International Journal of Vascular Surgery and Medicine
Incompetent Perforator Veins; To treat or not to treat
Thilina Gunawardena* and Nalaka Gunawansa
Cite this as
Gunawardena T, Gunawansa N (2019) Incompetent Perforator Veins; To treat or not to treat. Int J Vasc Surg Med 5(1): 001-004. DOI: 10.17352/2455-5452.000032The role of perforator vein incompetence (PVI) in the development of venous hypertension, chronic venous insufficiency (CVI) and ulceration has been well recognized for over a century. However, while minimally invasive endovenous ablative therapies have revolutionized the management of superficial truncal vein insufficiency, the definitive indications for intervention and management of PVI still remains somewhat debatable and unclear. This review will attempt to clarify the indications for intervention as well as look at the evidence for different methods of treatment in established PVI.
Introduction
Chronic Venous Insufficiency (CVI) and venous ulceration is a common health problem causing significant patient morbidity. Apart from the chronic physical and psychological disability caused to the individual, it also results in an enormous economic burden to the health care administration. Global prevalence rates of CVI are variable but may be as high as 40% among females and 17% among males [1]. The wide variation in global prevalence results from the wide variability in reporting, diagnosis and risk factors. Nevertheless, its morbidity and health care economic burden remain universal.
Homan in his landmark publication of 1917 described the pathophysiology of CVI caused by superficial and deep venous incompetence along with the importance of perforator incompetence in venous ulceration [2]. The importance of PVI in the manifestation of CVI and ulceration has since been well-recognized and widely studied. However, while the role of definitive management for junctional and truncal venous reflux in symptomatic CVI is well-established, the exact indications for management of PVI in isolation remains somewhat unclear.Pathophysiology of CVI
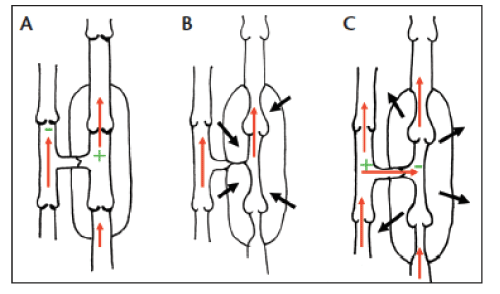
CVI is the result of lower extremity venous valvular incompetence causing inadequate venous return and eventual venous hypertension in the legs. While this may involve either the superficial venous system, deep venous system or both, the vast majority of such CVI results from junctional or truncal insufficiency of the superficial veins. Junctional reflux may occur at the sapheno-femoral junction or sapheno-popliteal junction while the truncal reflux may affect either the great saphenous vein (GSV) or small saphenous vein (SSV). Venous ulceration is the end result of CVI and chronic ambulatory venous hypertension causing overlying local tissue destruction (Figure 1).
Perforator veins traverse the deep fascia of the leg while forming communication channels between the superficial and deep venous systems. In physiology, perforator veins carry blood from the superficial to deep veins. Historically, these perforators have been classified according to their anatomical location, being named after their founders such as Hunter, Dodd, Boyd and Cockett. More recent studies by Mozes and colleagues have identified these perforator veins to be more anatomically complex than the original descriptions and thus have been categorized as direct and indirect [3]. Direct perforator veins are the classical perforators that connect superficial to deep veins while indirect perforators are more variable in their location and connect superficial veins to muscular veins.
Recent studies that attempted to define the anatomy of PVI have described that approximately 45% of all incompetent perforators are located within 10-15 cm of the medial malleolus and are located as medial perforators communication with the lower GSV [5]. It is postulated that once these perforator veins become incompetent, it results in reflux of blood from the deep veins to the superficial veins, contributing to superficial venous hypertension. Increased ambulatory venous pressure causes further incompetence of perforators resulting in a vicious cycle of events that result in CVI and ulceration. Stuart and colleagues (2000) demonstrated that the number and diameter of incompetent perforator veins was directly related to the eventual disease severity in CVI [6]. However, the role of PVI as a cause of CVI is not universally conclusive. Some authors have even described PVI as ‘normal’ allowing the blood from the refluxing superficial veins to return back to the superficial venous system (Figure 2) [7].
Historical description and evolution of treatment
In 1917, Homan described the role of PVI in the development of chronic venous ulceration and emphasized the importance of surgical disruption of such perforators [2]. Almost two decades later, Linton first described the medial fascial incision and perforator ligation as a means of managing PVI [8]. This technique remained the gold standard of managing PVI for almost half a century despite the associated morbidities including ulcer recurrence, wound breakdown and neuropathy. However, with the advent of newer less invasive treatment modalities, open surgical ligation has been largely abandoned.
Subfascial endoscopic perforator surgery (SEPS)
Hauer described the method of SEPS, which eventually displaced the open surgical perforator ligation due to significant reduction in operative morbidity and shorter hospital stay [9]. SEPS was shown to have success rates of up to 78% in closure of perforators during mid-term follow up. Tenbrook and colleagues performed a meta-analysis of 20 studies looking at the success of SEPS with or without concomitant superficial vein surgery [10]. They concluded that combination of SEPS resulted in ulcer healing rate of 88% overall with significant improvement in venous clinical severity scores. However, the same study also showed the associated adverse effects of SEPS including wound infection (06%), haematoma formation (09%), neuralgia (07%), and deep vein thrombosis (01%). These adverse effects along with the need for formal anaesthesia to perform SEPS prompted the search for a less invasive treatment modality for PVI.
More recent advances with image guided therapeutic maneuvers including endovenous ablative techniques have added to the surgeons’ armamentarium in managing PVI. With the advent of image guided catheter-based procedures, a minimally invasive treatment option was available to be performed under local anaesthesia. Percutaneous cannulation of perforators and catheter-based ablation using chemical or thermal ablation was able to achieve comparable success rates to SEPS with significantly less morbidity.
Ultra-sound guided foam sclerotherapy (UGFS)
UGFS uses sclerosant micro-foam injected in to the target perforators under direct ultra sound guidance. This remains the most widely used treatment modality for PVI. The advantages include relative ease in performing the procedure, minimal associated cost and non-requirement of tumescent anaesthetic infiltration. UGFS ablation of perforators has been described with reported successful closure rates up to 75% with associated improvement in venous clinical severity scores at 20 months [11].
Common reported side effects have been allergic reactions to the sclerosant, painful phlebitis and deep vein thrombosis. Although isolated case reports have shown potential of systemic embolization with associated transient visual disturbance and stroke, their incidence has been extremely rare compared to the number of procedures being performed throughout the world. Some case reports have also reported inadvertent injection to adjoining arteries with resultant skin necrosis [11]. However, with proper technical execution of the procedure with accurate ultra sound guidance, such adverse effects can be easily avoided.
Endo venous thermal ablation (EVTA)
EVTA has been performed for perforators using either RFA or EVLA. This involves a greater learning curve with the technology and higher costs of equipment. However, numerous studies have demonstrated higher perforator closure rates with the use of thermal ablation compared to UGFS [12]. The reported closure rates have been as high as 95% [13]. The obvious advantage over conventional surgical techniques and SEPS is the non-requirement for formal anaesthesia and the ability to be performed as day case or out-patient procedure. The reported short and mid-term closure rates have been excellent and thus it remains the current gold standard for treatment of PVI where the correct indication for treating incompetent perforators has been established.
Diagnosis
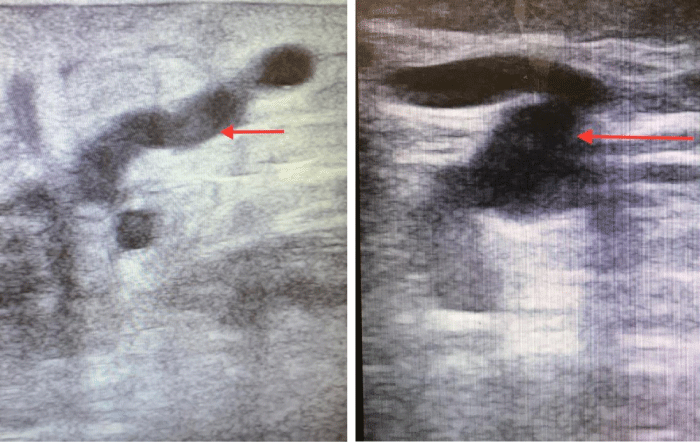
Duplex Ultra Sound (DUS) remains the gold standard in diagnosing CVI including PVI (Figure-2). However, a thorough understanding of the lower extremity venous anatomy is vital in detecting junctional, truncal and perforator incompetence by DUS. Unlike the main junctional and truncal reflux, DUS criteria in defining PVI are somewhat debatable and not well-defined. Nevertheless, there is growing consensus that perforators which are >4 mm in diameter and show reflux of >500 milliseconds upon calf compression should be categorized as incompetent [14,15]. Society for vascular surgery (SVS) guidelines define a ‘pathological’ perforator vein based on the anatomical location beneath an active or healed ulcer with a reflux lasting ≥ 500 milliseconds and a diameter ≥ 3.5 mm [16].
Indications for perforator ablation
Historically, some authors suggested that conventional treatment of the main truncal reflux (GSV or SSV) alone may suffice, causing the incompetent perforators to become competent or occlude in the absence of deep venous reflux. Stuart and colleagues demonstrated that successful surgery of truncal reflux resulted in a decline of incompetent perforators from 52% to 28% [17]. However, more recent studies have disproven this finding and showed that such ‘closure’ of the perforators following treatment of truncal reflux was transient and results in significantly higher rates of perforator recanalization [18]. This has been postulated as a major cause of ulcer recurrence following truncal venous ablation. Although some have argued that this recurrence is the result of undiagnosed deep venous reflux, Lafrati and colleagues demonstrated that such deep venous reflux had no direct correlation with ulcer healing or recurrence [19].
The role of perforator ablation in reducing ulcer recurrence has been demonstrated in several studies [20]. Ablation of all incompetent perforators was clearly linked to better healing rates and lower ulcer recurrence. Dillavou and colleagues (2016) demonstrated that simultaneous ablation of incompetent truncal and perforator veins results in significantly higher ulcer healing rates compared to ablation of truncal reflux alone [21,22].
Current guidelines by the American Venous Forum recommends treatment of incompetent perforators especially in Clinical, Etiological, Anatomical and Pathophysiological (CEAP) class 5 and 6 disease [23]. CEAP class-5 refers to PVI in the region of healed previous ulcer while CEAP class-6 refers to PVI in the region of an active ulcer.
Hager et al (2016) compared the current treatment modalities of radio-frequency ablation (RFA), endovenous laser ablation (EVLA) and ultra-sound guided foam sclerotherapy (UGFS) for isolated PVI in the absence of coexistent truncal reflux [13]. The results showed that RFA was the most reliable method of perforator closure at 1-year follow up. Thermal ablation with RFA or EVLA have been documented with significantly higher perforator closure rates at follow up compared to UGFS. Thermal perforator ablation has also been associated with significant improvement of venous clinical severity scores when performed in above discussed CEAP class-5 and 6 disease.
The case for perforator vein ablation in the absence of venous ulceration is less clear.
Although individual case series have implicated the possible role of persistent PVI in recurrence of symptomatic varicose veins after successful truncal ablation, there is no evidence from randomized controlled trials for this causative association [24]. Hence, at present, there is no conclusive evidence for the simultaneous ablation of incompetent perforators at the time of truncal vein ablation in the absence of active or past venous ulceration.
Conclusion
PVI is a well-recognized cause of CVI and resulting venous ulceration. It is often found co-existent with superficial or deep venous reflux and results in significant morbidity. In the absence of deep venous reflux, majority of such incompetent perforator disease is likely to improve with accurate ablation of superficial truncal veins. Although some studies have questioned this ‘spontaneous closure’ phenomenon, at present, there is no evidence for active ablation of perforators in CEAP class 1-4 disease. There is also no conclusive evidence that such remaining perforators contribute to recurrence of symptomatic superficial venous reflux. There has also been no benefit in the ablation of perforators in the presence of deep venous reflux in terms of ulcer healing or clinical symptomatic improvement. On the contrary, in patients with CEAP class-5 and class-6 disease with either a healed or active ulcer in relation to PVI, available evidence has shown that simultaneous ablation of such perforators results in significant reduction in ulcer recurrence and clinical improvement. Nevertheless, the common drawback in most studies pertaining to PVI is that isolated perforator incompetence in the absence of concomitant truncal reflux is difficult to define. Hence, better designed randomized trials looking at isolated PVI and its effect on CVI and ulceration should provide answers to many of the current controversies.
- Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D (2005) The Epidemiology of Chronic Venous Insufficiency and Varicose Veins. Ann Epidemiol 15: 175–184. Link: https://goo.gl/h5euY6
- Homan J (1917) The etiology and treatment of varicose ulcer of the leg. Surg Gynecol Obstet 24: 11.
- Mozes G, Gloviczki P, Menawat SS, Fisher DR, Carmichael SW, et al. (1996) Surgical anatomy for endoscopic subfascial division of perforating veins. J Vasc Surg 24: 800-808. Link: https://goo.gl/76qCws
- Fan C (2015) How I Decide to Ablate a Refluxing Perforator. Endovasc Today. Link: https://goo.gl/szvgRx
- O’Donnell TF (2010) The role of perforators in chronic venous insufficiency. Phlebology 25: 3-10. Link: https://goo.gl/pDeMMH
- Stuart WP, Adam DJ, Allan PL, Ruckley CV, Bradbury AW (2000) The relationship between the number, competence, and diameter of medial calf perforating veins and the clinical status in healthy subjects and patients with lower-limb venous disease. J Vasc Surg 32: 138-143. Link: https://goo.gl/o4PG8N
- Recek C (2016) Competent and incompetent calf perforators in primary varicose veins: a resistant myth. Phlebol J Venous Dis. 2016 31: 532-540. Link: https://goo.gl/RehxGm
- Linton RR (1938) The communicating veins of the lower leg and the operative technic for their ligation. Ann Surg 107: 582-593. Link: https://goo.gl/B2AzF7
- Hauer G (1985) Thr endoscopic subfascial division of the perforating veins - preliminary report (in German). Vasa - J Vasc Dis 14: 59-61. Link: https://goo.gl/qSygdw
- Ten Brook JA, Iafrati MD, O’Donnell TF, Wolf MP, Hoffman SN, et al. (2004) Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg 39: 583-589. Link: https://goo.gl/k7fcFJ
- Masuda EM, Kessler DM, Lurie F, Puggioni A, Kistner RL, et al. (2006) The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg 43: 551-557. Link: https://goo.gl/7RKR5a
- Kuyumcu G, Salazar GM, Prabhakar AM, Ganguli S (2016) Minimally invasive treatments for perforator vein insufficiency. Cardiovasc Diagn Ther 6: 593-598. Link: https://goo.gl/6z1kBr
- Hager ES, Washington C, Steinmetz A, Wu T, Singh M, et al. (2016) Factors that influence perforator vein closure rates using radiofrequency ablation, laser ablation, or foam sclerotherapy. J Vasc Surg Venous Lymphat Disord 4: 51-56. Link: https://goo.gl/wMrPxj
- Eidson JL, Bush RL (2010) Diagnosis and Current Management of Incompetent Perforator Veins. Semin Vasc Surg 23: 113-117. Link: https://goo.gl/EG42q6
- Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, et al. (2003) Definition of venous reflux in lower-extremity veins. J Vasc Surg 38: 793-798. Link: https://goo.gl/5R3WwB
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, et al. (2011) The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 53: 2S-48S. Link: https://goo.gl/2ZZMLm
- Stuart WP, Adam DJ, Allan PL, Ruckley CV, Bradbury AW (1998) Saphenous surgery does not correct perforator incompetence in the presence of deep venous reflux. J Vasc Surg 28: 834-838. Link: https://goo.gl/ZAMDcq
- Whiteley MS (2014) Part one: For the motion. Venous perforator surgery is proven and does reduce recurrences. Eur J Vasc Endovasc Surg 48: 239-242. Link: https://goo.gl/a9zeKQ
- Iafrati MD, Pare GJ, O’Donnell TF, Estes J (2002) Is the nihilistic approach to surgical reduction of superficial and perforator vein incompetence for venous ulcer justified? J Vasc Surg 36: 1167-1174. Link: https://goo.gl/2tbWsB
- Alden PB, Lips EM, Zimmerman KP, Garberich RF, Rizvi AZ, et al. (2013) Chronic Venous Ulcer: Minimally Invasive Treatment of Superficial Axial and Perforator Vein Reflux Speeds Healing and Reduces Recurrence. Ann Vasc Surg 27: 75-83. Link: https://goo.gl/gczqK6
- Dillavou ED, Harlander-Locke M, Labropoulos N, Elias S, Ozsvath KJ (2016) Current state of the treatment of perforating veins. J Vasc Surg Venous Lymphat Disord 4: 131-135. Link: https://goo.gl/kN2XZQ
- Harlander-Locke M, Lawrence P, Jimenez JC, Rigberg D, DeRubertis B,et al. (2012) Combined treatment with compression therapy and ablation of incompetent superficial and perforating veins reduces ulcer recurrence in patients with CEAP 5 venous disease. J Vasc Surg 55: 446-450. Link: https://goo.gl/HY1nYc
- O’Donnell TF, Passman MA, Marston WA, Ennis WJ, Dalsing M, et al. (2014) Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J Vasc Surg 60: 3S-59S. Link: https://goo.gl/LDKPaA
- O’Donnell TF (2014) Part Two: Against the Motion. Venous Perforator Surgery is Unproven and Does not Reduce Recurrences. Eur J Vasc Endovasc Surg 48: 242-246. Link: https://goo.gl/ffUHn7
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
