Journal of Clinical Research and Ophthalmology
Crosstalk between oxidative stress and ocular diseases
Lizette Gil del Valle1*, Mirtha Copello Noblet2 and Gregorio Martínez-Sanchez3
2Retinitis Pigmentosa National Reference Center. “Dr. Salvador Allende” Hospital, Havana, Cuba
3Independent Consultor, Ancona, Italy
Cite this as
Del Valle LG, Noblet MC, Sanchez GM (2020) Crosstalk between oxidative stress and ocular diseases. J Clin Res Ophthalmol. 7(1): 037-047. DOI: 10.17352/2455-1414.000071Context: Diverse findings reported during the past years has established the molecular mechanisms by which a disparity in the redox balance system in microenvironment and cells with high grade of Oxidative Stress (OS) can cause oxidation of macromolecules as nucleic acids, lipids, proteins and carbohydrates, thus bringing about their function alteration. These events also result in modulation of redox circuits herein signal transduction pathways and activation of transcription factors that can lead to chronic inflammation and contribute to several chronic diseases, including eye diseases and retinopathy.
Aims: The article proposes a review of the knowledge about OS pathways in eye’s diseases.
Methods: The search was carried in the LILACS, PAHO, SciELO, EMBASE, PubMed and Infomed databases, Google search engines and Google Scholar where the key words were placed to obtain information from original articles, theses, other articles of bibliographic review and high citation index journals published from 1980 to 2019, in Spanish or English.
Results: Four hundred seventy documents were identified, of which 91 were selected. The most important oxidant and reactive oxygen species produced by various physiological pathways, chemical and biological factors, including genetic and pathological conditions have been revised.
Conclusions: This document summarizes the evidences of crosstalk between OS and ocular diseases, including retinitis pigmentosa.
Introduction
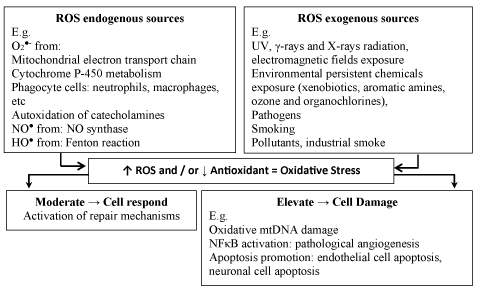
Oxidative Stress (OS) is defined as an imbalance between the generation of oxidants and the potential of antioxidant defenses of cells, as well as the ability of damage repair mechanisms to favor an excess of oxidants. This unbalanced state can cause tissue damage or lead to the production of toxic species to all cell components [1-5]. The oxidants include mainly the molecules with unpaired electrons (free radicals): superoxide anion radical (O2•-), hydroxyl radical (HO•), peroxyl radical, non-radical oxygen species, such as hydrogen peroxide (H2O2), an electronically excited form of molecular oxygen, termed singlet oxygen (1O2), nitric oxide radical (NO2•) and peroxynitrite (ONOO−) commonly known as Reactive Oxygen Species (ROS) [3,6-8]. OS may lead to disturbance in the normal oxidation-reduction state of a cell resulting in adaptation-resolving oxidant tone (eustress) or damage of the cellular components (distress).
Related literature indicates that OS is involved in several human diseases, such as inflammation, diabetes, arteriosclerosis, autoimmune disorders, skin diseases, hypertension, cancer, eye diseases, infectious diseases, among others [3,8-15]. Over the past decades, the OS theory has strongly stimulated interest in the role of antioxidant defense mechanisms in removing ROS, i.e. antioxidant enzymes and non-enzymatic antioxidants [13]. Concerns have been raised about the benefits of the regular physical activity of moderate intensity as the factor linked with adaptation of cells to OS and the ability to enhance the cell antioxidant system[16]. According to the World Health Organization report [17], about 347 million people were suffering from visual impairment worldwide in 2018. Among them, 46 million faced blindness and 300 millions suffered from moderate to severe vision impairments. The knowledge of the role of OS in the etiology of eye diseases, including cancer, mechanisms responsible for the counteraction of OS and its modulation action by bio-oxidative therapy, physical activity and as natural antioxidant may be useful in the prevention and therapy strategies. The present document will focus on OS participation in the eye diseases and the role of oxidant and defense systems, based on the recent scientific research.
Evidence Acquisition Terms included in the information search: oxidative stress, antioxidant, ocular physiology or diseases, retinoapaties, antioxidant, bioxidative therapy. Bibliographic databases consulted: MEDLINE/PubMed, SciELO, LILACS, PAHO, EMBASE and informed databases with search engines: Google and Google Scholar. Types of documents: original articles, published theses, clinical reports and bibliographic reviews. Languages: Spanish and English. Dates of publication: 1980 to 2019. Exclusion criteria: no free access to complete text due to financial constraints, studies on genetic variations unrelated to ocular diseases or physiology presenting inadequate scientific evidence.
The generation mechanisms of reactive oxygen
In aerobic cells, free radicals derived from molecular oxygen represent the most important group of ROS formed during the natural biologic processes in living cells. They are mainly produced by an electron transfer reaction through multiple pathways[18]. For example, the endogenous sources of the superoxide radical formation include: i) mitochondrial electron transport chain; ii) cytochrome P-450 metabolism; iii) autoxidation of catecholamines and hemoglobin; iv) oxidation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) by NADPH oxidase; v) oxidation of xanthine or hypoxanthine by xanthine oxidase; vi) reduction of molecular oxygen by NO synthase (NOS) isoforms (nNOS or eNOS) under arginine or tetrahydrobiopterin deficiency; vii) peroxisome metabolism; viii) phagocyte cells (neutrophils, macrophages, eosinophils), and iv) UV-light irradiation of tryptophan, eumelanin and pheomelanin. It is worth adding that during aerobic respiration, 1-5% of total oxygen consumed undergoes reduction to superoxide anion radical [19]. In turn, NO• arises from L-arginine with the participation of one of the three NOS isoforms. The superoxide radical is highly reactive towards NO• forming a very toxic oxidant ONOO‾ (the rate constant – 7.0 x 109 M-1s-1) [20], although the species is poorly reactive to biomolecules [14]. At physiological pH, superoxide radical undergoes the dismutation reaction catalyzed by superoxide dismutase (SOD) enzyme, forming H2O2. In contrast to superoxide radical, hydrogen peroxide can cross cell membranes and form highly reactive hydroxyl radicals via the Fenton reaction [21]:
Men+ + H2O2 → Me(n+1)+ + HO• + OH‾ (1)
(Me = Fe, Cu or Co free transition ion) and via the Haber-Weiss reaction:
O•-2 + H2O2 → O2 + HO• + OH‾ (2)
Reaction (2) may be catalyzed by free translation metal ions and the iron complexes in hemoglobin, myoglobin, transferrin and lactoferine [22]. Reactions (1) and (2) are commonly considered as the major sources of hydroxyl radical in vivo. Hydroxyl radical is a particularly strong oxidant that reacts rapidly and non-specifically with all biomolecules, including the DNA bases. It is well documented that oxidized DNA is inclined to alterations in transcriptions and genetic mutations by conformational changes of its structure, including single strand and double strand breaks, influences in hydrogen bonding, and decrease in fidelity DNA and/or RNA polymerase [23]. In aqueous medium, the superoxide radical occurs in the equilibrium state with hydroperoxy radical (HOO•). The HO• and HOO• radicals easily extract a hydrogen atom from unsaturated fatty acids. The process is followed by formation of a lipid radical (R•) and further on a lipid peroxyl radical (ROO•) after an addition of molecular oxygen. The latter radical is unstable, and abstracts a hydrogen atom from neighboring molecules or other lipid molecules, nucleic acids, forming lipid hydroperoxide (ROOH) and an alkyl radical (R•). The hydroperoxide as unstable molecule undergoes spontaneous metal catalyzed decomposition, generating alkoxy radical (RO•) and HO•.
The lipid peroxidation reaction is a chain reaction resulting in the production of a large number of free radicals, toxic aldehydes, polymerization and cross-linking reactions [24].
Additionally, the combination of two molecules of ROOH leads to the formation of a very strong oxidant 1O2, similar to the reaction of ROOH with superoxide radical [25]:
ROOH + O2 → 1O2 + LO + HO‾ (3)
The high toxicity of aldehydes has long been recognized. Aldehydes can react with amino groups of proteins, free amino acids, nucleic acids, and with the thiol (-SH) groups of proteins, resulting in cell damage [26]. The second source of ROS includes actions of the physical factors (UV, γ-rays and X-rays radiation, electromagnetic fields exposure) and environmental persistent chemicals exposure (xenobiotics, aromatic amines, ozone and organochlorines), and pathogens (reviewed previously by Klaunig, et al.) [9] (Figure 1). The increasing literature dealing with the role of ROS in cells has provided strong evidence of their participation in cell signaling processes, i.e. a communication between cells, response to extracellular signals and the expression of a number of genes [27].
Reactive oxygen species, particularly H2O2, have been recognized as the second messengers in signal transduction, and several transcription factors were reported to be modulated by these species: the nuclear factor (NF-κB), signal transducer and activator of transcription STAT3, activator protein-1 (AP-1), Mitogen-Activated Protein Kinase (MAPK) pathway, nuclear factor p53, hypoxia-inducible factor (HIF-1), the nuclear factor of activated T cells family (NFAT), and others [13]. The transductional factors can induce gene expression, cell growth and differentiation.
For example, AP-1 is important for cell growth and differentiation. NF-κB regulates genes involved in inflammation, cell proliferation, and survival. Their activity is found to be dependent on the presence of transition metal ion [28]. Another aspect related to ROS, especially HO• and 1O2, is their stimulating effect on the generation of NF-κB, the factor which, among other activities, activates protein kinase C (PKC) – the enzyme which stimulates the formation of proinflammatory cytokines, e.g. interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interferon. These cytokines have been reported to produce ROS in non-phagocytic cells [3].
It is generally recognized that ROS influence many stages in the cell signaling processes responsible for cell division, differentiation and apoptosis. It has been proven that ROS as a product of normal cellular metabolism play a dual role- beneficial and harmful in biological systems. The beneficial role includes the ROS control of physiological functions, including participation in the cellular reduction/oxidation reactions and protection of cells against OS. The damaging role of ROS appears when their production exceeds the cell’s antioxidant defense system capacity. There are several consequences of un-balanced production of ROS, like direct DNA oxidation, considered as an action responsible for the initiation of mutagenesis, carcinogenesis, and also ageing. The next consequence is lipid peroxidation resulting in disruption of structural and functional role and/or formation of the hydroperoxides, having the ability to affect signaling pathways. Another outcome of OS is oxidation of proteins and their amino acids (residues) that may lead to the generation of disulfide linkages between proteins, protein-SH groups and low molecular weight thiols, mainly glutathione (GSH), compounds containing carbonyl groups, and protein hydroperoxides. For example, the aldehydes malondialdehyde (MDA) and 4-hydroxy-2- nonenal are the most commonly detected end-products of lipid peroxidation or polyunsaturated fatty acid residues of phospholipids caused by ROS. These biomarkers of oxidative damage of lipids and fatty acids have been found to be mutagenic and carcinogenic, owing to their reaction with lysine, histidine and cysteine of the cellular protein’s residues [1]. The next sensitive biomarker of the human diseases with participation of OS is the product of DNA damage 8-hydroxy-2’-deoxyguanosine (8-oxo-dG) resulting from hydroxylation of the C-8 position of guanine. Another determinant of the proteins oxidative damage associated with human diseases is production of mixed disulphides between the –SH groups of their amino acid residues and GSH and also a higher concentration of protein containing carbonyl groups (for exhaustive details, the reader is referred to a paper of Dalle-Donne, et al. [29]). Due to multiple mechanisms by which ROS may alter signaling processes, the activity of growth factors, enzymes or transcription factors may be modified that, in turn, modulates gene expression or induces apoptosis. The permanent modification of genes consists of the first step involved in mutagenesis, carcinogenesis and ageing [30].
Antioxidants
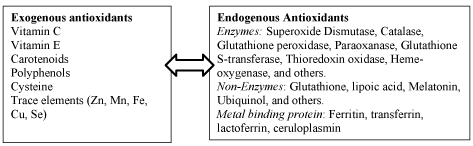
The level of cellular ROS is kept under control due to the presence of a number of antioxidants operating in hydrophilic and hydrophobic environment of the cell. Antioxidants are molecules able to maintain good cellular functions owing to the ability of stabilization or deactivation of the ROS, thereby protecting the cells against auto-oxidative damage. Rahman [31] has reported that “An ideal antioxidant should be readily absorbed and quench free radicals, and chelate redox metals at physiological relevant levels. It should also work in both aqueous and/or membrane domains and effect gene expression in a positive way”. The water-soluble antioxidants (hydrophilic) prevent the cell cytosol and the blood plasma against oxidants, whereas those soluble in lipids (hydrophobic) protect cell membranes against lipid peroxidation. Defense mechanisms rely mainly on deactivation of superoxide radical and decomposition of H2O2 before their contact with iron does not support the formation of HO• in the Fenton reaction that, in turn, prevents against lipid peroxidation and allows sustaining the proper redox environment of a cell [3]. The endogenous defense mechanisms include enzymatic and non-enzymatic antioxidants. The major endogenous enzymatic antioxidants include superoxide dismutases (SOD), glutathione peroxidase (GPx), catalase (CAT), and thioredoxin reductase [32]. SOD and cytochrome C catalyze the superoxide radical dismutation to H2O2 and O2. In turn, thioredoxin (TRX) reductase reduces this radical to H2O outside of the cell. The toxic product H2O2 is removed from the cell by its decomposition in a reaction catalyzed by catalase (2H2O2 → 2H2O + O2) and the selenium dependent GPx. Also, the large amounts of H2O2 formed in cytoplasm are removed with the participation of the GPx glutathione reductase system. It is also worth mentioning indoleamine 2,3-dioxygenase (the enzyme that uses superoxide radical as a substrate for the oxidation of tryptophan), metal complexes, and metalloenzymes of which the metal ions are in a higher oxidation state, allowing to oxidize the radical to O2•-. Other noteworthy enzymatic chelators are transferrin, lactotransferrin and ceruloplasmin. The endogenous nonenzymatic antioxidants include the reduced form of glutathione (GSH), α-tocopherol (vitamin E), ascorbic acid, vitamin D, proteins, free amino acids, and peptides [32].
Glutathione is the major thiol antioxidant that is present in high concentration in the cytosol, nuclei, and mitochondria (1-11 mmol/L; 3-15 mmol/L; 5-11 mmol/L, respectively) [3]. Compounds containing thiol group are essential for protection against ROS mediated oxidation of biomolecules. They act by scavenging free radicals and are a substrate for several enzymes. Glutathione disulfide (GSSG) (GSH in the oxidized state) is accumulated inside the cell under OS conditions. The ratio of GSH/GSSG is often used as a measure of OS in an organism [33]. In healthy cells, more than 90% of the total glutathione is present in the reduced form. Valko, et al. [3]. listed several main preventive actions of this antioxidant against oxidation: an ability to act as a cofactor for several detoxifying enzymes e.g. GPx, participation in transport of amino acids through the plasma membrane, regeneration of vitamins E and C back to their antioxidant state, and reduction of the tocopherol radical. The second thiol antioxidant thioredoxin possesses two –SH groups and the oxidoreductase activity in its reduced form. The next thiol antioxidant α-lipoic acid is also a disulphide and occurs in the cellular membranes and the cytosol of eukaryotic and prokaryotic cells. All thiol-containing antioxidants are powerful scavenging oxygen radicals and 1O2, decompose H2O2, and lipid peroxides. Their antioxidant potential is due to the sulfur atom:
H2O2 + 2GSH → GSSG + 2H2O (4)
ROOH + 2GSH → ROH + H2O + GSSG (5)
Further, melatonin – an indoleamine neurohormone that is synthesized in the pineal gland and is also acquired in diet, exhibits an efficient scavenging of ROS. The hormone is able to inhibit the metal ion-catalyzed oxidation [34]. Many researches have been carried out so far on the influence of nutrients on ROS production and removal. It has been found that high intake of proteins and animal fat can stimulate the generation of ROS, which, in turn, triggers lipid peroxidation [35]. Conversely, the diet rich in vegetables and fruits prevents against OS [36]. Additionally, the research has documented that products of natural origin play an important preventive role against cancer development [37]. The natural exogenous antioxidants are present in vegetables, fruits, and grain cereals. An important class of antioxidants is carotenoids present in plants. This group of natural pigments occurring in photosynthetic organelles has been commonly recognized as very efficient scavengers of ROS and quenchers. They are also present in human and animal tissues. Epidemiological and intervention studies and also clinical trials suggest that carotenoids play a protective role in a number of diseases of the ROS etiology, from certain forms of cancer, cardiovascular Diseases, and macular degeneration, among others [38]. The antioxidant property is due to a number of conjugated double bonds in carotenoids’ molecules and results from the ability to delocalize the unpaired electrons. Supplementation with carotenoids can affect cell growth and modulate gene expression and immune responses [39].
The next very important group of antioxidants that have received a lot of attention are (poly) phenolic compounds. Over the last 40 years, several reports have demonstrated the ability of phenolic compounds to scavenge ROS. Epidemiologic studies suggest that dietary polyphenol intake is associated with a lower incidence of several non-communicable diseases [40]. Plants are rich in several classes of the phenolic compounds like flavonols, flavones, flavanols, tannins, phenolic acids, etc., that are grouped into flavonoid and non-flavonoid compounds. The antioxidant capacity of (poly) phenolic compounds results from the ability of their hydroxyl groups (OH) to donate a hydrogen atom to a free radical and/or to donate one electron to an alkyl free radical. This leads to breaking of the chain reaction of lipid peroxidation, by removing the toxic free radicals and protecting cell membranes. In addition, phenolic compounds play a key role in sequestration of redox active ions preventing their participation in reactions generating ROS [41]. Interestingly, tea leaves, particularly green tea, are rich in polyphenols aminoacids, vitamins and minerals, like Zn (II), Ca (II), and K (I) [42]. The health and medicinal benefits of tea drinking is known since ancient times. This is due to the fact that green tea is a rich source of flavonoids and anthocyanins. Recent molecular studies have revealed that green tea polyphenols protect against ocular disorders and cancer [43,44] due to their antioxidant and anti-inflammatory properties. For example, polyphenol compounds sourced from plants, commonly found in green tea, red wine and cocoa, like epigallocatechin gallate and quercetin have been recognized to have therapeutic benefit in several diseases of the OS etiology, including ocular disease e.g. glaucoma and ocular hypertension[44]. Besides directly removing free radicals, many above mentioned dietary antioxidants can act on the signaling pathways, including the important agents in cell transformation and tumor promotion, for example cyclooxygenase (COX-2), AP-1 or NF-κβ [15]. Unfortunately, some antioxidants can act synergistically with other antioxidants and exert pro-oxidant action. Similarly, the pro-oxidant behavior of carotenoids was observed at their high concentration [45]. Recent literature findings have showed that molecular hydrogen (H2) exhibits antioxidative activity and exerts anti-inflammatory and anti-allergic activities of high potency [46]. The protective and therapeutic applications of H2 have been found in 38 diseases (citation for Ohta [47]), including ocular diseases. H2 is the only antioxidant that crosses the blood-brain and blood-ocular barriers. It quickly penetrates through tissue due to its small molecular size and effectively removes ROS, mainly hydroxyl radicals and peroxynitrite. Apart from its antioxidant effects, H2 also displays anti-inflammatory, antiapoptotic, cytoprotective and mitohormetic properties (Figure 2) [48].
Oxidative stress in eye diseases
Numerous researches have demonstrated that OS has been implicated in the pathogenesis of several ocular conditions and diseases, such as cataract, retrolental fibroplasia, glaucoma, age-related macular degeneration, diabetic retinopathy, autoimmune and inflammatory uveitis endothelial corneal dystrophy, and granular corneal dystrophy type 2, primary open-angle glaucoma, retinal light damage, retinopathy of prematurity, and cancer [7,49-51]. Further, OS has been widely accepted as playing a key role in the pathogenesis of dry eye and ageing [49]. The eye is one of the major targets of ROS attack because it is environmentally exposed to UV radiation, irritants, high pressure of molecular oxygen, pollutants, industrial smoke, driving fumes, temperature, and wind. These environmental factors can shift the redox status of the ocular surface towards oxidizing conditions, due to inactivation of antioxidant enzymes, followed by damage of the lipid layer of ocular surface. The statement that OS is involved in eye diseases is based on a fact that recognized risk factors for OS contribute to the development of eye diseases; moreover, the inhibitors of OS play an important role in eye diseases prevention and therapy [52]. The most important oxidant occurring in the anterior uvea and aqueous humor is H2O2. This active species and O2•- are present in the eye tissues at high concentrations [53]. Due to its ability to diffuse in aqueous medium across membrane and to produce HO• radicals (reactions 1 and 2), its unbalanced concentration has been reported to be deleterious to the lens [54].
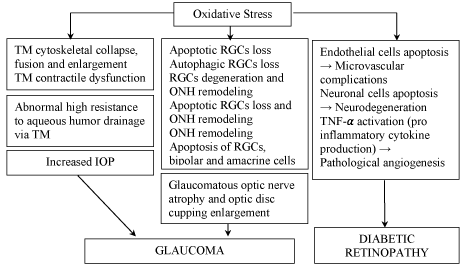
It is a commonly considered hypothesis that OS is a key factor in cataract formation. The disease is characterized by damage of crystalline proteins, nucleic acids, lipids, and polysaccharides with consequent opacity of the eye lens [51]. The main factors leading to cataract are UV rays-induced ROS formation and reduction in GSH-Px activity. It has been observed that the concentration of H2O2 in aqueous humour is strongly increased when this oxidant concentration is high in lens [55]. The role of H2O2 in cataract development has also found a confirmation in the reduction of the defense enzymes activities, like GSH-Px, SOD and glutathione reductase in the lens of patients with cataract. In addition, an increased concentration of lipid peroxidation products in aqueous humour of age-related cataract has been observed. It is widely accepted that OS plays an important role in the aging process. As Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/Kelch-like erythroid-cell-derived protein with CNC homology (ECH)-associated protein 1 (Keap1) system is considered as one of the main cellular defense mechanisms against oxidative stresses. In cataracts Nrf2 suppressors may augment oxidative stress of the lens, however Nrf2 inducers may decrease the oxidative stress and prevent the cataract formation [56]. Several reports have demonstrated that an accumulation of products of the lens components oxidation, the decreased potential of antioxidants with age, and repair mechanisms are relevant to the development of age-related cataract [55]. Another eye disease in the pathogenesis of which OS plays a major role is glaucoma – an optic neuropathy linked with changes in the optic nerve [57]. An elevated intraocular pressure as a result of disturbance in normal aqueous outflow is considered as a key risk factor of primary open angle glaucoma (POAG). Studies have reported a high level of lipid peroxidation products and DNA damage in tissues from the trabecular meshwork of patients with POAG. Moreover, meta-analysis indicates that POAG patients had a lower TAS in the blood and higher levels of SOD, GPX, and CAT in the aqueous humor [58]. As support for this, it is reviewed that antioxidant such as polyphenols, can contribute, not only theoretically, to neuroprotection but which are also able to counteract the metabolic pathways that lead to glaucomatous damage [59].
Other ocular disease in which OS has been involved is diabetic retinopathy (DR). The worldwide prevalence of DR has been estimated to be 35%, and the prevalence of vision-threatening DR (VTDR) has been estimated to be 10% [60]. As reviewed by these authors, there is a relationship between high concentration of blood glucose, OS, and an initiation of retinopathy, owing to a high susceptibility of retina to damage from ROS. Retina is made up of specialized nerve cells that are highly sensitive to light. Light after passing through lens is focused on the retina and being involved in photosensized reactions in the presence of the chromophores (molecules that absorb light) produces highly reactive electronically excited states of the chromophores, 1O2, and free radicals, thus inducing OS [14]. The next major source of ROS in retina owes to the autoxidation of sugars and formation of sugar adducts to proteins. As presented above, these toxic products are involved in peroxidation of biomolecules, such as the polyunsaturated fatty acids, lipids, DNA, and RNA. An important consequence of lipid peroxidation may be alternations in retinal blood flow, which leads to hypoxia in the retina and alterations in retinal blood flow [61]. Kowluru & Chan [62] have summarized the potential mechanisms that may link OS to damage of retinal blood vessels during hyperglycemia. They include, among others, i) synthesis of NO; ii) expression of protooncogene NF-κβ which, in turn, activates protein kinase C (the enzyme mediating the generation of proinflammatory cytokines); iii) secretion of proinflammatory cytokine like IL- 1β; iv) formation of lipid peroxides; v) activation of retinal caspase-3, which, in turn, increases apoptosis, and vi) glutamate concentration in retina.
OS has also been identified to play a role also in retinitis pigmentosa (RP), varied group of inherited eye disorders, characterized by progressive rod photoreceptor apoptosis with consequent gradual death of cone cells, leading to blindness. Early symptoms can already occur in childhood or adolescence and, usually, consist of night blindness. Rods are about 95% of all photoreceptors while only 5% are made up by cones; oxidative metabolism of fatty acids is their main energy source. RP inherited in autosomal dominant (15–25%), autosomal recessive (5–25%) and X-linked (5–15%), while about 40% of forms are still uncharacterized. Cones degeneration is a late event and it is supposed to result from cytotoxic effects of high oxygen levels in retina after rods reduction; so oxidative damage is considered the first cause of cones apoptosis and progressive vision loss. It is noted that Komeima, et al. [63], observed that antioxidants slow death of the photoreceptor cells in mouse in experimental RP. Others authors demonstrate that Retinal Pigment Epithelium (RPE) provides many vital functions for photoreceptor cells [64]. Despite that, several experimental studies have demonstrated support that UV irradiation and tobacco smoking are responsible for oxidative damage to the retinal epithelium, the precise mechanisms of progression of the disease are not well known [65]. Recent report based on comparative whole transcriptome analysis of human RPE cells, treated with 100 μg/ml of oxLDL and untreated, at different time points, explore OS pathways in RP. As previously mentioned, redox disruption could impact on gene expression by diverse transcription factors. The results pointed to over-expressed and down-expressed genes; 23 of them were already known to be RP causative ones and related to redox circuits. Intersection analyses highlighted new 77 candidate related genes (49 over-expressed and 28 down-expressed) involved in cell cycle regulation, vesicular trafficking, cell migration, endoplasmic reticulum stress, chaperones activity, small GTPase signaling, retinoic acid cycle, epigenetics, microvascular impairments, chromosome instability, circadian rhythms related to fatty acids metabolism, synapses integrity, JUN complex and retinal cells rescue. Results demonstrate complexity in molecular etiopathogenesis related to OS in RP [66].
Another group of eye disease in which ROS may play a key role in the pathogenesis under the OS conditions are autoimmune and inflammatory uveitis [52]. Experimental findings have shown the presence of the mitochondrial proteins elicited by OS in the retina and down-regulation of ATP synthase, providing important evidence that ROS participate in retinal damage. Oxidative damage resulting from these toxic species has been implicated in the pathogenesis of age-related macular degeneration (AMD) [52]. The disease is characterized by death of photoreceptor cells, resulting from atrophy of the retinal pigment epithelium. AMD is a leading cause of blindness in patients over 65 years of age in Western countries [67]. As presented by research findings, genetics, age, high body mass index (BMI), exposure to light radiation, consumption of high fat diets, and smoking were recognized as the major risk factors for AMD [68]. The main cause of damage to retina is OS, which increases the level of the strong oxidants, such as oxygen free radicals and 1O2, particularly during UV light exposure. A high susceptibility of the retina to damage is linked to its high consumption of oxygen and concentration of polyunsaturated fatty acids, and also with the presence of photosensitizers. OS may be an important causal risk factor for AMD, as evidence from literature clearly supports that a diet poor in vegetables and fruits and low levels of antioxidants in plasma or exposure of the retinal pigment epithelium to H2O2 may lead to damage and death of these cells [51,69]. Conversely, antioxidants such as lutein and zeaxanthin, and omega-3 essential fatty acids exhibit preventive effects in patients with AMD and those with disease of retina [70]. Furthermore, numerous studies have demonstrated that a great number of chemicals in tobacco smoke are toxic and can cause several eye diseases, including AMD and cataract [68]. Another noteworthy finding was a population-based cohort study carried out by Cheung, et al. [71], that examined the relationship between early AMD and cancer mortality. The authors have identified 464 cases of AMD among a cohort of 10,029 individuals aged 49 to 73 years who were free of cancer. During the 10 years study period of 464 cases of AMD, 234 cases died of various forms of cancer. An increased risk of death was significant in African American individuals (RR=1.68; 95% CI=1.03-2.73) but not in white cases. The authors in their conclusion suggest that common pathogenic mechanisms may be involved in early AMD and cancer disease in African American patients.
Furthermore, an imbalance between the pro-oxidants formation and the level of antioxidants in cells might elicit an increased production of ROS which could cause dry eye disease and inflammation [72]. Dry Eye Disease (DED) is a distressing multifactorial condition of major impact on patients’ vision and quality of life, with disease symptoms that can seriously hinder daily activities. This condition affects between 5% and 35% of adults worldwide [72]. The disease is characterized by a problem in tear fluid deficiency, and is considered as a multifactorial disease of the tear film layer and ocular surface. Although the detailed mechanisms operating in this disease have not been fully elucidated, the body of evidence available from basic and clinical research indicates that inflammation and ocular surface immunology play a key role in the onset and development of dry eye disease. The ocular surface consists of a tear-film layer, lacrimal, meibomian, accessory glands, corneal conjunctiva, and nasolacrimal ducts responsible for the homeostasis of the ocular surface and protection against oxidative and xenobiotic (e.g. drugs, food additives, environmental pollutants, pathogens) stress. Products of xenobiotic oxidation may be involved in ROS production. Disruption in the homeostasis causes a disorder in the integrity of the ocular surface, and the inflammatory reactions being an important source of ROS are originated, resulting in dry eye disease [73].
A number of studies have been performed examining a role of OS in the induction of inflammation. Inflammation is defined as “a pathological condition characterized by mononuclear immune cell infiltration (monocytes, macrophages, lymphocytes, and plasma cells), tissue destruction and fibrosis”. Chronic inflammation is involved in a disease development through messenger molecules (cytokines, prostaglandins, chemokines), excessive generation of ROS and other oxidants, and decrease in the antioxidants level [74]. Given these findings, OS can promote inflammation by activating the immune inflammatory signaling pathway stimulating cells in the ocular surface to generate redox-sensitive proinflammatory cytokines, such as IL-1, IL- 6, IL-8, TNF-α, and chemokine in the tear fluid, lacrimal glands, and conjunctiva. For extensive discussion of the role of inflammation in ocular and OS in dry eye disease, the recent excellent reviews by Rhee and Mah [75] and Pinazo-Durán, et al. [76], are illustrative. As reported previously, the increased frequency of eye surface infections and inflammation has been found to result from reduced level of lactoferrin in the tear film of an antioxidant that binds free iron, thus preventing against production of HO• in the Fenton reaction. Also, Ibrahim, et al. [77], demonstrated experimentally that lack of SOD enzyme caused an increased lipid peroxidation and damage to DNA in meibomian gland of the mouse model of dry eye. As noted earlier, there is growing evidence and general agreement for the OS theory of aging. The theory of aging proposes that damage to DNA, lipids and protein from ROS generated by both, the exogenous and endogenous sources, as mentioned earlier, accumulates within the cycle of life and its rate increases with age [78]. This process has been observed in dry eye disease as a change in the quality and quantity of the tear fluid with age, and is closely related to earlier discussed eye diseases. Today, it is still unclear whether OS is an initiator of eye diseases, but it is a general agreement that ROS participate in the propagation of the cell and tissue damages being involved in biochemical, physical, molecular and also pathological cellular events (Figure 3).
OS is involved in inflammation and a variety of cancers, including eye. Chronic inflammation can increase cell proliferation and differentiation, limit apoptosis and form new blood vessels [79]. Cancer as a multistage and multistep process involves multiple molecular and cellular reactions that transform a normal cell to a malignant neoplastic cell [7,79]. Cancer cells generate more free radicals compared to normal cells. At least three stages of carcinogenesis are distinguished: initiation, promotion, and progression. Based on cumulative knowledge over the past three decades, chronic OS, particularly arising during inflammation, is involved in carcinogenesis and two mechanisms regarding its participation have been suggested. The first – direct effect includes oxidation, nitration, and halogenations of DNA, RNA, and lipids by ROS. The second - indirect effect includes stimulation of the redox-sensitive cellular signaling pathways participating in cell growth/proliferation, differentiation, cell survival, protein synthesis, glucose metabolism, and inflammation [10,15]. These authors have specified actions of H2O2, superoxide and hydroxyl radicals in the induction of cellular signaling as regulation of protein activity by reversible oxidation of phosphatases in site cysteine or by oxidation of kinases with consequent activation of several signaling cascades, such as NF-κB activation, the mitogen activated protein kinase (MAPK) cascades, activation of transcription factors, e.g. AP-1, and others. The creation of additional signaling cascades, alteration of certain genes, expression, uncontrolled cell proliferation and growth of cells populations may lead to cancer development. It is worth to mentioning the NF-κB factor, which is an example of transcription factor being sensitive on redox modulation. Because this factor plays a key role in the regulation of genes involved in immune, inflammatory, and anti-apoptotic responses, it can exert a basic role in cell growth, differentiation, proliferation, and survival. These actions determine an important role of NF-κB in inflammation and cancer [69].
Detailed mechanisms related to specific functions of ROS in carcinogenesis have been revised by different authors [9,10,15,69]. In detail, eye cancer manifests itself as a tumor arising from uncontrolled growth of abnormal cells in/or around the eye. The eye cancer is a rare disease, the average annual incidence rate (new cases) in the U.S. is about 1/100,000 population and the estimated prevalence rate (total cases) is about 12/100,000 population [80]. Two types of cancers are observed, primary intraocular cancers that start in the tissues of eye and secondary intraocular cancers that start somewhere else in the body and spread or are metastasized to the eye. The second type of cancer is a more common type of cancer and is usually caused by breast and lung cancers. Primary eye cancers can develop in the eyeball (an intraocular cancer), the orbit or in the adnexal structures like the ducts and the eyelid. The most common type of primary intraocular cancer that starts in the eyeball is melanoma. Melanomas develop more frequently in white than black people. This may be due to higher sensitivity to sunlight. The suggested risk factors for eye melanoma include eye color, large number of moles on the skin, too much exposure to sunlight and exposure to UV radiation. Some researchers have observed that people with blue, green or grey eyes develop eye melanomas more frequently compared to those with brown eyes [81]. This type of cancer starts in the pigment producing cells called melanocytes (biomolecules producing the dark-coloured pigment melanin) usually in the uveal tract. The tract contains the choroid, iris and ciliary body; they are coloured with melanin. They protect against too much irradiation. For example, the choroid prevents light reflection, and the iris controls the amount of light entering the eye. The second type of primary intraocular cancer is retinoblastoma. This cancer develops in the light-sensitive tissue – retina, a nervous tissue. Retinoblastoma is a rare pediatric cancer of the eye that accounts for about 3% of cancer in children younger than 15 years. For example, in the UK about 45 children develop retinoblastoma in early life. It is estimated that 40% of retinoblastoma is due to an inherited faulty gene [82]. Using in vitro models, scientists have demonstrated that levels of ROS and expression of NF-κβ and p53 are higher in carcinoma tissue than in normal tissue [83]. In addition, there is large epidemiological and laboratory evidence of inverse correlation between the concentration of antioxidants present in tissues and cancer. For example, Vandhana, et al. [84], carried out an experimental study that analyzed expression of genes responsive on OS in cultured non-neoplastic retinoblastoma cells and retinoblastoma tumor tissues, under the influence of H2O2. They found a higher concentration of ROS and minimal expression of antioxidant genes in the tumor tissues with invasion of choroid, optic nerve and retinal pigment epithelium than in those with choroidal cancer only. These findings may suggest involvement of redox signaling pathways in the pathogenesis of retinoblastoma. Another cancer that can affect the eye is lymphoma. This cancer begins in immune system cells (lymphocytes) of individuals who have problems with their immune system, for example, individuals suffering from HIV, AIDS, or auto-immune disease [85].
OS is implicated in the pathogenesis of cancer mainly through excessive production of ROS and deficiency of antioxidant enzymes and non-enzymatic antioxidants, conducting to disruption of signaling pathways and gene expression [69]. For that reason, it is important to note that lutein and its isomer zeaxanthin, being members of the carotenoids group, are present in the lens and macula of retina and prevent against free radical damage. Their mode of action includes a screening of highly energetic blue light and scavenging/inhibiting of ROS arising during light irradiation [86]. Research findings have shown that dietary intake of lutein significantly reduces eye diseases, such as AMD and cataracts. Mode of action of dietary antioxidants on several types of cancer has been recently listed by Valluru, et al. [83]. It is worth noting that both, ROS and antioxidants are implicated in cancer therapy and prevention [39]. Examples of the ROS application in cancer therapy are chemotherapy and radiation therapy [51,57, 59,65,66]. Usage of ROS in cancer therapy involves the production of free radicals to cause damage of cancer cells and their necrosis. In turn, usage of antioxidants in chemotherapy helps protect normal tissues against damage from cytokines generated by ROS. Unfortunately, radiation therapy based on usage of X- and γ-rays to treat cancer includes ROS formation that kills tumor cells with simultaneous threat of the integrity and survival of the surrounding normal cells [87]. Usage of combinations of antioxidants with radiation or chemotherapy was reported to increase ROS levels within tumor, and also to reduce toxicity in the normal cells and to increase survival time [88]. In order to reduce the risk of free radical damage of surrounding normal cells during cancer chemotherapy and to improve the distribution of the drug in the exact target sites, bioactive nanoparticles (e.g. nanoparticles, nanofibers, nanocapsules, nanotubes and others) have been projected as nanoplatforms for delivery of the drug [89].
Bio-oxidative therapy based on recognized molecular pathophysiology could produce adaptation of cells to OS. This action includes the increased generation of antioxidants (SOD, GSH) and the increase of the cells’ resistance against the ROS driven reactions. These benefits are suggested to result from low level of ROS formed in systemic via through diverse modalities that is able to induce the expression of antioxidant enzymes and other protective systems [90]. The molecular mechanism involves up-regulation of the NF-κβ transcription factor (for the mechanism of protection against OS on the NF-κβ signaling way. The next important property of bioxidative therapy activity is an improvement in immune function by its positive effect on monocytes, neutrophils, lymphocytes and eosinophil and also can decrease the concentration of pro-inflammatory markers, such as IL-6, TNF-α, and C-Reactive Protein (CRP) [91].
In a particular way, ozone by rectal insufflation application in retinitis pigmentosa has been evaluated in Cuba and demonstrated its effectiveness even with protective effects in several organs [92]. Another possible alternative is the regular practice of exercise, similar to bioxidative therapy could generate an increase in antioxidant response which reduced oxidant tone enough to reduce BMI. Physical exercise actions has e dual role: protection against the ROS damage by regular-moderate physical activity and damaging effect through mediation of OS by endurance exercise without adaptable physical training [52]. Otherwise, OS is related to the eye diseases as confirmed by several animal and human studies, future research is encouraged in order to elucidate the detailed mechanisms of cellular processes linked to ROS and participating in the eye diseases etiology and prevention, to evaluate efficacy and safety of particular modulated strategies, as well as the specific bio-oxidative therapy enhancing the antioxidant system and effecting adaptation of cells to OS.
Conclusions
There are evidences of the role of OS in eye diseases development, including RP. Some interventions as bioxidative therapy, exercises and ingestion of the natural antioxidants target the redox sensitive pathways and transcription factors contributing to adaptation and stress resistance. Due to common agreement that RP is caused by genetic bases, this could start from oxidative damage to DNA with consequent alteration of the gene expression. It is important to promote the healthy life style in order to prevent OS.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This work was partially supported by the Ministry of Public Health, Republic of Cuba (Project No. 2003009). Author thanks Dr. Mark Weiser from Ozone therapy group, California, USA for its critical reading and the grammatical correction of the manuscript.
- Sies H (2018) On the history of oxidative stress: Concept and some aspects of current development. Current Opinion in Toxicology 7: 122-126. Link: https://bit.ly/37MUKbL
- Jensen SJK (2003) Oxidative stress and free radicals. J Mol Struct 666-667: 387-392.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44-84. Link: https://bit.ly/2AAj04J
- Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46: 531-542. Link: https://bit.ly/2BhrJsc
- Durackova Z (2010) Some current insights into oxidative stress. Physiol Res 59: 459-469. Link: https://bit.ly/2UTpm5V
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1-40. Link: https://bit.ly/2N71vvq
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49: 1603-1616. Link: https://bit.ly/2Y7ddMD
- Serra JA, Dominguez RO, Marschoff ER, Guareschi EM, Famulari AL, et al. (2009) Systemic oxidative stress associated with the neurological diseases of aging. Neurochem Res 34: 2122-2132. Link: https://bit.ly/2UM0UDB
- Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38: 96-109. Link: https://bit.ly/3da2QMg
- Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44: 479-496. Link: https://bit.ly/30SmUAq
- Odutan OA, Mashige KP (2011) A review of the role of oxidative stress in the pathogenesis of eye diseases. S Afr Optom 70: 191-199. Link: https://bit.ly/3hzaGmj
- Rios-Arrabal S, Artacho-Cordon F, Leon J, Roman-Marinetto E, Del Mar Salinas-Asensio M, et al. (2013) Involvement of free radicals in breast cancer. Springerplus 2: 404. Link: https://bit.ly/2AGHHw4
- Sies H, Berndt C, Jones DP (2017) Oxidative Stress. Annu Rev Biochem 86: 715-748. Link: https://bit.ly/2Y8B7aH
- Kruk J, Duchnik E (2014) Oxidative stress and skin diseases: possible role of physical activity. Asian Pac J Cancer Prev 15: 561-568. Link: https://bit.ly/2Y8tK2X
- Nourazarian AR, Kangari P, Salmaninejad A (2014) Roles of oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev 15: 4745-4751. Link: https://bit.ly/2Bk2CoD
- McLeay Y, Stannard S, Houltham S, Starck C (2017) Dietary thiols in exercise: oxidative stress defence, exercise performance, and adaptation. J Int Soc Sports Nutr 14: 12. Link: https://bit.ly/3d7L6Bl
- WHO (2018) Visual impairment and blindness, Updated September 2018. In.: WHO Media Centre. Link:
- Nicco C, Batteux F (2017) ROS Modulator Molecules with Therapeutic Potential in Cancers Treatments. Molecules 23: 84. Link: https://bit.ly/37zR78G
- Chiste RC, Freitas M, Mercadante AZ, Fernandes E (2015) Superoxide Anion Radical: Generation and Detection in Cellular and Non-Cellular Systems. Curr Med Chem 22: 4234-4256. Link: https://bit.ly/3fzVDai
- Ferrer-Sueta G, Campolo N, Trujillo M, Bartesaghi S, Carballal S, et al. (2018) Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem Rev 118: 1338-1408. Link: https://bit.ly/2UQZ6Js
- Liu C, Chen W, Qing Z, Zheng J, Xiao Y, et al. (2016) In Vivo Lighted Fluorescence via Fenton Reaction: Approach for Imaging of Hydrogen Peroxide in Living Systems. Anal Chem 88: 3998-4003. Link: https://bit.ly/30M3wFn
- Ems T, Huecker MR (2019) Biochemistry, Iron Absorption. In: StatPearls. edn. Treasure Island.
- Leser M, Chapman JR, Khine M, Pegan J, Law M, et al. (2019) Chemical Generation of Hydroxyl Radical for Oxidative 'Footprinting'. Protein Pept Lett 26: 61-69. Link: https://bit.ly/2Y8G1EP
- Maiorino M, Conrad M, Ursini F (2018) GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal 29: 61-74. Link: https://bit.ly/2UP1Ir6
- Ahmad R, Ahsan H (2019) Singlet oxygen species and systemic lupus erythematosus: a brief review. J Immunoassay Immunochem 40: 343-349. Link: https://bit.ly/3fuNzYc
- Ahmed Laskar A, Younus H (2019) Aldehyde toxicity and metabolism: the role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab Rev 51: 42-64. Link: https://bit.ly/37ChScE
- Suzuki T, Yamamoto M (2017) Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem 292: 16817-16824.
- Herbein G, Varin A, Fulop T (2006) NF-kappaB, AP-1, Zinc-deficiency and aging. Biogerontology 7: 409-419. Link: https://bit.ly/3eb98wy
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52: 601-623. Link: https://bit.ly/2YFqhIp
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, et al. (2016) ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev 2016: 4350965. Link: https://bit.ly/2Y60Al0
- Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2: 219-236. Link: https://bit.ly/2UTMEZz
- Neha K, Haider MR, Pathak A, Yar MS (2019) Medicinal prospects of antioxidants: A review. Eur J Med Chem 178: 687-704. Link: https://bit.ly/2URp9jK
- Enns GM, Cowan TM (2017) Glutathione as a Redox Biomarker in Mitochondrial Disease-Implications for Therapy. J Clin Med 6: 50. Link: https://bit.ly/2CcDFvT
- Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, et al. (2018) Mitochondria: Central Organelles for Melatonin's Antioxidant and Anti-Aging Actions. Molecules 23: 509. Link: https://bit.ly/37BeG0Z
- Tsuji M, Tamai Y, Wada K, Nakamura K, Hayashi M, et al. (2012) Associations of intakes of fat, dietary fiber, soy isoflavones, and alcohol with levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Causes Control 23: 683-689. Link: https://bit.ly/3fuqipg
- Alissa EM, Ferns GA (2015) Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr 57: 1950-1962. Link: https://bit.ly/3hDNV0n
- Peng C, Luo WP, Zhang CX (2017) Fruit and vegetable intake and breast cancer prognosis: a meta-analysis of prospective cohort studies. Br J Nutr 117: 737-749. Link: https://bit.ly/3daYTqI
- Elvira-Torales LI, Garcia-Alonso J, Periago-Caston MJ (2019) Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants (Basel) 8: 229. Link: https://bit.ly/3hAGjfm
- Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2017) Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174: 1290-1324. Link: https://bit.ly/2Y9m68t
- Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, et al. (2019) Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 25: 63. Link: https://bit.ly/3eb7tXM
- Russo GL, Spagnuolo C, Russo M, Tedesco I, Moccia S, et al. (2019) Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem Pharmacol 2019: 113719.
- Suganuma M, Saha A, Fujiki H (2011) New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci 102: 317-323. Link: https://bit.ly/2YGHwcn
- Fujiki H, Watanabe T, Sueoka E, Rawangkan A, Suganuma M (2018) Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: from Early Investigations to Current Focus on Human Cancer Stem Cells. Mol Cells 41: 73-82. Link: https://bit.ly/2Bhoz7O
- Patel S, Mathan JJ, Vaghefi E, Braakhuis AJ (2015) The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 253: 1841-1850. Link: https://bit.ly/2YDqls2
- Ribeiro D, Freitas M, Silva AMS, Carvalho F, Fernandes E (2018) Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem Toxicol 120: 681-699. Link: https://bit.ly/3hCt79y
- Iida A, Nosaka N, Yumoto T, Knaup E, Naito H, et al. (2016) The Clinical Application of Hydrogen as a Medical Treatment. Acta Med Okayama 70: 331-337. Link: https://bit.ly/2ULXCAf
- Ohta S (2012) Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta 1820: 586-594. Link: https://bit.ly/30VFXtT
- Cejka C, Kubinova S, Cejkova J (2019) The preventive and therapeutic effects of molecular hydrogen in ocular diseases and injuries where oxidative stress is involved. Free Radic Res 53: 237-247. Link: https://bit.ly/3ed7bQu
- Dogru M, Kojima T, Simsek C, Tsubota K (2018) Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest Ophthalmol Vis Sci 59: DES163-DES168. Link: https://bit.ly/3egfD1q
- Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, et al. (2017) Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci 131: 2865-2883. Link: https://bit.ly/3ddpN17
- Tangvarasittichai O, Tangvarasittichai S (2018) Oxidative Stress, Ocular Disease and Diabetes Retinopathy. Curr Pharm Des 24: 4726-4741. Link: https://bit.ly/2CfIl4h
- Kruk J, Kubasik-Kladna K, Aboul-Enein HY (2015) The Role Oxidative Stress in the Pathogenesis of Eye Diseases: Current Status and a Dual Role of Physical Activity. Mini Rev Med Chem 16: 241-257. Link: https://bit.ly/3eb6M0C
- Hashimoto H, Arai K, Takahashi J, Chikuda M (2019) Effects of astaxanthin on VEGF level and antioxidation in human aqueous humor: difference by sex. J Clin Biochem Nutr 65: 47-51. Link: https://bit.ly/30M5vJL
- Ivanov IV, Mappes T, Schaupp P, Lappe C, Wahl S (2018) Ultraviolet radiation oxidative stress affects eye health. J Biophotonics 11: e201700377. Link: https://bit.ly/2AIk06K
- Gil-del-Valle L, Gravier-Hernández R, Delgado-Roche L, León-Fernández O (2015) Oxidative Stress in the Aging Process: Fundamental Aspects and New Insights. In: Oxidative Stress: Diagnostics, Prevention, and Therapy. edn. Edited by al. He: American Chemical Society 177–219. Link: https://bit.ly/2USnk67
- Liu XF, Hao JL, Xie T, Malik TH, Lu CB, et al. (2017) Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 16: 934-942. Link: https://bit.ly/2YM40Zg
- Canto A, Olivar T, Romero FJ, Miranda M (2019) Nitrosative Stress in Retinal Pathologies: Review. Antioxidants (Basel) 8: 543. Link: https://bit.ly/3fw1bCp
- Tang B, Li S, Cao W, Sun X (2019) The Association of Oxidative Stress Status with Open-Angle Glaucoma and Exfoliation Glaucoma: A Systematic Review and Meta-Analysis. J Ophthalmol 2019: 1803619. Link: https://bit.ly/3hDjQOs
- Sacca SC, Corazza P, Gandolfi S, Ferrari D, Sukkar S, et al. (2019) Substances of Interest That Support Glaucoma Therapy. Nutrients 11: 239. Link: https://bit.ly/2N61uHW
- Melo LGN, Morales PH, Drummond KRG, Santos DC, Pizarro MH, et al. (2018) Current epidemiology of diabetic retinopathy in patients with type 1 diabetes: a national multicenter study in Brazil. BMC Public Health 18: 989. Link: https://bit.ly/2USXwXv
- McMonnies C (2018) Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J Optom 11: 3-9. Link: https://bit.ly/3hyh9xL
- Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007: 43603. Link: https://bit.ly/2Ca3Ovf
- Komeima K, Rogers BS, Campochiaro PA (2007) Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol 213: 809-815. Link: https://bit.ly/2N4rx2b
- Narayan DS, Wood JP, Chidlow G, Casson RJ (2016) A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol 94: 748-754. Link: https://bit.ly/3fBpYVT
- Yumnamcha T, Devi TS, Singh LP (2019) Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front Neurosci 13: 1065. Link: https://bit.ly/2Y6G3wE
- Donato L, Scimone C, Nicocia G, D'Angelo R, Sidoti A (2019) Role of oxidative stress in Retinitis pigmentosa: new involved pathways by an RNA-Seq analysis. Cell Cycle 18: 84-104. Link: https://bit.ly/2YL3afx
- Al-Zamil WM, Yassin SA (2017) Recent developments in age-related macular degeneration: a review. Clin Interv Aging 12: 1313-1330. Link: https://bit.ly/30MXVyw
- Nita M, Grzybowski A (2017) Smoking and Eye Pathologies. A Systemic Review. Part II. Retina Diseases, Uveitis, Optic Neuropathies, Thyroid-Associated Orbitopathy. Curr Pharm Des 23: 639-654. Link: https://bit.ly/37IhUQj
- Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, et al. (2018) Role of ROS and Nutritional Antioxidants in Human Diseases. Front Physiol 9: 477. Link: https://bit.ly/2ULVDvN
- Singh N, Srinivasan S, Muralidharan V, Roy R, Raman R (2017) Prevention of Age-Related Macular Degeneration. Asia Pac J Ophthalmol (Phila) 6: 520-526. Link: https://bit.ly/3eaC2gq
- Cheung N, Shankar A, Klein R, Folsom AR, Couper DJ, et al. (2007) Atherosclerosis Risk in Communities Study I: Age-related macular degeneration and cancer mortality in the atherosclerosis risk in communities study. Arch Ophthalmol 125: 1241-1247. Link: https://bit.ly/2UR2l3o
- Baudouin C, Irkec M, Messmer EM, Benitez-Del-Castillo JM, Bonini S, Figueiredo FC, et al. (2018) Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol 96: 111-119. Link: https://bit.ly/3d96Ywf
- Seen S, Tong L (2018) Dry eye disease and oxidative stress. Acta Ophthalmol 96: e412-e420. Link: https://bit.ly/3d8jOuO
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, et al. (2016) Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev 2016: 7432797. Link: https://bit.ly/3fvBge7
- Rhee MK, Mah FS (2017) Inflammation in Dry Eye Disease: How Do We Break the Cycle? Ophthalmology 124: S14-S19. Link: https://bit.ly/3fuk5tt
- Pinazo-Duran MD, Gallego-Pinazo R, Garcia-Medina JJ, Zanon-Moreno V, Nucci C, et al. (2014) Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging 9: 637-652. Link: https://bit.ly/3d4sK45
- Ibrahim OM, Dogru M, Matsumoto Y, Igarashi A, Kojima T, et al. (2014) Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS One 9: e99328. Link: https://bit.ly/2AAk8oZ
- Cabello-Verrugio C, Simon F, Trollet C, Santibanez JF (2017) Oxidative Stress in Disease and Aging: Mechanisms and Therapies 2016. Oxid Med Cell Longev 2017: 4310469. Link: https://bit.ly/2USlOkr
- Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, et al. (2018) Chronic diseases, inflammation, and spices: how are they linked? J Transl Med 16: 14. Link: https://bit.ly/3hz4RVZ
- Kleinstein RN, Lehman HF (1977) Incidence and prevalence of eye cancer. Am J Optom Physiol Opt 54: 49-51. Link: https://bit.ly/3fujMyP
- Patel DR, Patel BC (2019) Cancer, Ocular Melanoma. In: StatPearls. edn. Treasure Island.
- Ortiz MV, Dunkel IJ (2016) Retinoblastoma. J Child Neurol 31: 227-236. Link: https://bit.ly/3fznAyC
- Valluru L, Dasari S, Wudayagiri R (2014) Role of free radicals and antioxidants in gynecological cancers: current status and future prospects. Oxid Antioxid Med Sci 3: 15-26. Link: https://bit.ly/3eayWsH
- Vandhana S, Lakshmi TS, Indra D, Deepa PR, Krishnakumar S (2012) Microarray analysis and biochemical correlations of oxidative stress responsive genes in retinoblastoma. Curr Eye Res 37: 830-841. Link: https://bit.ly/3d4simp
- Maheshwari A, Finger PT (2018) Cancers of the eye. Cancer Metastasis Rev 37: 677-690. Link: https://bit.ly/3frDKKo
- Mares J (2016) Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu Rev Nutr 36: 571-602. Link: https://bit.ly/3d7FR4D
- Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, et al. (2018) Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin Transl Oncol 20: 975-988. Link: https://bit.ly/3ebqw4f
- Kma L (2013) Synergistic effect of resveratrol and radiotherapy in control of cancers. Asian Pac J Cancer Prev 14: 6197-6208. Link: https://bit.ly/2YGsvr0
- Riley RS, June CH, Langer R, Mitchell MJ (2019) Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 18: 175-196. Link: https://bit.ly/2Y9v5Xt
- Couroucli XL (2018) Oxidative stress in the retina: Implications for retinopathy of prematurity. Current Opinion in Toxicology 7: 102-109. Link: https://bit.ly/3eaAKlA
- Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, et al. (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6: 183-197. Link: https://bit.ly/2N1hivI
- Copello M, Menéndez S, Hernández F (2012) Ozone Therapy in Retinitis Pigmentosa Patients: Clinical Evolution and Oxidative Stress Behavior in Retinitis Pigmentosa Patients Treated with Ozone Therapy over 20 Years. Ozone: Science Engineering 34: 476-483. Link: https://bit.ly/3ftQ2C8

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
