Journal of Clinical Research and Ophthalmology
Evaluation of clinical efficacy and safety of Bibrocathol 2% eye ointment in the treatment of Chronic Blepharoconjunctivitis
Elena Yani1, Sergey Astakhov2, Vladimir Brzheskiy3, Evgeniy Egorov4, Alexey Seleznev5, Dorothea Groß6* and Heidi Opitz6
2Federal State Budgetary Educational Institution of Higher Education "Pavlov First Saint Petersburg State Medical University" of the Ministry of Healthcare of the Russian Federation, Saint-Petersburg, Russia
3Federal State Budgetary Educational Institution of Higher Education “Saint Petersburg State Pediatric Medical University” of the Ministry of Healthcare of the Russian Federation, Saint-Petersburg, Russia
4Federal State Budgetary Educational Institution of Higher Education “Russian National Research Medical University n.a N.I. Pirogov” of the Ministry of Health of the Russian Federation, Moscow, Russia
5Regional Budgetary Healthcare Institution “Ivanovo Regional Clinical Hospital”, Ivanovo, Russia
6URSAPHARM Arzneimittel GmbH, Saarbrücken, Germany
Cite this as
Yani E, Astakhov S, Brzheskiy V, Egorov E, Seleznev A, et al. (2022) Evaluation of clinical efficacy and safety of Bibrocathol 2% eye ointment in the treatment of Chronic Blepharoconjunctivitis. J Clin Res Ophthalmol 9(1): 008-014. DOI: 10.17352/2455-1414.000096Copyright License
© 2022 Yani E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Purpose: To assess the efficacy and safety of bibrocathol 2% eye ointment in patients with chronic blepharoconjunctivitis.
Materials and methods: This was a multi-center, randomized, double-masked, placebo-controlled, parallel-group, phase III study. Patients with chronic blepharoconjunctivitis were randomized to apply either bibrocathol 2% (n = 100) or placebo (n = 100) three times a day for 14 days. The primary efficacy endpoint was the change from baseline to Day 15(+1 day) in the total score of signs summarizing the investigators’ assessment of the severity of lid oedema, lid erythema, debris, hyperemia, and pouting of Meibomian glands based on slit-lamp examination (modified Intention-to-treat (mITT) set). Safety endpoints included visual acuity, intraocular pressure, and adverse events. Investigators and patients performed an overall assessment of treatment tolerability.
Results: On Day 15(+1 day) the least square (LS) mean change from baseline in the total sum score of signs was -8.62 (95% CI: -9.16; -8.08) in the bibrocathol group and -6.00 (95%CI: -6.54; -5.45) in the placebo group. The LS means the difference between treatment groups was statistically significant in favor of bibrocathol (-2.63 [95% CI: -3.36, -1.89], p < 0.001). Bibrocathol was statistically significantly superior to placebo in reduction of the individual ocular signs scores and the patient’s-assessed ocular discomfort severity (p < 0.001). No safety issues were observed concerning visual acuity, intraocular pressure, and the occurrence of adverse events.
Conclusions: The study showed superior efficacy of two weeks of treatment with bibrocathol versus placebo in reducing signs and symptoms of chronic blepharoconjunctivitis. Treatment with bibrocathol 2% eye ointment was safe and well-tolerated.
Introduction
Blepharoconjunctivitis is a common eye condition, characterized by inflammation of both the anterior and posterior eyelid margin (blepharitis) and conjunctiva (conjunctivitis). Predominant symptoms are burning, itching, irritated eyes, watery eyes, photophobia, blurred vision, and red eyes [1,2]. A pathophysiological component of a chronic blepharoconjunctivitis is blepharitis. Anterior blepharitis (affecting the outer part of the eyelid margin) is frequently a result of a bacterial infection and/or sebaceous gland activity. Posterior blepharitis (affecting the inner part of the eyelid margin) is frequently associated with a dysfunction of the Meibomian glands [3,4]. Though the pathophysiology of anterior and posterior blepharitis may be different, the treatment options are similar.
The classification and clinical picture of blepharitis are complex [2] and a definition of chronic blepharitis is difficult to find. According to McCulley, et al. patients suffering symptoms for at least six months and who did not undergo any treatment for at least two weeks may have a chronic blepharits [2]. The treatment of chronic blepharoconjunctivitis is similar to that of blepharitis including eyelid hygiene (warm compresses and eyelid massage), topical antibiotics, topical corticosteroids [5], as well as antiseptics and non-steroidal anti-inflammatory drugs [6]. Due to the crucial role which infection plays in the etiology of chronic blepharoconjunctivitis, the use of antiseptic agents seems to be a reasonable treatment option [7,8]. The lack of antibiotic resistance and the lower risk of side effects could be an additional advantage of antiseptics in comparison to antibiotics in the treatment of eyelid infections.
Bibrocathol (4,5,6,7-Tetrabromo-2-hydroxy-1,3,2-benzo-dioxabismole) is a substance with well-established use but only a few controlled clinical data are available. Bibrocathol with its known topical antiseptic activity is commonly used in treating the signs and symptoms of chronic blepharoconjunctivitis. It is a bismuth-containing substance with antiseptic, astringent, and secretion-inhibiting properties (on mucous membranes). The mechanism of action is due to its molecular structure containing a phenolic derivate with tetrabromopyrocatechol and bismuth hydroxide. Bibrocathol causes the precipitation of proteins and the shrinking of surficial layers of tissue thus forming a protective membrane against pathogenic invasion. In addition, this astringent effect on small vessels reduces local inflammation and secretion [9]. As a consequence, no resistance to this antiseptic agent can develop. As bibrocathol is almost not soluble in water the probability of the occurrence of systemic side effects upon topical application is considered to be below [9]. Since the eye ointment base comprising white vaseline, liquid paraffin, and wool fat is water-free no preservatives are required, therefore improving its local tolerability.
Eye ointments containing 2% or 5% bibrocathol have been marketed since 1967 for the treatment of eye irritation, chronic blepharitis, and non-infected corneal injuries. Reports of clinical experience with bibrocathol for the treatment of blepharitis exist since the beginning of the 20th century [10]. Vaughan, et al. [11]. Confirmed in 1983 the bacteriostatic and bacteriocidal action of bibrocathol due to its protein denaturation effect. The authors concluded that an additional anti-inflammatory and secretion inhibitory effect can be beneficial in the treatment of non-specific, non-severe irritations of the conjunctiva and eyelid margin.
A first Good Clinical Practice (GCP) compliant clinical study of bibrocathol was published in [12]. This was a randomized, double-masked, placebo-controlled study aimed to assess the efficacy and safety of a 2-week treatment with bibrocathol 5% eye ointment (Noviform®) in patients with acute blepharitis. The study showed the superiority of bibrocathol over placebo in improving the total symptom score, especially in patients with primary severe symptoms. Bibrocathol treatment was well tolerated and safe. In another randomized, double-masked, placebo-controlled study published in 2012 [13] the efficacy and safety of bibrocathol 2% eye ointment (Posiformin® 2%) was investigated in patients with signs and symptoms of moderate acute blepharitis. Bibrocathol demonstrated high efficacy and a favorable safety profile.
The purpose of this study was to assess the efficacy and safety of bibrocathol 2% eye ointment (Posiformin® 2%, trade name in Russia: Bibrocathol-POS® 2%) in patients with chronic blepharoconjunctivitis. The bacteriostatic and bactericidal effects of bibrocathol as described by Vaughan, et al. [11] were not the focus of this study and are not further addressed in this publication.
Materials and Methods
Study design
This multi-center, randomized, double-masked, placebo-controlled, parallel-group study was conducted at 5 investigational sites in the Russian Federation between 15 January 2018 and 15 February 2019 (https://www.isrctn.com/ no. ISRCTN 14084351). The study was conducted under the Declaration of Helsinki, International Council for Harmonization Good Clinical Practice guidelines, and applicable Russian regulatory guidelines. It was also approved by the Ethics Council at the Ministry of Healthcare of the Russian Federation and by Independent Ethics Committees at each investigational site. All patients provided written informed consent.
Male or female patients aged ≥18 years with chronic blepharoconjunctivitis and a total sum score of signs ≥13 at baseline were eligible for inclusion in the study. The blepharoconjunctivitis total sum score was calculated from the 5 objective signs assessed by the investigator using the slit lamp examination (severity of lid oedema, lid erythema, debris, hyperemia, and pouting of Meibomian glands) as previously published [13].
Patients were not included in the study if they had any of the following: antibiotic-requiring or therapy-resistant chronic blepharoconjunctivitis; acute ocular and/or follicle- or lid- infection or active ocular inflammation other than blepharoconjunctivitis; irritations of the outer eye that are related to corneal damage (e.g. erosions, injuries, burns); abnormal eye-lid anatomy; ocular surgery within 90 days before enrollment; severe dry eye syndrome; acute allergic eye diseases; glaucoma; intraocular pressure ≥21mmHg; patients with only one eye; known hypersensitivity to the ingredients of investigational product; severe systemic disease; history of malignancy of any organ system within 5 years before enrollment; use of oral or topical ocular antibiotics or corticosteroids or non-steroidal anti-inflammatory drugs within 2 weeks prior to the trial (low doses of oral acetylsalicylic acid and occasional use of painkillers were allowed); use of topical ocular antihistamines or α-sympathomimetics within 1 month before enrollment.
Eligible patients were randomized in a 1:1 ratio to double-masked treatment with either bibrocathol 2% or vehicle (placebo) for 14 days. Randomized patients were to self-administer the eye ointment (about 5 mm) into the conjunctival sac and the eyelid of the affected eye(s) three times daily (morning, midday, and evening). Eyelid hygiene (warm compress followed by circular self-massage of the eyelids) should be performed in the morning and evening before applying the eye ointment. No eye ointment was to be applied less than 3 hours before the scheduled examinations at study visits. The use of eye makeup as well as wearing contact lenses was prohibited. In cases of bilateral blepharoconjunctivitis, both eyes were to be treated. The eye with the highest total sum score at baseline was chosen as the study eye. If the total sum scores were equal, the right eye was chosen as the study eye. All patients were asked to record each application of the investigational product over the 2-week treatment period in the patient diary provided.
A randomization list was generated by an independent biostatistician before the start of the study. All patients and investigators were blinded to treatment identity. Investigational products were packaged in anonymized cardboard boxes (processing units) in which eye ointment tubes were identical in size, weight, and appearance. Since verum and placebo differed in color (bibrocathol is yellow, placebo is white), the investigational products were distributed to the patients by qualified site personnel not involved in the measurement of any trial efficacy or safety parameters. This personnel was instructed not to reveal the identity of the investigational product to the investigator or study monitors. The patients were not informed about the appearance or color of the eye ointment and were instructed to open their masked investigational processing units at home. The investigators and the patients were not allowed to talk about the appearance of the investigational product during the study.
Clinical study visits were conducted on days -2 to 1 (Visit 1, screening), Day 1 (Visit 2, randomization/baseline), Day 7(±1 day) (Visit 3), and Day 15(+1 day) (Visit 4). Screening and randomization visits could be performed on the same day.
Efficacy assessments
Efficacy assessments included ocular signs and the patient’s assessment of ocular discomfort. The investigator-assessed five ocular signs during the slit-lamp examination at Visit 2 (baseline) and each study visit thereafter. Ocular signs included lid oedema, lid erythema, debris, hyperemia, and pouting of Meibomian glands. The severity of each sign was measured on a 5-point scale from 0 (“none”) to 4 (“severe” for hyperemia and “very severe” for other ocular signs). Depending on the clinical signs, each category was assessed using an individual definition. Hence, the composite (total sum) score of signs could range from 0 to 20. At Visits 2, 3, and 4 patients were asked to rate the degree of ocular discomfort on a 0-100 mm visual analogue scale (VAS).
The primary efficacy endpoint was the change from baseline to Day 15(+1 day) (Visit 4) in the total sum score of signs. Secondary efficacy endpoints included the change from baseline to Day 15(+1 day) (Visit 4) in individual signs and the patient-assessed ocular discomfort.
Safety assessments
Safety endpoints included visual acuity (VA), intraocular pressure (IOP), and adverse events (AEs). VA was evaluated according to the standard method used in the investigational site (Visits 1 and 4). IOP was measured using the non-contact tonometer (Visits 2 and 4). As for safety evaluations, physical examination and assessment of vital signs were also conducted (Visits 1 and 4). All AEs observed by the investigator, elicited during study visits, or spontaneously reported by the patient were collected from the first application of the investigational product until the last study visit. At Visits 3 and 4, the patients were asked to rate their overall assessment of local tolerability by using the 4-point scale (3 = very good, 2 = good, 1 = moderate, 0 = poor). A similar evaluation was done by the investigator at Visit 4.
Sample size
Estimation of sample size was based on data from a previously conducted clinical study [12] in which the effect size of Cohen’s d = 0.43 for the difference between bibrocathol and placebo for slightly different primary efficacy variables was reported. Assuming that the revised primary efficacy measure was as sensitive as the outcome measure of the referenced trial, 86 patients/group were required to detect such an effect size with a power of 80% at the two-sided significance level of 5%. To account for non-evaluable data due to early drop-outs or other protocol violations of about 15%, a total of 2×100= 200 patients were to be recruited in this study.
Statistical analysis
Efficacy was evaluated in the modified Intention-to-treat (mITT) set and the per protocol (PP) set. The mITT set consisted of all patients randomized that received at least one dose of the investigational product and had at least one post-baseline assessment of the primary efficacy variable. The PP set comprised all the mITT-patients who completed the study according to the protocol and did not show major deviations from the protocol procedures that might have an impact on the study outcome. The Safety set consisted of all randomized patients who applied the investigational product at least once and was used to evaluate safety and tolerability data.
Comparison of bibrocathol to placebo to the primary endpoint was carried out using an analysis of covariance (ANCOVA) model with treatment and center as fixed factors and baseline sum score as a covariate. The treatment contrast was presented by the least square (LS) mean for the difference between the two treatments with its 95% confidence interval and the p - value for the hypothesis that the contrast is 0. The primary analysis was computed for the mITT (last observation carried forward, LOCF) set and the PP set. Changes in five individual sign scores (lid oedema, lid erythema, debris, hyperemia, and pouting of Meibomian glands) and ocular discomfort severity were analyzed separately using the same statistical methodology as for the primary endpoint in the mITT (LOCF) set. Additionally, for the continuous efficacy primary and secondary endpoints, the treatment-group differences in the mean change from baseline at each post-baseline assessment were conducted using a Mixed Model for Repeated Measures (MMRM) analysis with restricted maximum likelihood estimation and an unstructured covariance matrix in the mITT without missing data imputation.
Results
Study population
A total of 200 patients were screened and randomized into two treatment groups, each of 100 patients. 14 patients (7.0%) prematurely discontinued the study. AEs were the primary reason for discontinuation (bibrocathol, n = 3; placebo, n = 6), other reasons included the withdrawal of consent (n = 2 in each group) and loss to follow up (placebo, n = 1). Four (4) of these patients did not start the study: one patient in the bibrocathol group who withdrew the consent and three patients in the placebo group (withdrawal of consent [n = 2] and loss to follow up [n = 1]). Both treatment groups were well balanced to demographic and other baseline characteristics (Table 1). A total of 189 patients were included in the mITT set and were analyzed for efficacy (bibrocathol, n = 95; placebo, n = 94). Eleven (11) patients were excluded from the mITT set because they did not start treatment (n = 4) or were missing all post-baseline assessments of the primary endpoint (n = 7). The PP set included 165 patients (bibrocathol, n = 88; placebo, n = 77).
Efficacy
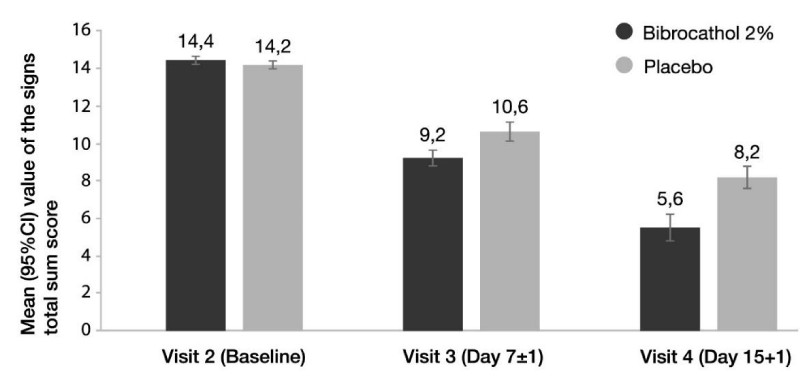
Figure 1 shows the mean values of the total sum score of signs from baseline until the last study visit (descriptive statistics). During treatment, the score improved in each group, but the improvement was more considerable in the bibrocathol group compared to the placebo group. The mean changes from baseline in total sum score and individual signs scores at Day 15 (+1 day) (Visit 4) based on ANCOVA are shown in Table 2. At Visit 4, the LS mean change from baseline in the signs total sum score was -8.62 (95% CI: -9.16; -8.08) in the bibrocathol group and -6.00 (95% CI: -6.54; -5.45) in the placebo group. The LS means the difference between treatment groups was statistically significant in favor of bibrocathol (-2.63 [95% CI: -3.36, -1.89], p < 0.001).
Similar results were obtained for the PP set in which the LS mean change from baseline was -8.80 (95% CI: -9.33; -8.27) in the bibrocathol group and -6.32 (95% CI: -6.89; -5.75) in the placebo group, the LS means the difference between treatment groups was statistically significant (-2.48 [95% CI: -3.21; -1.75], p < 0.001).
Table 2 shows a superior efficacy of bibrocathol over placebo for all secondary efficacy endpoints. For both the individual signs scores and ocular discomfort severity, the differences between the LS means were statistically significant with p < 0.001.
The MMRM analysis showed the differences between groups in the mean changes from baseline for the total sum score, individual signs scores, and ocular discomfort severity was statistically significant (p < 0.01) in favor of bibrocathol at each post-baseline assessment (Visits 3 and 4) (no data shown).
Safety
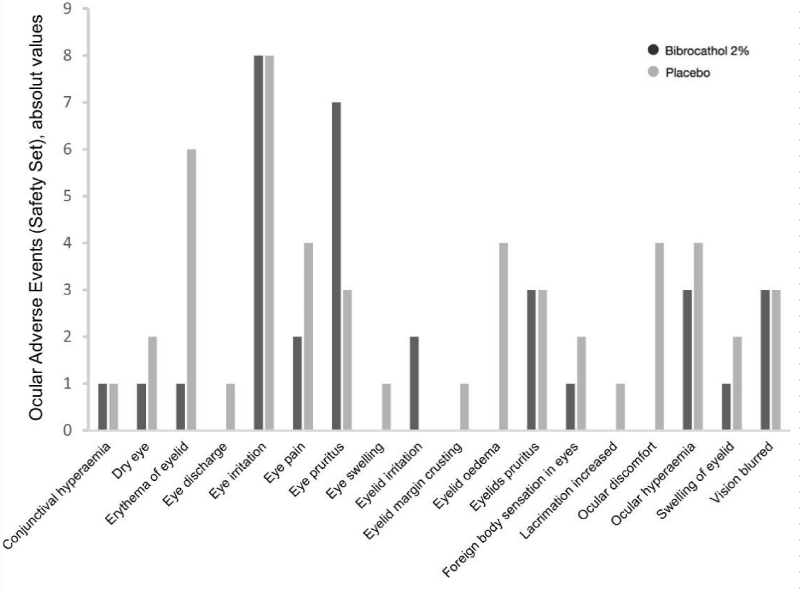
18 (18.2%) patients of the bibrocathol group and 21 (21.6%) patients of the placebo group reported at least one ocular AEs during the study (Table 3 and Figure 2). The majority of patients reported mild ocular AEs (bibrocathol, 17.2%; placebo, 20.6%). Moderate ocular AEs were experienced by one patient of each treatment group; erythema of eyelid in the bibrocathol group and dry eye, eye irritation, and eyelid oedema in the placebo group. All ocular AEs were assessed by the investigators as treatment-related events. Three (3) patients in the bibrocathol group and 6 patients in the placebo group experienced ocular AEs that led to study discontinuation. Additionally, the treatment of two patients in the placebo group was temporarily interrupted due to ocular AEs.
The incidence of non-ocular AEs was low (bibrocathol, 2.0%; placebo, 3.1%). All cases of non-ocular AEs in the bibrocathol group (swelling face, respiratory tract infection) were assessed as moderate in severity. Those in the placebo group (abdominal pain upper, viral respiratory tract infection, anxiety) were considered mild AEs. Two non-ocular AEs were assessed by the investigators as treatment-related events: swelling face (bibrocathol: n = 1) and anxiety (placebo, n = 1). No clinically relevant findings were obtained in other safety-relevant examinations (VA, IOP, vital signs, physical examination).
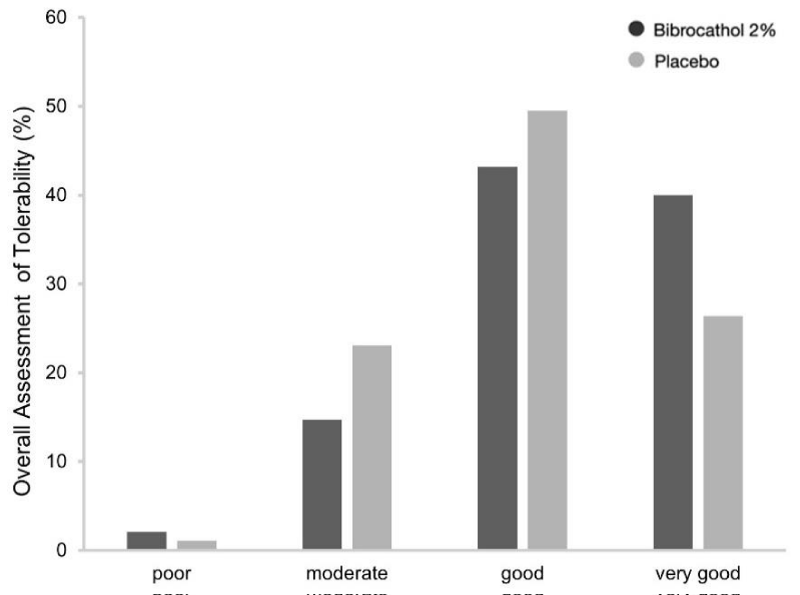
Approximately 83% and 76% of patients in the bibrocathol and placebo groups, respectively, assessed the treatment tolerability as either “good” or “very good” at Visit 4 (Figure 3). Investigator’s assessments were more favorable for bibrocathol: at least “good” tolerability was reported for approximately 92% and 76% of patients in the bibrocathol and placebo groups at Visit 4, respectively.
Discussion
Blepharoconjunctivitis is a type of inflammation of the ocular surface and eyelids that involves changes in the eyelids, dysfunction of the Meibomian glands, and inflammation of the conjunctiva. Chronic inflammation can lead to scarring of the eyelid and loss of proper eyelid function with time and secondary damage to the ocular surface. While the etiology of the disease is complex and not fully understood, bacterial infection and inflammation are believed to contribute to the pathology. Long-term management of symptoms may include daily eyelid hygiene and the use of therapeutic agents that reduce infection and inflammation. Although several therapeutic options have been proposed to manage this multifactorial disease, to date there is no definitive therapy [14].
Bibrocathol 2% eye ointment has been successfully used for decades to achieve an antiseptic, astringent, and secretion-inhibiting effect on the ocular surface and the lid margin. However, a limited number of clinical studies have been conducted so far [12,13].
The purpose of this randomized, double-masked, placebo-controlled study was to assess the efficacy and safety of a 2-week treatment with bibrocathol 2% eye ointment in patients with chronic blepharoconjunctivitis. Patients aged 18 years and older with moderate or severe chronic blepharoconjunctivitis but not requiring antibiotic therapy were enrolled in the study. Bibrocathol 2% or vehicle (placebo) was applied three times daily for two weeks; the participants were asked to perform eyelid hygiene before applying the ointment in the morning and evening. The study results showed a statistically significant superiority of bibrocathol over placebo for the primary efficacy endpoint and all secondary efficacy endpoints. The higher efficacy of bibrocathol was demonstrated both for the investigator’s-assessed ocular signs (the severity of lid oedema, lid erythema, debris, hyperemia, and pouting of Meibomian glands based on slit-lamp examination) and for subjective ocular discomfort reported by the patients. For all efficacy endpoints, a statistically significant difference in favor of bibrocathol was found both after 7 and 14 days of treatment.
Treatment with bibrocathol 2% eye ointment was well-tolerated and not associated with any major safety issues. Most of the AEs recorded during the study were mild and also common signs and symptoms of blepharoconjunctivitis. The incidence of AEs in the placebo group was higher than in the bibrocathol group (21.6% vs. 18.2%). Moreover, more patients in the placebo group prematurely withdrew from the study because of AEs (n = 6 vs. n = 3). Additionally, for two patients in the placebo group, the study treatment was temporarily interrupted due to ocular AEs. The safety results in the placebo group can be explained by low efficacy, which led to the deterioration of blepharoconjunctivitis signs and symptoms during the study. Similar results were obtained in other placebo-controlled studies of bibrocathol eye ointment [12,13]. Bezdetko, et al. [13]. Suggested the application of eye ointment could cause application site discomfort, which may be perceived more intensely by patients with no or slow symptom improvement than by patients with fast symptom improvement.
Overall, our findings support those of previous studies demonstrating that bibrocathol eye ointment is effective and safe in the treatment of blepharitis [12,13]. Previous studies were conducted on patients with acute blepharitis. To our knowledge, our study is the first that assesses the efficacy and safety of bibrocathol eye ointment in the treatment of chronic blepharoconjunctivitis according to the classification of McCulley, et al. [2].
A potential limitation of this study was the relatively short follow-up time of two weeks, which precluded the long-term outcome evaluation [14]. However, the chosen period of two weeks corresponds to the usual duration of treatment for chronic blepharoconjunctivitis. Lack of evaluation of the antibacterial effect of bibrocathol may also be considered a potential weakness in the study setup, however, this was not in the scope of the project.
As initial treatment of chronic blepharoconjunctivitis antibiotics are frequently used. However, this – among others – causes the development of resistance which continuously increase in its frequency. Therefore, it is of high clinical relevance that the antiseptic bibrocathol confirmed its suitability as an efficacious alternative treatment.
In conclusion, bibrocathol 2% eye ointment applied three times daily for two weeks is an effective and safe option for the treatment of patients with chronic blepharoconjunctivitis. Further studies on the clinical efficacy of bibrocathol eye ointment in patients with inflammatory eye diseases are warranted.
Acknowledgment
Medical writing assistance was provided by Eugenia Radkova, PhD, OCT Rus, Russia, and was funded by URSAPHARM Arzneimittel GmbH, Germany.
Author disclosure statement
Elena Yani, Sergey Astakhov, Vladimir Brzheskiy, Evgeniy Egorov, Alexey Seleznev have received research support and honoraria fees from URSAPHARM Arzneimittel GmbH. Dorothea Groß and Heidi Opitz are employees of URSAPHARM Arzneimittel GmbH.
Funding information
The study was sponsored by URSAPHARM Arzneimittel GmbH, Germany.
- Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009 Apr;7(2 Suppl):S1-S14. doi: 10.1016/s1542-0124(12)70620-1. PMID: 19383269.
- McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982 Oct;89(10):1173-80. doi: 10.1016/s0161-6420(82)34669-2. PMID: 6218459.
- McCulley JP. Blepharoconjunctivitis. Int Ophthalmol Clin. 1984 Summer;24(2):65-77. doi: 10.1097/00004397-198424020-00009. PMID: 6233233.
- Kaercher T, Brewitt H. Blepharitis. Der Ophthalmologe. 2004;101(11):1135-1148.
- Zhao L, Sun YJ, Pan ZQ. Topical Steroids and Antibiotics for Adult Blepharokeratoconjunctivitis (BKC): A Meta-Analysis of Randomized Clinical Trials. J Ophthalmol. 2021 Jan 8;2021:3467620. doi: 10.1155/2021/3467620. PMID: 33520297; PMCID: PMC7817233.
- Librando A, Carlesimo SC, Albanese G, Albanese GM, Migliorini R, Pacella E. Effectiveness of 0.1% topical salicylic acid on blepharoconjunctivitis affecting glaucoma patients treated with topical prostaglandin analogues: a prospective randomized trial. Int J Ophthalmol. 2018 Dec 18;11(12):1936-1940. doi: 10.18240/ijo.2018.12.10. PMID: 30588426; PMCID: PMC6288534.
- McCulley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea. 2000 Sep;19(5):650-8. doi: 10.1097/00003226-200009000-00010. PMID: 11009317.
- Behrens-Baumann W. Antibiotics and antiseptics from the perspective of the ophthalmologist. Article in German. Augenärztl Fortbildung. 1991;14:27–31.
- Federal Health Office. Monography Bibrocathol, circularized on 29.01.1992.
- von Liebermann L. Zur Therapie der Lidrandentzündungen. DMW. 1912;11.
- Vaughan D, Asbury T. Ophthalmologie. Berlin Heidelberg New York Tokio: Springer Verlag; 1983.
- Behrens-Baumann W, Niederdellmann C, Jehkul A, Kohnen R. Bibrocathol-Augensalbe ist wirksam bei Blepharitis. Ergebnisse einer randomisierten, doppelblinden, kontrollierten klinischen Studie [Bibrocathol eye ointment is efficacious in blepharitis. Results from a randomized, double-blind, controlled clinical trial]. Ophthalmologe. 2006 Nov;103(11):960-5. German. doi: 10.1007/s00347-006-1426-4. PMID: 17003947.
- Bezdetko PA, Sergienko N, Dyomin Y, Korol A, Nikitin N, Merzbacher M, Groß D, Kohnen R. Successful treatment of blepharitis with bibrocathol (Posiformin® 2 %). Graefes Arch Clin Exp Ophthalmol. 2012 Dec;250(12):1869-75. doi: 10.1007/s00417-012-2001-0. Epub 2012 Apr 25. PMID: 22527308; PMCID: PMC3501175.
- Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012 May 16;2012(5):CD005556. doi: 10.1002/14651858.CD005556.pub2. PMID: 22592706; PMCID: PMC4270370.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
