Journal of Clinical Research and Ophthalmology
The analysis of characteristics of anti-SARS-CoV-2 antibodies in clinically COVID-19 patients
Wenzheng Guo, Siyuan Yu, Guangbo Li, Qiankun Xuan, Simin Yang, Donghua Wen and Wenjuan Wu*
Donghua Wen, Department of Laboratory Medicine, Shanghai East Hospital, Tongji University School of Medicine, No.1800, Yuntai Road, Shanghai, China, E-mail: [email protected]
Cite this as
Guo W, Yu S, Li G, Xuan Q, Wu W, et al. (2020) The analysis of characteristics of anti-SARS-CoV-2 antibodies in clinically COVID-19 patients. J Clin Res Ophthalmol. 7(2): 081-086. DOI: 10.17352/2455-1414.000077Background: The novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had spread all of China and many other countries. The COVID-19 patients exhibit typical clinical symptoms, and most cases could be diagnosed by nucleic acid testing and imaging. Recent studies have reported that serum antibody testing can be used for diagnostic screening of SARS-CoV-2 infection. However, the rules and standards of antibody production in serum of discharged COVID-19 patients remain ambiguous.
Methods: We examined both nucleic acid and antibody detection of 139 non-severe (mild and common) patients who were diagnosed with COVID-19.
Results: Although the nucleic acid of the discharged patient has turned to negative, some patients have not detected antibodies, including IgG (n=11) and IgM (n=27) .19.4% (27/139) discharged patients’ IgM detection was negative. In the IgM positive discharged patients, 72.8% (83/114) have an onset time over 30 days. And, there was no statistically significant difference in IgM concentration of discharged patients from 4 to 9 weeks after the onset of disease. In addition, we also detected IgM and IgG in nucleic acid test positive patients (n=12) within two weeks after the onset of disease. The concentration of IgM was peaked about on the sixth day and then decreased, while the IgG concentration was continuously increasing.
Conclusions: Our study demonstrated the rules and standards of antibody production in serum of discharged COVID-19 patients which will provide more ideas for COVID-19 research.
Abbreviations
COVID-19: The Coronavirus Disease 2019; SARS-COV-2: Severe Acute Respiratory Syndrome Coronavirus 2; BALF: Broncho Alveolar Lavage Fluid
Introduction
The outbreak of the Coronavirus Disease 2019 (COVID-19), designated by WHO in February, 2020, quickly spread all over the world [1-3], which caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV) [4,5], As well as SARS-CoV in 2003 [6,7] and MERS-CoV in 2012 [8], SARS-CoV-2 infection can also cause severe pneumonia. As of July 28, 2020, there were more than 16.3 million confirmed cases of COVID-19 and more than 650 thousands death cases worldwide (https://covid19.who.int/). It has become a public health emergency of international concern. Although COVID-19 has been effectively controlled and cured in China, the global situation is still grim [9,10].
Detecting the specific anti-SARS-CoV2 antibodies could effectively and quickly identify the COVID-19 patients [11-13]. Antibody testing has been incorporated into COVID-19 diagnosis in China according to the Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance (http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4 cef80dc7f5912eb1989.shtml). As known, during virus infection, the body produces IgM antibody firstly, and then produces IgG antibody with high adaptive affinity, which are important for long-term immunity and immune memory [14]. Based on the experience in combating MERS and SARS-CoV infection, we have known that IgM could be detected in patient blood after 3-6 days and the IgG could be detected after 8 days [15-17]. So we speculate that rapid detection of IgG and IgM antibodies will play an important role in assessing the disease status of admitted patients and the prognosis of discharged COVID-19 patients.
Herein, we detected the nucleic acid and antibody of 139 non-severe COVID-19 patients from Molin Rehabilitation Center and Dongxihu Fangcang hospital in Wuhan, China. All admitted patients were diagnosed as COVID-19 through positive nucleic acid test and symptoms. When they discharge, we detected their antibody (IgG and IgM) in serum. Besides, we also detected the antibody concentration in COVID-19 patients at different time point. We objectively describe these data here, aiming to provide more ideas for COVID-19 research.
Materials and methods
Patients and sample collection
According to the discharge criteria:1) Normal temperature for over 3 days; 2) Respiratory symptoms improved markedly; 3) Pulmonary imaging showed significant absorption and improvement; 4) Negative nucleic acid tests for COVID-19, from 2 consecutive respiratory specimens over one day apart. Specimens from 139 non-severe (mild and common) patients diagnosed with COVID-19 and discharged from hospital after treatment from March 9 to March 11, 2020, were collected, including 41 patients from Dongxihu Fangcang Hospital and 98 patients from Wuhan Molin Rehabilitation Center. Among them, 80 were male patients, 59 were female patients. The age was ranging from 9 to 71 years old, and the average age was 48.81 (± 15.34). The duration of disease day was ranging from 18 to 68 days, and the mean duration of disease day was 34.74 (± 8.58). The CT imaging result of 139 discharged patients were negative.
Nucleic acid detection
Samples were detected by Realtime Reverse transcription polymerase chain reaction ( RT-PCR) (rRT-PCR) testing within 24 hours. A 20 µL reaction buffer was set up containing 1.5 µL 2019-nCoV enzyme-mix and 18.5 µL 2019-nCoV reaction buffer in BGITM One-step Quantitative RT-PCR system. Thermal cycling was performed at 50℃ for 20 minutes followed by an initial denaturation at 95℃ for 10 minutes and 40 cycles of amplification at 95℃ for 15 seconds and 60℃ for 30 seconds.
Detection of joint IgM/IgG anti-SARS-CoV-2 antibodies
Wondfo SARS-CoV-2 Antibody Test Kit (Fluorescence immunochromatography) to detect joint IgM/IgG antibodies in serum. After reading the ID chip, pipette 10 L of serum into the sample dilution solution, then add 75 µL of the mixed solution into the sample well. After 10 minutes of incubation at room temperature, read the value on the Wondfo immunofluorescence detector (FS113). In our lab, we valued the Wondfo SARS-CoV-2 antibody Test Kit’s precision/sensitivity and accuracy to make sure effective of our results in this work.
Data analysis
The result of antibody detection can be obtained directly on the machine, where the negative result of antibody detection shows <1.0. All data graph was processed in GraphPad Prism5.0.
Results
Detection of IgM and IgG in discharged COVID-19 patients
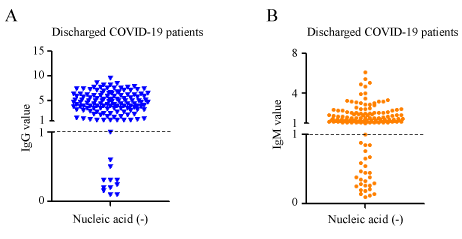
A total of 139 non-severe COVID-19 discharged patients from Fangcang hospital and rehabilitation centerin Wu Han (Table 1; Supplementary data 1) were collected. All admitted patients were diagnosed as COVID-19 through positive nucleic acid test and symptoms. After clinical treatment, these patients met the discharged criteria: 1) Normal temperature for over 3 days; 2) Respiratory symptoms improved markedly; 3) Pulmonary CT imaging showed significant absorption and improvement; 4) Negative nucleic acid tests for COVID-19 from 2 consecutive respiratory specimens one day apart. We confirmed that their nucleic acid tests were negative. At the same time, we also performed antibody detection. As shown in Figure 1A, although 92.08% (128/139) patients produce the IgG antibody, there are also 7.90% (7/139) patients who did not detected IgG. 80.57% (112/139) patients detected the IgM antibody production, and there are still 19.42% (27/139) patients whose IgM antibodies were not detected (Figure 1B).
IgM exist long time in most non-severe COVID-19 discharged patients
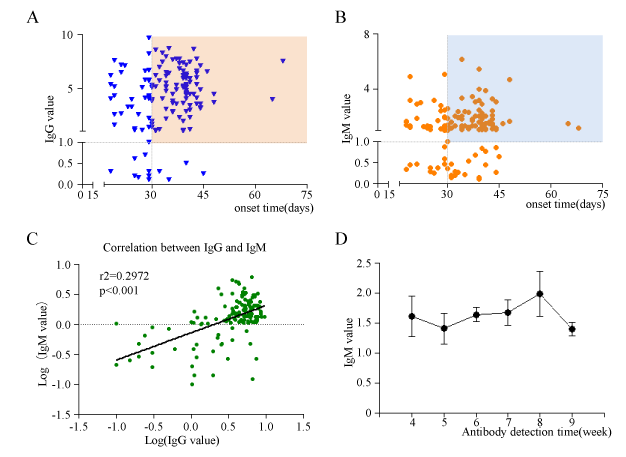
Based on knowledge of viral infection, we speculated that IgM positive indicates recent exposure to SARS-CoV-2, whereas IgG positive indicates virus exposure for longer time. Then, we detected the antibodies of discharged non-severe COVID-19 patients whose onset time ranges from 18 days to 68 days. In discharged patients whose nucleic acid of SARS-CoV-2 turned to negative, there are 92.08% (128/139) patients producing IgG antibody (value>1.0 ). Among IgG positive patients, 76.56% (98/128) have onset time for more than 30 days, which is also in line with the physiological characteristics of IgG, that is, it can exist in the rehabilitation patient for long time (Figure 2A; Supplementary data 2). Unexpected, only 19.42 %(27/139) of discharged patients’ IgM detection were negative, which seems to violated with the IgM representing acute virus infection. In the IgM positive discharged patients, 74.10% (83/112) had an onset time of more than 30 days (Figure 2B; Suppl data 2).This means that in non-severe COVID-19 patients, IgM does not disappear in a certain period of time. The above results suggest that although COVID-19 patients have been cured and discharged, there are still some patients whose IgG or IgM antibodies are undetectable. The correlation analysis also showed that IgM and IgG had a positive correlation (r2=0.2972, p<0.001) (Figure 2C; Supplementary data 2). The COVID-19 patients with high IgG level in discharged patients generally had high IgM level. In order to further explore the rule of IgM remaining time in discharged COVID-19 patients' serum. We analyzed the relationship between serum IgM levels and the onset time of antibody detection in discharged COVID-19 patients, and found that there was no statistically significant difference in IgM concentration (Figure 2D) from 4 to 9 weeks after the onset of disease in discharged COVID-19 patients.
Detection of IgM and IgG in nucleic acid positive patients
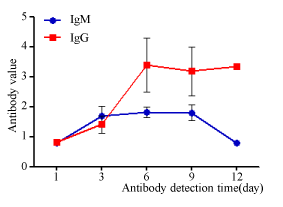
In order to understand about the rule of antibody production in infected patients, serum specimens from 12 SARS-CoV-2 nucleic acid positive patients diagnosed by admission. The concentration of IgM and IgG were detected at continuous different time when they were transferred and admitted to Shanghai Public Health Clinic Center for quarantine and advanced treatment. As shown in Figure 3, within a week of infection, both IgM and IgG increased, and the concentration of IgM reached its peak on the sixth day. During the original infection period, the concentration of IgG showed a continuously increasing trend (Figure 3). All of above data demonstrated that the SARS-CoV-2 infection in the early stage (within two weeks) was similar to other common virus infection.
Discussion
Here, we detected the IgM and IgG antibody in 139 non-severe COVID-19 patients from Molin Rehabilitation Center and FangCang hospital in Wu Han. Although the nucleic acid turned to negative in discharged patients, there are 7.90% (7/139) patients whose IgG antibodies were undetectable. Besides, there are 19.42% (27/139) patients whose IgM antibodies were undetectable. We also found that IgM in serum of discharged COVID-19 patient didn’t disappear in a short time (<30 days), which suggests that IgM detection may not have an advantage in predicting recent infection with SARS-CoV-2.
Nucleic acid detection and CT imaging are the current diagnostic standards for COVID-19, but they also have some shortcomings: sampling affects nucleic acid results; nucleic acid detection requires special experimental conditions; the turnaround time takes several hours. Besides, CT imaging need expensive facility and is not fit to detect at the early stage of COVID-19. Serological antibody detection can achieve rapid bedside detection, which are beneficial for COVID-19 identification and subsequent processing [18,19]. SARS-CoV-2 infection starts at the deep of lungs. In the early infection stage, throat swab or sputum may not detect the virus [20]. Although broncho alveolar lavage fluid (BALF) can increase the positive detection rate, not all patients are suitable for sampling, and even less suitable for COVID-19 screening [21,22]. The antibody detection also reduced occupational exposure during sampling. Certainly, there are some limitations during antibody detection. High concentrations of RF in plasma lead to false positives in antibody detection in fever clinics non-COVID-19 patients. Also, individual non-COVID-19 patients also have elevated antibodies without explanation. Several Chinese IVD companies are developing products ranging from IgM only and IgM-IgG joint detection, so we believe this limitation will be resolved in the near future.
During virus infection, the body first produces IgM antibody, and then produces IgG antibody with high adaptive affinity, which are important for long-term immunity and immune memory [14,23]. Based on previous SARS-CoV antibody research experience, IgG seroconversion can start as early as 4 days after the onset of infection. Detection of IgM would not provide earlier evidence for SARS-CoV infection compared with detection of IgG [24]. As reported, the IgM was still at low level at 100 days after the onset of infection, although a previous study showed IgM disappeared after 12 weeks [24-26]. A recent research reported that both IgM and IgG antibodies were detected 5 days after infection with SARS-CoV-2, so using serology antibody detection may facilitate the diagnosis of COVID-19 [27,28]. In our study, we found that 19.42% patients did not detected IgM when they discharged. Besides, we also detected IgG antibody in these patients. In the IgM negative discharged patients, there are also 37.03% (10/27) patients whose IgG antibodies were undetectable. We did not track the immune status of these patients. It is possible that the application of immunosuppressant suppresses the production of antibodies or keeps antibodies at a lower concentration. In the IgM positive discharged patients, 72.8% have an onset time for more than 30 days. And there was no statistically significant difference in IgM concentration from 4 to 9 weeks after the onset of disease in discharged patients. As shown in Figure 2, we did not detect the value of IgG or IgM over a short period of time (within two weeks), so we cannot judge the timing of antibody production. We can only conclude that the antibody concentration has not changed within 4-9 weeks. So, IgM can last long time in serum, and detecting IgM may not be a good strategy to distinguish the acute infection of SARS-CoV-2 in this period. In order to understand about the rule of antibody production in infected patients, we detected the concentration of antibody in SARS-CoV-2 nucleic acid positive patients (n=12) diagnosed by admission (within two weeks) as shown in Figure 3, which demonstrated that the concentration of IgM reached its peak on the sixth day.
As shown, we only detected the antibody levels of discharged patients ranging from 30-60 days. This level throughout the disease cycle may be at its peak, rising or falling, and it does not indicate the time latitude of the antibody change in COVID-19 patients. In order to understand more clearly the law of antibody production in infected patients, we collected 12 nucleic acid positive patients diagnosed on admission. Within a week of infection, both IgM and IgG increased, and the concentration of IgM reached its first peak on the sixth day. Based on the above results, we speculated that the decreased IgM after one week may have a second increase, reaching a second peak in a certain period. Therefore, this also suggests that we need to carry out large-scale epidemiological investigations, especially in patients with non-severe COVID-19 infection, which will have a better understanding of disease assessment and the immune law of SARS-CoV-2 in the population. Based on the diverse, some coronavirus proteins are conserved through the different clades while others, like Spike proteins, are quite species-specific [29].Of the 61 samples of presumably fully seroconverted COVID-19+ patients (i.e. two weeks after symptom onset) tested on the five antigens, 100 % cross-reacted with SARS-CoV-1 Nucleocapsid protein and 45.9 % also cross-reacted with SARS-CoV-1 Spike protein. Notably, only 2 (3.3%) of the 61 samples cross-reacted with MERS-CoV Nucleocapsid [30]. So, antibody detection may give false-positive reactions in some coronaviruses, which is also one of the urgent points to be resolved.
In conclusion, we detected the antibody (IgG and IgM) in discharged COVID-19 patients. There are some patients whose IgG antibody or IgM antibody are undetectable. Within a week of infection, the concentration of both IgM and IgG increased, and the concentration of IgM reached its first peak on the sixth day. But after a month, for example 4 to 9 weeks, IgM can last long time in serum as well as IgG, so detecting IgM in this period is not a good strategy to distinguish the acute infection of SARS-CoV-2.
This work was supported by grants from National Nature Science Foundation of China 81971990(WJW), 81902338(WZG), 81972830(DHW); The Top-level Clinical Discipline Project of Shanghai Pudong PWY gf 2018-05; Shanghai "Rising Stars of Medical Talent" Youth Development Program- Clinical Laboratory Practitioners Program.
ICMJE statement
Contributors Wenjuan Wu was responsible for the organization and coordination of the trial. Wenzheng Guo and Siyuan Yu were the chief investigator and responsible for the data analysis. Guangbo li,Qiankun Xuan,Simin Yang and Donghua Wen developed the trial design. All authors contributed to the writing of the final manuscript.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, et al.(2020) A new coronavirus associated with human respiratory disease in China. Nature 579: 265-269. Link: https://bit.ly/3hSYfRM
- Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, et al. (2020) Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J 133: 1015-1024. Link: https://bit.ly/2Xa52OG
- Chen H, Guo J, Wang C, Luo F, Yu X, et al. (2020) Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395: 809-815. Link: https://bit.ly/3gePyAy
- Wu C, Chen X, Cai Y, Xia J, Zhou X, et al. (2020) Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 180: 934-943. Link: https://bit.ly/313N5m2
- Wu Z, McGoogan JM (2020) Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. Link: https://bit.ly/30ctaT5
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, et al. (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953-1966. Link: https://bit.ly/30a4yu4
- Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, et al. (2003) Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362: 263-270. Link: https://bit.ly/3149lMT
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367: 1814-1820. Link: https://bit.ly/3hR4JQV
- Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, et al. (2020) Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol 30: 313-324. Link: https://bit.ly/2Xa2e4i
- Zhai P, Ding Y, Wu X, Long J, Zhong Y, et al. (2020) The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 55: 105955. Link: https://bit.ly/3fdr3Cv
- Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, et al. (2020) Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 52: 971-977. Link: https://bit.ly/39ETs3j
- Liu X, Wang J, Xu X, Liao G, Chen Y, et al. (2020) Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect 9: 1269-1274. Link: https://bit.ly/3159XSg
- Döhla M, Diegmann C (2020) On the usefulness of point-of-care antibody tests for severe acute respiratory syndrome coronavirus 2 in community screening settings. Public health 185: 30. Link: https://bit.ly/3hQoyrI
- Racine R, Winslow GM (2009) IgM in microbial infections: taken for granted? Immunol lett 125: 79-85. Link: https://bit.ly/2PaQV7I
- Lee HK, Lee BH, Seok SH, Baek MW, Lee HY, et al. (2010) Production of specific antibodies against SARS-coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J Vet Sci 11: 165-167. Link: https://bit.ly/3hP5KsF
- Yin Y, Wunderink RG (2018) MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 23: 130-137. Link: https://bit.ly/2CSj2WJ
- Chafekar A, Fielding BC (2018) MERS-CoV: Understanding the Latest Human Coronavirus Threat. Viruses 10: 93. Link: https://bit.ly/2XdYr6a
- Liu M, Cheng SZ, Xu KW, Yang Y, Zhu QT, et al. (2020) Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. 369: m2195. Link: https://bit.ly/2EyBpAq
- Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I (2020) CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurology(R) Neuroimmunology Neuroinflammation 7. Link: https://bit.ly/2Xd3weZ
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395: 497-506. Link: https://bit.ly/3hI79Bo
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, et al. (2020) Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 382: 970-971. Link: https://bit.ly/2Xbdiht
- Zou L, Ruan F, Huang M, Liang L, Huang H, et al. (2020) SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 382: 1177-1179. Link: https://bit.ly/3jTYPAp
- Wan Y, Shang J, Sun S, Tai W, Chen J, et al. (2020) Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J Virol 94: e02015-19. Link: https://bit.ly/30Yw2C0
- Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC (2004) Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 10: 1062-1066. Link: https://bit.ly/3gbT6Uo
- Hsueh PR, Hsiao CH, Yeh SH, Wang WK, Chen PJ, et al. (2003) Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg Infect Dis 9: 1163-1167. Link: https://bit.ly/313QlxM
- Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, et al. (2003) Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361: 1767-1772. Link: https://bit.ly/3gfi4lx
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, et al. (2020) Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes Infections 9: 386-389. Link: https://bit.ly/39HGJg5
- Li Z, Yi Y, Luo X, Xiong N, Liu Y, et al. (2020) Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J Med Virol. Link: https://bit.ly/3fdDT3M
- Sudhakar A, Robin G, Boyd L, Eric F, Vineet D, et al. (2014) Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J Infect Dis 209: 995-1006. Link: https://bit.ly/39H6I7A
- Ahidjo A, Guillaume T, David M, Edouard T, Raisa R, et al. (2020) Multiplex detection and dynamics of IgG antibodies to SARS-CoV2 and the highly pathogenic human coronaviruses SARS-CoV and MERS-CoV. J Clin Virol 129: 104521. Link: https://bit.ly/3fdDYEC

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
