Journal of Clinical Research and Ophthalmology
Usefulness of Intravitreal dexamethasone implant in treatment of persistent cystoid macular edema due to Irvine–Gass syndrome
Mya Thida Ohn*, Thompson E, Waghmare A, Chandra A and Karia N
Cite this as
Ohn MT, Thompson E, Waghmare A, Chandra A, Karia N (2019) Usefulness of Intravitreal dexamethasone implant in treatment of persistent cystoid macular edema due to Irvine–Gass syndrome. J Clin Res Ophthalmol 6(1): 001-006. DOI: 10.17352/2455-1414.000055Introduction: Pseudophakic cystoid macular edema (CME) is one of the commonest causes of visual impairment after uneventful cataract surgery. There is no standardized protocol for pseudophakic CME and cases with refractory or persistent CME remained therapeutic challenge.
Purpose: To investigate outcomes of intravitreal dexamethasone implant for the treatment of pseudophakic cystoid macular edema (CME) not responding to topical therapy or periocular steroids.
Method: Retrospective, single-practice data analysis from an electronic medical record system of 30 eyes (30 patients) with persistent pseudophakic CME receiving intravitreal dexamethasone implants were studied. Patients with history of uveitis, diabetes, previous intraocular surgery, complicated cataract surgery, retinal vascular diseases and macular telangiectasia (MACTEL) were excluded. Full demographic information including age, gender was entered. The cystoid macular edema (CME) was assessed by clinical examination and optical coherence tomography (OCT) scan.
Results: Mean age was 73.70 years ranged from 54 to 85 with 40% male and 60%female. All cases had routine phacoemulsification-IOL and received at least 4 weeks treatment with topical steroid with or without topical NSAIDs after been diagnosed with Irvine-Gass syndrome. Mean duration of CME before receiving the first implant was 22.87 weeks. 13.33% also had history of periocular steroid therapy.
Mean best corrected visual acuity (BCVA) improved from 57.4 ETDRS letters to 68.23 ETDRS letters on week 4-8 and 67.87 letters on week 52. Mean central subfield thickness (CST) at fovea improved from 469.67um to 269um on week 52. 46.67% had complete resolution of CME after the first implant and patients were able to be discharged on weeks 52. 53.33% had refractory CME within six months after the dexamethasone implant. Re-treatment was given when visual acuity reduced to 6/12 or worse. Out of 30 cases, 26.67% required two implants and 10% required three on week 52.
Conclusion: This study concluded that intravitreal dexamethasone implant is useful and safe as a therapy of persistent pseudophakic CME. Elevated intraocular pressure was found in 13.33% and only half of those required topical antiglaucoma therapy.
Introduction
Irvine-Gass syndrome, pseudophakic cystoid macular edema (PCME) is one of the most common causes of vision impairment after the cataract surgery [1,2]. Small incision cataract surgery and phacoemulsification reduce the risk of PCME, however number of cataract surgery performed each year is significant and PCME is still a commonly encountered morbidity [2].
Pseudophakic CME, Irvine-Gass syndrome was first reported by A. Ray Irvine Jr., MD in 1953 and fluorescein angiographic diagnosis was first made by J. Donald M. Gass, MD, in 1969 [3,4].
Diagnosis of Irvine-Gass syndrome can either be through clinical examination, fluorescein angiogram or by optical coherence tomography. Of which, optical coherence tomography has the highest sensitivity, followed by angiography and then clinical examinations. The incidence of PCME measured by OCT and fluorescein angiogram after uneventful cataract surgery can be as high as 41 and 30 percent, respectively [5,6]. Presence of PCME is not always directly related to visual acuity. In the past, the condition was diagnosed by clinical examination and reduce in visual acuity and the incidence was 1 to 2 percent [7]. Development of OCT technology brings the incidence of PCME with reduced vision up to 14 percent [8,9].
There is no standardized protocol for Prophylaxis and Treatment of PCME. Lack of large studies and strong randomized clinical trials could be due to the possible outcome of spontaneous resolution of PCME. However, there has been persistent and recurrent cases which lead to chronic CME and the treatment remains a challenge [2].
Pseudophakic CME based on the pathogenesis of inflammatory cascade and its pathophysiology leads to treatment with Corticosteroids which reduce inflammation by inhibiting phospholipase A2, and nonsteroidal anti-inflammatory drugs (NSAIDs) by inhibiting COX [10]. A course of topical steroids and non-steroidal anti-inflammatory agents (NSAIDs) helps in the resolution of a large proportion of cases. However, a certain number of patients do not respond to topical therapy. A step ladder approach for the management for this group of patients includes oral carbonic anhydrase inhibitor therapy, peribulbar or intravitreal steroids, anti-VEGF agents, immunomodulators, and, in cases secondary to vitreous traction, Nd: Yag laser vitreolysis or pars plana vitrectomy are used. Prophylaxis is suggested for those with diabetes and uveitic cataract, in which spontaneous resolution of CME is unlikely [11].
Intravitreal implant containing 0.7 mg (700 mcg) of dexamethasone comes under the name of OZURDEX has been introduced as a product of Allergan. It is a preloaded single-use rod-shaped solid polymer with sustained-released drug delivery system that can be directly implanted into the vitreous cavity and it leaves no residue in the eye. It delivers dexamethasone inside the vitreous for six months with peak around day 60 and has returned to baseline by month six [12]. Intravitreal dexamethasone implant, OZURDEX is effectively used in diabetic macular edema [13], macular edema due to retinal vein occlusion [14] and non-infectious uveitis [12]. OZURDEX is said to be particularly effective in vitrectomized eyes [12], however it can also have high efficacy with safety in eyes with persistent pseudophakic CME in which patients have continuous visual symptoms due to cystoid macular changes which is not responding to topical therapy.
The TNF-alpha, pro-inflammatory cytokine tumor necrosis factor-alpha plays an important role in mucosal inflammation and is a key mediator in the inflammatory cascade. Infliximab, a monoclonal antibody against TNF-alpha has shown high efficacy in controlling inflammatory process [15]. Regression of Type 2 diabetic related Sight-Threatening Macular Edema Following Treatment with Infliximab has been reported [16] and the therapy is also found to be effective in treating non-infectious uveitis [17].
Objectives
The objective of the study was to investigate visual and anatomical outcomes of intravitreal dexamethasone implant for the treatment of post-operative cystoid macular oedema (PCME) not responding to topical therapy and/or periocular steroids.
Operational definitions
Pseudophakic cystoid macular edema: PCME was diagnosed by presence of intraretinal fluid or cystoid macular changes at Optical Coherence Tomography (OCT).
Materials and Methods
We retrospectively reviewed the clinical records of 46 eyes of 42 patients by using an electronic medical record system at Southend and Orsett Hospitals. 30 eyes of 30 patients were eligible for the study. Patients were diagnosed with post-operative or pseudophakic cystoid macular edema (PCME), Irvine-Gass syndrome. Patients had routine phacoemulsification and intraocular lens implant four to twelve weeks before they presented with PCME and patients had symptoms with blurred vision and the visual acuities were 6/12 or less. Diagnosis was made by dilated fundus examination and Heidelberg Spectralis Optical Coherence Tomography (OCT). After the diagnosis of PCME, patients received topical treatment with steroids, Pred Forte 1% four time daily for four weeks or Dexamethasone 1% four times daily and NSAID (Acular) three times daily for at-least four weeks. Review was made four to eight weeks after starting topical treatment and best corrected visual acuity (BCVA), dilated fundus examination and OCT were performed at each visit. Patients who had no significant improvement in their visual symptoms, visual acuity 6/12 or worse with persistent PCME at OCT after using the topical treatment for at least 4 weeks regardless of their good compliance received intravitreal dexamethasone implants. PCME was identified by presence of intraretinal fluid at OCT. Some patients received repeated topical treatment longer than four weeks and some received periocular steroids but no resolution of PCME was noted during their follow up. We identified those patients as non-responding group to topical and periocular therapies. Patients` OCT findings proved that patients persistently had CME to the date they received intravitreal dexamethasone implant. Patients received intravitreal dexamethasone implant 0.7mg in view of the underlying pathology involving inflammatory process of non-infectious aetiology.
Data collection procedure
Informed consent was obtained. BCVA was recorded by using ETDRS scoring method. Clinical examination, Intraocular Pressure (IOP) check by using Goldman Applanation Tonometry and measurement of Central Subfield Thickness (CST) by using OCT were made before starting therapy with intravitreal dexamethasone implant. BCVA, clinical examination, IOP measurement and OCT scan were repeated during follow up visits.
Sample selection
Inclusion Criteria: Patients with persistent pseudophakic CME who received intravitreal dexamethasone implants between January 2014 and December 2016 were included in the study.
Resolution, recurrence and re-treatment criteria: Resolution of cystoid macular oedema was defined as the complete disappearance of retinal cysts, retinal thickening, and intraretinal fluid (IRF) along with a restoration of the normal foveal contour and a reduction of central subfield thickness (CST) according to findings on OCT and clinical examination, associated with an improvement in BCVA. Recurrence was defined as the re-appearance of retinal thickening, cysts and intraretinal fluid after complete resolution of the pseudophakic CME with therapy associated with a reduce in BCVA to 6/12 or worse, with or without metamorphopsia.
Exclusion Criteria: Patients with other causes of cystoid macular oedema such as uveitis, diabetes, previous intraocular surgery except uncomplicated cataract surgery, complicated cataract surgery, retinal vascular diseases and MACTEL were excluded. Cases who had other co-morbidity, steroid responders and history of glaucoma or ocular hypertension were also excluded from the study. Duration of pseudophakic CME more than 52 weeks were also excluded.
Intravitreal dexamethasone implant injection: The conjunctival cul-de-sac was anesthetized by using minims proxymetacaine hydrochloride (0.5%) eye drops three times. A drop of povidone iodine (5%) was instilled in the cul-de-sac prior to the procedure. Subconjunctival Lignocaine 1% was given. The injection was carried out in the treatment room by a trained doctor using aseptic measures and a standardized surgical technique. The implant (0.7mg) was injected in the infero-temporal quadrant 3.5-4.0 mm from the limbus. A drop of minim chloramphenicol was instilled in the conjunctival cul-de-sac at the end of the procedure. No post-operative antibiotic or other eye drop was given to take home.
Postoperative assessments were performed 4- 8 weeks after the procedure then eight weekly up to 52 weeks, unless complications necessitated a more frequent follow-up. The BCVA, IOP, and a complete ocular examination along with the OCT scan were performed at each visit. The CST was measured at every follow up.
Outcome measures: Visual outcome was identified by letter gain by using ETDRS scoring system. Anatomical outcome was by taking measurement of central subfield thickness (CST) at fovea and presence or absence of IRF and/or cystic changes at OCT.
Data was analysed for the pre-treatment visit and review visits at 4 to 8 week, 24 weeks and 52 weeks after the first intravitreal dexamethasone implant for each patient. Recurrence of pseudophakic CME and complications were recorded. Patients who had recurrence of PCME and who were eligible for re-treatment according to criteria such as symptoms with blurred vision, BCVA 6/12 or less and OCT evidence of recurrent PCME underwent further treatment with intravitreal dexamethasone implant. Patients who had remission from PCME after the first implant were recorded. In group of patients who developed recurrent PCME, number of implants required within the duration of 52 weeks were also recorded.
Data analysis procedure: Data was entered to SPSS version 10.0 and analysed through it. Quantitative variables like age and CST were presented as mean and standard deviation. BCVA was in mean and medium. Qualitative variables of the study were gender and presence of PCME. Frequency and percentages were calculated for qualitative variables. Stratification with respect to age and gender was done on these variables. Post stratification chi-square test was applied. P value was considered significant at < 0.05.
Results
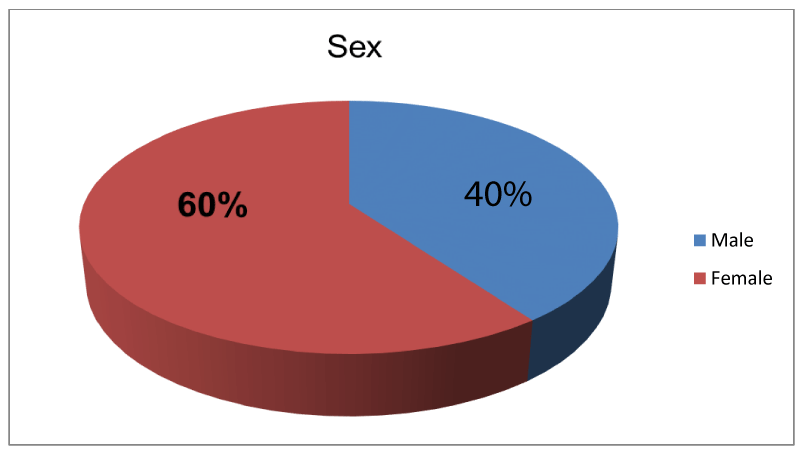
A total of 30 eyes of 30 patients were retrospectively recruited and were analysed. Mean age of this cohort of patients was 73.70 years ranged from 54 to 85 with 40% male and 60% female. (Figure 1) Male to female ratio was 1:1.5. Mean duration of pseudophakic CME before the first intravitreal dexamethasone implant was 22.87 weeks (Medium was 23) and 13.33% of cases previously received treatment with periocular steroid in addition to topical therapy.
Visual outcome
Mean best corrected visual acuity (BCVA) improved from 57.4 ETDRS letters to 68.23 ETDRS letters on week 4-8 and 67.87 letters on week 52 (P<0.0001).
Anatomical outcome
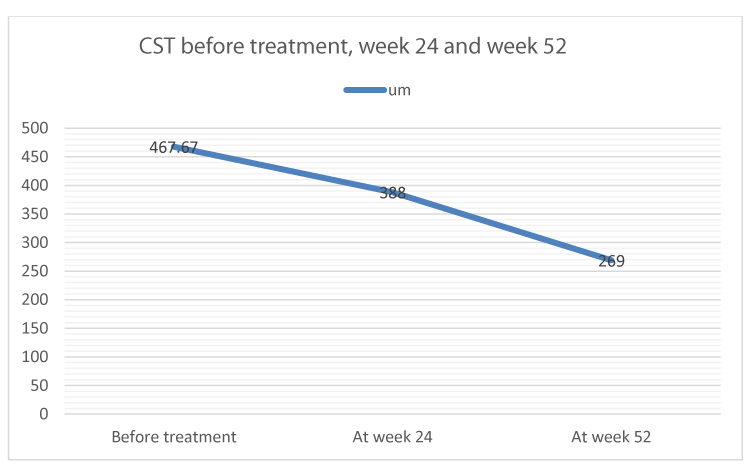
Mean central subfield thickness (CST) improved from 469.67um at baseline to 269um at week 52 (Figure 2).
Resolution, recurrence and re-treatment
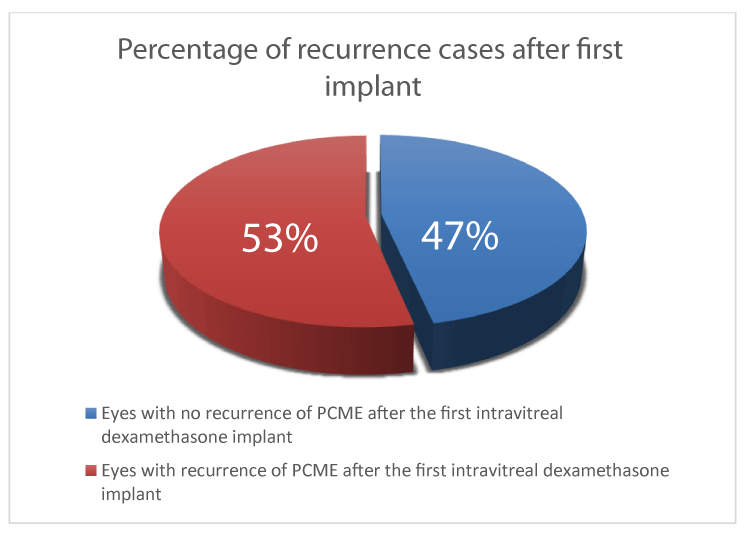
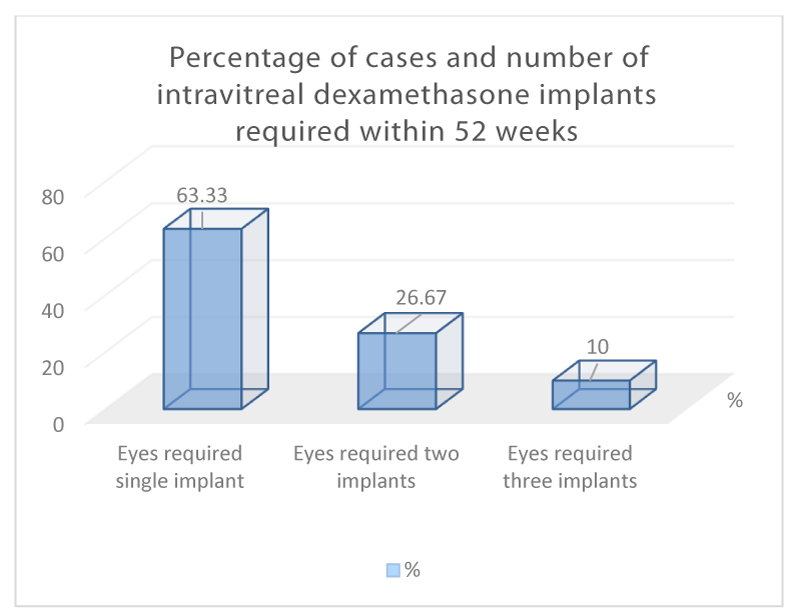
After the first intravitreal dexamethasone implant, 46.67% of eyes had resolution of pseudophakic CME as defined by the absence of intraretinal (IRF) or cystic changes on OCT without further recurrence and were able to discharge on week 52. 53.33% had recurrence of PCME within 24 weeks of their first treatment. (Figure 3) Re-treatment was given when visual acuity reduced to 6/12 or worse with presence of IRF on OCT. Overall, out of 30 cases, 26.67% required two intravitreal dexamethasone implants and 10% required three at week 52. (Figure 4) Mean CST at week 24 was 388um (P<0.0001).
Incidence and management of intraocular pressure (IOP) elevation
Four cases (13.33%) had IOP higher than 21mmHg of which two were between 22 to 30mmHg and the other two had IOP more than 30mmHg. Patients with postoperative IOP higher than 30mmHg received topical treatment to lower IOP and the intraocular pressures were controlled at week 52.
Other complications
No other complications related to intravitreal dexamethasone implant were noted during the study.
Discussion
Therapeutic treatment and interventions of pseudophakic CME are based on the proposed pathogenesis, mainly inflammation and vitreous traction [2]. NSAIDs are potent inhibitors of prostaglandins and are approved by the Food and Drug Administration for postoperative CME. Cyclooxygenase isoform COX-2 is the predominant isoform in the retinal pigment epithelium and NSAIDs inhibit cyclooxygenase isoforms COX-1 and COX-2 [18]. However, limited data is known about the long-term effects (>twelve months) of NSAIDs in treatment of persistent pseudophakic CME [19]. Combination therapy with topical NSAIDs and corticosteroid may appear superior to either individual therapy. There was a small, randomized control trial in 2000 compared topical NSAIDs to topical prednisolone to combination therapy for the treatment of pseudophakic CME [20].
For pseudophakic CME which has been refractory or not responding to topical therapy, periocular corticosteroids given sub-Tenon’s or subconjunctivally provides as sustained release therapy. Intravitreal triamcinolone acetonide, dexamethasone implant (Ozurdex, Allergan) and fluocinolone acetonide implant (Retisert, Bausch + Lomb) have also been used in this subset of patients [2]. However, strong reports or data are not known for the cases of pseudophakic CME. There has been reports on their efficacy in macular edema but the reports mainly concentrate on cases of diabetic or retinal vein occlusion. Side effects of periocular and intravitreal corticosteroids include endophthalmitis and elevated intraocular pressure.
Anti-VEGF with intravitreal bevacizumab (Avastin, Genetech) injection has been shown effective in treating refractory pseudophakic CME in view of suppressing the action of vascular endothelial growth factor [21]. Vascular endothelial growth factor causes breakdown of the blood-retinal barrier and contributing to the development of macular edema by increasing vascular permeability.
Carbonic anhydrase inhibitors (oral acetazolamide) have affect on fluid pumping across retinal pigment epithelial cells. They have been reported effective in treating macular edema in cases of retinitis pigmentosa and aphakia, but their efficacy in pseudophakic CME is unknown.
Surgical intervention is often the next step to lyse vitreous adhesions when medical therapy fails to resolve PCME. Removal of malpositioned intraocular lens [22], Nd:YAG laser and pars plana vitrectomy can be used. Release of vitreomacular traction is believed to allow resolution of pseudophakic CME and pars plana vitrectomy had improved visual acuity compared to controls [23]. Small pilot series reported as therapies which are effective for refractory pseudophakic CME include intravitreal infliximab (Remicade, Centocor Ortho Biotech) [24], intravitreal diclofenac 500μm/0.1 ml [11] and subcutaneous interferon alpha (Imgenex) [25].
There was a subgroup analysis in a Phase II study that investigated efficacy of intravitreal dexamethasone implant in the treatment of persistent macular edema resulting from uveitis or pseudophakic CME. This Phase II study reported an improvement in BCVA of at least 10 letters on day 90 in 42% in the 350ug group and 54% in the 700ug group [26].
Intravitreal dexamethasone implant in treatment of recurrent cystoid macular oedema (CME) due to Irvine–Gass syndrome was mentioned in a case series by Sudhalkar et al [27]. Case series of Sudhalkar et al included patients with pseudophakic CME previously resolved by topical therapy and periocular steroids. Their case series reported improved visual acuity after therapy with intravitreal dexamethasone implant and it also reported majority of patients required single implant within one year with very low recurrence rate of CME [27].
In our study, patients presented with persistent pseudophakic CME which did not respond to topical therapy or periocular steroids although they do not have other causes or risk factors that could develop cystoid changes on the macula. Intravitreal dexamethasone implant has shown effective to resolve persistent CME due to Irvine-Gass syndrome. Refractory CME after the first intravitreal dexamethasone implant was as high as 53.33%. Among all cases, 26.67% required 2 intravitreal dexamethasone implants and 10% required 3 within the period of 52 weeks. Two out of 30 cases required topical therapy to lower intraocular pressure.
Intravitreal triamcinolone injection using 8 mg has been reported to treat refractory chronic pseudophakic CME in a case series. Benhamou et al. reported the first study using 8 mg of intravitreal triamcinolone to treat 3 cases in 2003. The results showed improvement of visual acuity and macular thickness. However, the effects were transient [28]. There were other similar case serie reported by Conway et al. This case series treated PCME with 4 mg of intravitreal triamcinolone and it showed transient effect with subsequent requirement of multiple injections [29]. Other case series also reported transient improvement of visual acuity at 1 month by using 4 mg of intravitreal triamcinolone, however there was no sustained benefit [30-32]. Ahmadabadi et al. reported in a controlled study in 2010 by randomizing 41 diabetic eyes with moderate nonproliferative diabetic retinopathy randomized to routine phacoemulsification or that with intravitreal triamcinolone injection. This study showed the treatment group with intravitreal triamcinolone had better visual improvements at 1 month, however the visual benefits on month 6 were not significant [33].
Clearly, one of the major limitations of intravitreal triamcinolone injection is the transient effect that requires repeated injections. By using the sustained-released intravitreal dexamethasone implant, OZURDEX for treatment of persistent pseudophakic CME can overcome this limitation and achieve a sustained better visual outcome as well as the macular morphology with less frequent need for therapy which will definitely be more convenient for the patients.
Conclusion
Intravitreal dexamethasone implant 0.7mg administered in cases of persistent cystoid macular edema due to Irvine-Gass syndrome has improved visual acuity and macular morphology. After the first implant, patients with resolved CME without recurrence was identified in significant percentage of cases. In recurrent cases, subsequent therepy by using 2nd or 3rd intravitreal dexamethasone implant can be given. The results were favourable both in terms of safety and efficacy till the end of the follow-up period on week 52. This treatment can prevent visual consequences caused by chronic CME due to Irvine-Gass syndrome.
Summary
What was known before: Intravitreal dexamethasone implant 0.7mg has been found to be an effective therapy for vision impairment associated with recalcitrant cystoid macular oedema caused by Irvine-Gass syndrome in case reports and case series, considered insufficiently responsive to available therapies in a subset of patients.
What this study adds: In this study, efficacy findings confirm that the implant is a useful treatment option for cases with persistent cystoid macular edema due to Irvine-Gass syndrome. The implant is effective in eliminating persistent pseudophakic CME with a good safety profile in patients.
- Flach AJ (1998) The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 96: 557-634. Link: https://goo.gl/4jXRFo
- Yonekawa Y, Kim IK (2012) Pseudophakic cystoid macular edema. Curr Opin Ophthalmol 23: 26-32. Link: https://goo.gl/gpKgpx
- Irvine SR (1953) A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol 36: 599-619. Link: https://goo.gl/C7hPHL
- Gass JD, Norton EW (1969) Follow-up study of cystoid macular edema following cataract extraction. Trans Am Acad Ophthalmol Otolaryngol 73: 665-682. Link: https://goo.gl/fVLtfU
- Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG (2004) Macular alterations after small-incision cataract surgery. J Cataract Refract Surg 30: 752-760. Link: https://goo.gl/Wi59E9
- Flach AJ (1998) The incidence, pathogensis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 96: 557-634. Link: https://goo.gl/ZvWWYd
- Wright PL, Wilkinson CP, Balyeat HD (1988) Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol 106: 740-744. Link: https://goo.gl/96Ytk4
- Kim SJ, Belair ML, Bressler NM (2008) A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 28: 870-876. Link: https://goo.gl/JSa4P9
- Lally DR, Shah CP, Boston (2004) Pseudophakic CME remains a common cause of reduced vision after cataract surgery. A look at its causes and treatment. Link: https://goo.gl/qVCEqr
- Shelsta HN, Jampol LM (2011) Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina 31: 4-12. Link: https://goo.gl/8oCxdP
- Soheilian M, Karimi S, Ramezani A, Peyman GA (2010) Pilot study of intraviteal injection of diclofenac for treatment of macular edema of various etiologies. Retina 30: 509-515. Link: https://goo.gl/g5c55N
- Haghjou N, Soheilian M, Abdekhodaie MJ (2011) Sustained Release Intraocular Drug Delivery Devices for Treatment of Uveitis. J Ophthalmic Vis Res 6: 317–329. Link: https://goo.gl/Pwbrmf
- Mehta H, Gillies M, Fraser-Bell S (2015) Perspective on the role of Ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema. Ther Adv Chronic Dis 6: 234–245. Link: https://goo.gl/gGqhTD
- Justus G, Zandi S (2016) Retinal vein occlusion and the use of a dexamethasone intravitreal implant (Ozurdex®) in its treatment. Arch Clin Exp Ophthalmol 254: 1257-1265. Link: https://goo.gl/soGxka
- Danese S (2008) Mechanisms of action of infliximab in inflammatory bowel disease: an anti-inflammatory multitasker. Dig Liver Dis 40: S225-S228. Link: https://goo.gl/VhDs8u
- Sfikakis PP, Grigoropoulos V, Emfietzoglou I (2010) Infliximab for Diabetic Macular Edema Refractory to Laser Photocoagulation. Diabetes Care 33: 1523-1528. Link: https://goo.gl/We8VGr
- Ikewaki J, Kono H, Shinoda K (2010) Cystoid Macular Edema: Possible Complication of Infliximab Therapy in Behçet's Disease. Case Rep Ophthalmol 1:14-19. Link: https://goo.gl/TeZquq
- Chin MS, Nagineni CN, Hooper LC, Detrick B, Hooks JJ (2001) Cyclooxygenase-2 gene expression and regulation in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42: 2338-2346. Link: https://goo.gl/4Vefjb
- Flach AJ, Jampol LM, Weinberg D (1991) Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol 112: 514-519. Link: https://goo.gl/aYVgHU
- Heier JS, Topping TM, Baumann W, Dirks MS, Chern S (2000) Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 107: 2034-2039. Link: https://goo.gl/T1AJfN
- Arevalo JF, Maia M, Garcia-Amaris RA (2009) Pan-American Collaborative Retina Study Group (PACORES). Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: The Pan-American Collaborative Retina Study Group results. Ophthalmology 116: 1481-1487. Link: https://goo.gl/QwbMgx
- Shepard DD (1979) The fate of eyes from which intraocular lenses have been removed. Ophthalmic Surg 10: 58-60. Link: https://goo.gl/1YFqGg
- Fung WE, Vitrectomy-ACME Study Group (1985) Vitrectomy for chronic aphakic cystoid macular edema. Results of a national, collaborative, prospective, randomized investigation. Ophthalmology 92: 1102-1111. Link: https://goo.gl/ur9LXc
- Wu L, Arevalo JF, Hernandez-Bogantes E, Roca JA (2012) Intravitreal infliximab for refractory pseudophakic cystoid macular edema: Results of the pan-american collaborative retina study group. Int Ophthalmol 32: 235-243. Link: https://goo.gl/Nv6UvG
- Deuter CM, Gelisken F, Stubiger N (2011) Successful treatment of chronic pseudophakic macular edema (Irvine-Gass syndrome) with interferon alpha: A report of three cases. Ocul Immunol Inflamm 19: 216-218. Link: https://goo.gl/GH6jkP
- Williams GA, Haller JA, Kuppermann BD (2009) Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol 147:1048-1054. Link: https://goo.gl/HssJ2R
- Sudhalkar A, Chhablani J, Vasavada A (2016) Intravitreal dexamethasone implant for recurrent cystoid macular edema due to Irvine–Gass syndrome: a prospective case series 30: 1549-1557. Link: https://goo.gl/TPNi6R
- Benhamou N, Massin P, Haouchine B (2003) Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol 135: 246-249. Link: https://goo.gl/cCzJbu
- Conway MD, Canakis C, Livir-Rallatos C, Peyman GA (2003) Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg 29: 27-33. Link: https://goo.gl/HFn1eg
- Giganti M, Beer PM, Lemanski N (2010) Adverse events after intravitreal infliximab (Remicade). Retina 30: 71-80. Link: https://goo.gl/h1vHtJ
- Steinert RF, Wasson PJ (1989) Neodymium:YAG laser anterior vitreolysis for Irvine-Gass cystoid macular edema. J Cataract Refract Surg 15: 304-307. Link: https://goo.gl/vjkzau
- Katzen LE, Fleischman JA, Trokel S (1983) YAG laser treatment of cystoid macular edema. Am J Ophthalmol 95: 589-592. Link: https://goo.gl/ThAvP7
- Ahmadabadi HF, Mohammadi M, Beheshtnejad H, Mirshahi A (2010) Effect of intravitreal triamcinolone acetonide injection on central macular thickness in diabetic patients having phacoemulsification. J Cataract Refract Surg 36: 917-922. Link: https://goo.gl/XGLy9V

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
