that the two independent predictors of SSI in this cohort were the wound class (p=0.002) and the duration of procedure (p=0.049). Fehr et al observed a longer duration of the operation to be significant risk factor for SSI [23] Surgeon’s skills and number of people in operating theater during the procedure did not continue to be important factors in multivariate analysis showing there were other factors contributing to this effect.
Conclusion
SSI rates were more likely to occur in Gynecological patients than General surgical patients. This may be attributed to the difference in wound class in gynecological procedures clean contaminated versus relatively more clean surgeries for general surgery procedures. The observed high rates of SSI demands a change in the practice and preventive measures on the perioperative factors. A review of antibiotic protocols, to using more seemingly effective antibiotics in our center has been advised. The study outlines important challenges and identifies factors in our environment and similar settings that can provide guidance and thus must be considered in the perioperative period.
This project was made possible by the MTRP in collaboration with the HRSA-funded KCMC MEPI grant # T84HA21123-02; U.S. National Institutes of Health.
- Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, et al. (2012) Prevalence of Healthcare-Associated Infections in Acute Care Hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 33: 283–291. Link: https://goo.gl/Gv8cJe
- Awad SS (2012) Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt) 13: 234-237. Link: https://goo.gl/T6MJXL
- Awan MS, Dhari FJ, Laghari AA, Bilal F, Khaskheli NM (2011) Surgical Site infection in Elective Surgery. Journal of surgery Pakistan 16: 33-37. Link: https://goo.gl/Eq986P
- Lilani SP, Jangale N, ChawdharyA, Daver GB (2005) Surgical site infection in clean and clean-contaminated cases. Indian J Med Microbiol 23: 249-252. Link: https://goo.gl/Kel3XD
- Mu Y, Edward JR, Horan TC, Berrios-Torres SI, Fridkin SK (2011) Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 32: 970-986. Link: https://goo.gl/FpfbFV
- Oni A, Ewete AF, Gbaja AT, Kolade AF, Mutiu WB, et al. (2006) Nosocomial infections: surgical site infection in UCH Ibadan, Nigeria. Nigerian Journal of surgical Research 8: 19-23. Link: https://goo.gl/yqtl31
- Kassavin DS, Pascarella L, Goldfarb MA (2011) Surgical site infections: incidence and trends at a community teaching hospital. Am J Surg 201: 749–753. Link: https://goo.gl/MEHPMh
- Eriksen HM, Chugulu S, Kondo S, Lingaas E (2003) Surgical site infections at Kilimanjaro Christian Medical Center. J Hosp 55: 14-20. Link: https://goo.gl/KEpH7D
- Fer J , Christopher H, Isaac S, Kibatala P, Urassa H et al. (2006) Risk Factors for Surgical Site Infection in a Tanzanian District Hospital: A Challenge for the Traditional National Nosocomial Infections Surveillance System Index. Infection control and Hospital epidemiology 27: 1401-1404. Link: https://goo.gl/YGPqog
- Mawalla B, Mshana SE, Chalya PL, Imirzalioglu C, Mahalu W (2011) Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surgery 11: 21. Link: https://goo.gl/amKndu
- Akoko LO, Mwanga AH, Fredrick F, Mbembati NM (2012) Risk Factors of Surgical Site Infection at Muhimbili National Hospital, Dar es Salaam, Tanzania. East and Central African Journal of Surgery 17: 12-17. Link: https://goo.gl/XoGhPy
- Mangram AJ, Horan TC, Pearson ML, Silver LC, William R (1999) Guideline for Prevention of Surgical Site Infection,1999. American Journal of Infection Control 27: 97-134. Link: https://goo.gl/9GfXsV
- Fehr J, Hatz C, Soka I, Kibatala P, Urassa H, et al. (2006) Antimicrobial prophylaxis to prevent surgical site infections in a rural sub-Saharan hospital. Clinical Microbiology and Infection 12:1224-1227. Link: https://goo.gl/VZZBfL
- Owens CD, Stoessel K (2008) Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 70: 3-10. Link: https://goo.gl/RtKq4t
- Deverick AJ, Kaye KS (2009) Staphylococcal Surgical Site Infections. Infect Dis Clin North Am 25: 53-72. Link: https://goo.gl/s1w9Yg
- Hafez S, Saied T, Hasan E, Elnawasany M, Ahmad E (2012) Incidence and modifiable risk factors of surveillance of surgical site infections in Egypt. Am J Infect Control 40: 426-430. Link: https://goo.gl/WxhGej
- Korol E, Johnston K, Waser N, Sifakis F, Jafri HS, et al. (2013) A Systematic Review of Risk Factors Associated with Surgical Site Infections among Surgical Patients. PLoS ONE 8: e83743. Link: https://goo.gl/ZFpYjq
- Kilimanjaro Christian Medical Centre (TZ). Welcome to Kilimanjaro Christian Medical Centre [Internet]. KCMC; [cited 2016 December 29]. Link: https://goo.gl/wUPwVJ
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20: 271-274. Link: https://goo.gl/Pc16ZF
- Ortega G, Rhee DS, Papandria DJ, Yang J, Ibrahim AM, et al. (2012) An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res 174: 33-38. Link: https://goo.gl/Xv96pP
- Jones DJ, Bunn F, Bell-Syer SV (2014) Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev 3: CD005360. Link: https://goo.gl/NXJoJm
- Nguyen D, MacLeod WB, Phung DC, Cong QT, Nguyen VH, et al. (2001) Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol 22: 485-492. Link: https://goo.gl/1rfz2G
- Fehr J, Christoph H, Isaac S, Kibatala P, Urassa H, et al. (2006) Risk Factors for Surgical Site Infection in a Tanzanian District Hospital: A Challenge for the Traditional National Nosocomial Infections Surveillance System Index. Infect Control Hosp Epidemiol 27: 1401-1404. Link: https://goo.gl/DI4DWZ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
