Imaging Journal of Clinical and Medical Sciences
Gadolinium Brain Deposition in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in oncologic pediatric patients
Jonathan Carabin1, Amine Bouhamama1*, Ana Maria Mandache1, Alexandre Basle1, Edouard Marie1, Cécile Faure-Conter2, Pierre Leblond2, Didier Frappaz2, Audrey Lardy-Cleaud3 and Frank Pilleul1
2Department of Pediatric Oncology, Institut d’Hématologie et d’Oncologie Pédiatrique, 1 Place Professeur Joseph Renaut, 69008 Lyon, France
3Biostatistics Unit, DRCI, Centre Léon Bérard, 28 Prom. Léa Et Napoléon Bullukian, 69008Lyon, France
Cite this as
Carabin J, Bouhamama A, Mandache AM, Basle A, Marie E, et al. (2022) Gadolinium Brain Deposition in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in oncologic pediatric patients. Imaging J Clin Medical Sci 9(1): 018-025. DOI: 10.17352/2455-8702.000138Copyright License
© 2022 Carabin J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Gadolinium deposition in the human brain, especially in the Globus Pallidus (GP) and the Dentate Nucleus (DN) has been reported after the administration of linear Gadolinium-Based Contrast Agents (GBCAs). This study aims to determine whether repeated injections of macrocyclic GBCA are associated with an increased Signal Intensity (SI) on T1-weighted sequences in the Globus Pallidus (GP) and Dentate Nucleus (DN) in oncologic pediatric patients.
Materials and methods: This retrospective monocentric cohort study included consecutive children with at least two MRIs performed after administration of macrocyclic GBCAs. The SI measurements were determined on unenhanced T1-weighted images by drawing a circular region of interest (ROI) within GP and Thalamus (TH) and DN, Pons (P), at baseline, and at last MRI. SI ratios and the differences in ratios (GP/TH) and (DN/P) between the first and last MRI calculated.
Results: Out of 413 consecutive children attending Leon Berard cancer center, 50 patients were included, and data analysis showed significantly increased GP/TH SI ratios between the first and the last MRI (p =0.0305). The DN/P SI ratios did not significantly differ between the first and the last MRI (p=0.2668).
Conclusion: This study showed an increased SI in GP after several intravenous administrations of macrocyclic GBCAs but no increased SI was identified in the DN. Although no clinical adverse effects have been reported so far, the gadolinium deposits in the brain should be carefully monitored, especially in children who still undergo neurodevelopment.
Introduction
Gadolinium-Based Contrast Agents (GBCAs) have been widely used as contrast media for magnetic resonance imaging (MRI) for the diagnosis and monitoring of many diseases and an estimate of more than 300 million doses have been administrated since their first use in 1988 [1-3]. Gadolinium (Gd) belongs to the family of heavy metals, free Gd is highly toxic to humans. To prevent consequences related to free Gd, GBCAs are chelated with various ligands [4-6]. In vivo, many factors such as kinetic stability, temperature, and pH can lead to the deceleration of GBCA (7,8). Despite some adverse reactions, such as rare nephrogenic systemic fibrosis, which have been reported in the past, GBCAs are considered safe [9-18]. Nowadays, the main adverse reaction risk from GBCA is an allergic reaction [16]. The seven GBCA currently available belong to two broad categories, linear and macrocyclic, which are further subdivided into ionic or nonionic agents [19]. Macrocyclic GBCAs are more stable than linear GBCAs, and ionic agents provide a more stable structure than nonionic agents [20]. Gadolinium brain deposition in patients with the normal renal function was first described in 2014 and more specifically localized in the Globus Pallidus (GP) and in the Dentate Nucleus (DN) [21,22].
To date, many studies in adults [23,24] and more rarely in children [25-28] revealed increased T1 signal intensity in the GP and DN on unenhanced T1-weighted MRI after multiple administration of linear GBCA, as supported by post-mortem studies [29-31]. After multiple administrations of macrocyclic agents, most studies reported no increased T1 signal intensity MRI in patients with normal renal function [32,33].
To our knowledge, no specific symptoms or diseases related to macrocyclic GBCA have been described. This cohort study aims to determine whether multiple injections of macrocyclic GBCA increase signal intensity on T1-weighted MRI in pediatric patients.
Material and methods
This retrospective monocentric cohort study enrolled 413 consecutive patients from 1 to 18 years, at the time of their first MRI, who was attending the Leon Berard regional cancer center in Lyon (France) between February 2011 and December 2018 for brain MRI, and who experienced at least two MRI examinations in their follow-up period with the administration of macrocyclic GBCAs were eligible for inclusion.
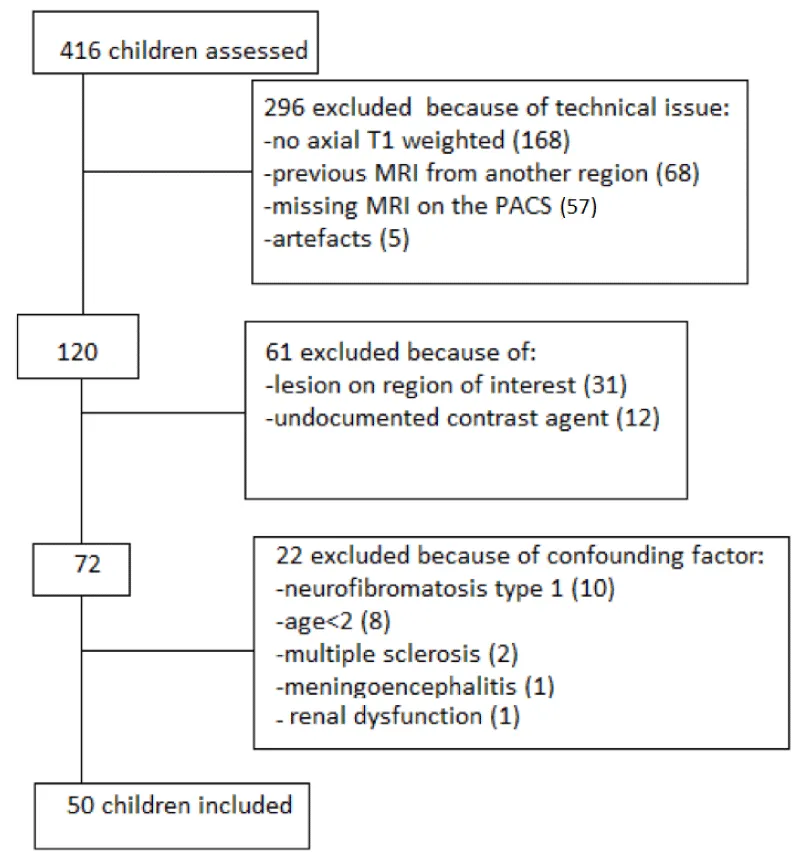
Exclusion criteria were patients with abnormal renal function (≥90 ml/min/ m2) (n=1), patients with neurofibromatosis type I (n=18), multiple sclerosis (n=2), meningo-encephalitis (n=1), patients with a history of previous use of contrast MRI for another localization (n=68), or with linear contrast agent or contrast agent type not specified (n=12), or insufficient imaging quality artifacts on MRI (n=5), lack of T1 images in the axial plane (n=168), or tumoral invasion of Regions of Interest (ROI) preventing Signal Intensity (SI) ratio determination (n=31), failure in back-up /storage of MRI on the PACS (n=57).
(Figure 1). This retrospective study received local approval from the ethics committee Lyon Sud-Est IV.
For each patient, demographic data (gender, date of birth), type of tumor, medical reason for MRI, number of MRI, date of first and last MRI, number of GBCAs administered, and history of brain radiotherapy and chemotherapy were collected [Table 1].
MRI acquisition
All MRI examinations were performed with a 1.5-T MRI (AchievaTM, PhilipsTM Amsterdam, The Nederlands) by using a standard head matrix coil. All examinations included an unenhanced axial T1-weighted spin-echo sequence or turbo Spin echo techniques, or an unenhanced T1-weighted three-dimensional sequence. All protocols included a pre-contrast axial turbo T1-weighted sequence with the following parameters: repetition time/echo time: 513ms /10ms; section thickness: 4 mm; field of view: 240mm; matrix size: 288; flip angle: 65°.
Imaging analysis
MR images were imported on PACS (Centricity, GE Healthcare, Chicago, Illinois) and reviewed, blind with respect to clinical data, by one radiology student (J.C) and two experienced neuroradiologists working independently (5 (AMM) and 6 (AB) years experience). Each observer placed ROIs on all exams from all 50 patients.
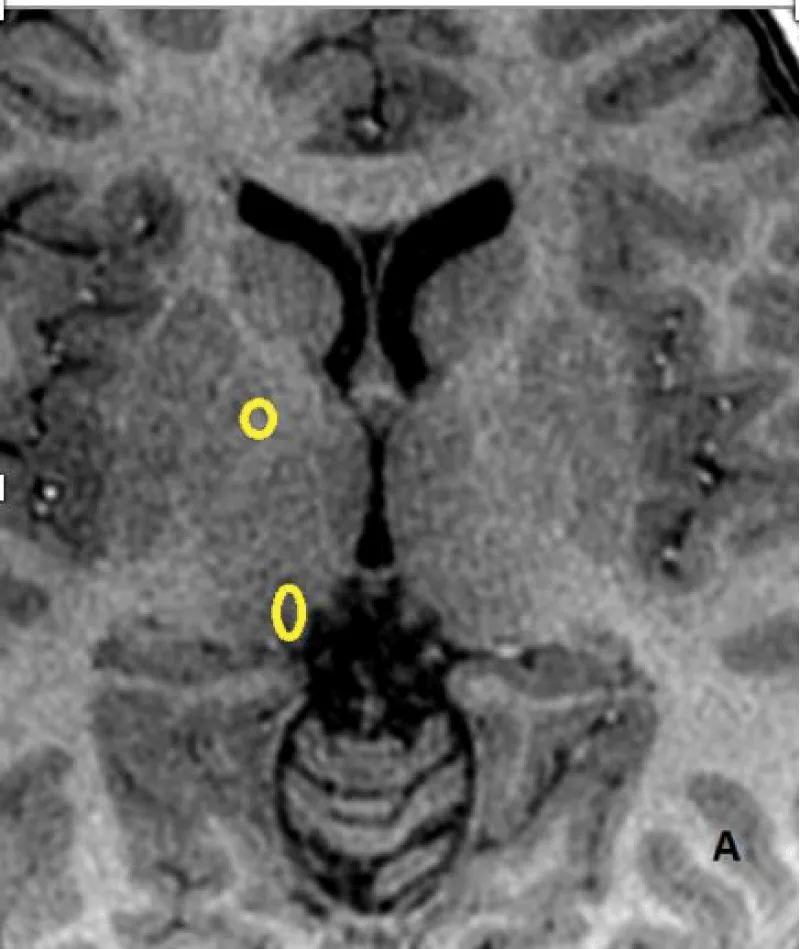
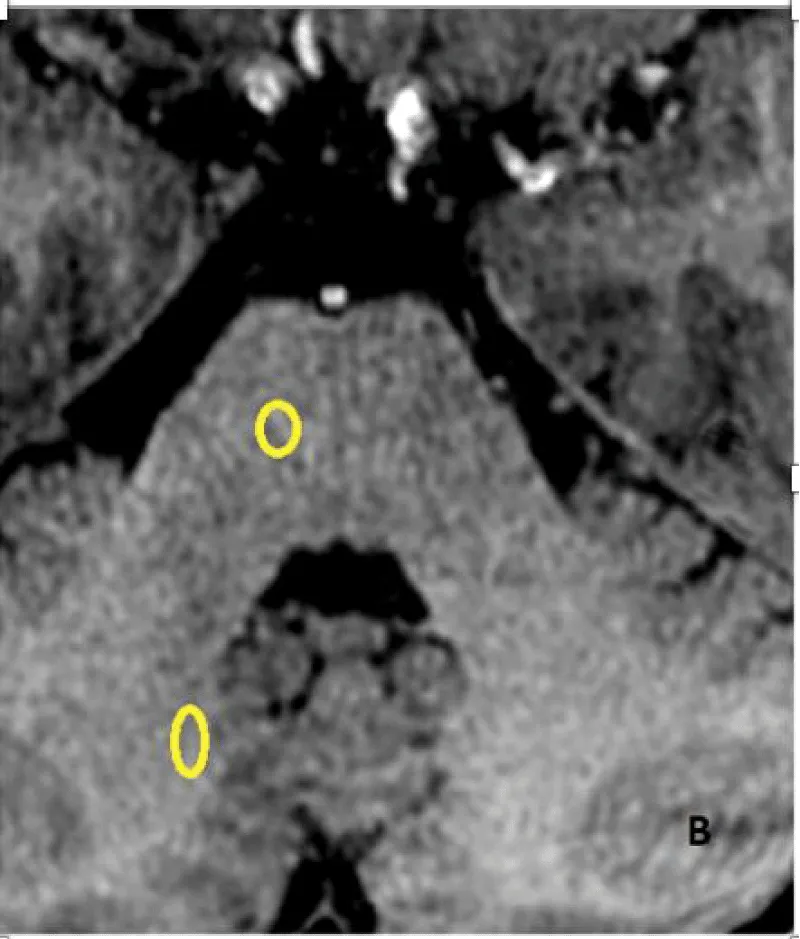
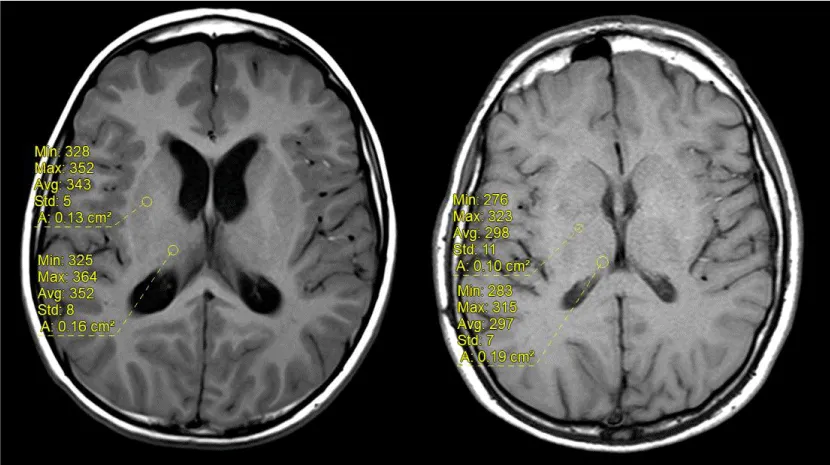
The signal intensity was measured on unenhanced T1-weighted images by drawing a circular or ovoid Region of Interest (ROI) with a size adapted for each patient, on the right side, including the Dentate Nucleus (DN), Pons (P), Globus Pallidus (GP) and Thalamus (TH), (mean diameter 4mm, range 3–9 mm) to include the anatomic structures as much as possible. The ROI was drawn without any knowledge of patient outcomes and randomly placed in each nucleus. Measures at baseline and at last MRI were performed (Figure 2A,2B). The left side was used if the right side could not be evaluated.
The ROI signal intensity ratios for the Globus Pallidus to the Thalamus (GP/TH) and Dentate Nucleus to Pons (DN/P) were calculated for all patients at baseline and at the last MRI. The differences in signal intensity ratio were calculated by subtracting from the last MRI examination the value of the signal intensity ratio of the first MRI on unenhanced T1 weighted images on the axial plane. If artifacts, brain lesions, or tumoral invasion prevent measurement of one of the four regions of interest to be assessed, and the corresponding ratio, only one ratio was assessed (GP/TH or DN/P) (n=17).
Statistical analysis
Patient’s characteristics are described with qualitative data expressed as n (%), and quantitative data with median (min-max).
Considering the three readers involved in measurements and repeated measurements, a general linear model was used to assess the ratio differences considering a potential patient effect.
We used a general linear model to explore the impact on the signal intensity GP/TH and DT/P ratio values at the last MRI of the potential confounding factors (gender, tumor type, age at the first MRI, number of MRI, reader, signal intensity values at the first MRI, brain radiotherapy, chemotherapy). A P value ≤0.05 was considered statistically significant
All the analyzes were carried out with SAS software version 9.4.
Results
Population
Eligibility criteria were assessed for 416 consecutive patients, and 366 patients were excluded for technical reasons (n=296) : lack of T1 images in axial plane (n=168), artifacts on MRI (n=5), previous MRI contrast agent used for another localisation (n=68) ,failure in MRI PACS accessing (n=57); for potential confounding factors (n=22): (abnormal renal function (n=1), neurofibromatosis type I (n=10), multiple sclerosis (n=2), meningo-encephalitis (n=1),; and for because of tumoral invasion of regions of interest preventing SI ratio determination (n=31) or undocumented contrast (n=12), the study included 50 patients (Figure 1).
40 patients were male (80%), the median age at the first MRI was 7 years (range: 1-18) and the median age at the last MRI was 8.9 years (range 1-18) (Table 1). The median number of contrast-enhanced MRIs was 6 (range 2-31). The median delay between the first and the last MRI was 11 months (range 0-111). Twenty-seven patients (54%) had brain radiotherapy and 14 patients (28%) had chemotherapy. Solid cancer or hematologic malignancies included 14 gliomas (28%), 5 medulloblastoma (13%), 13 acute leukemia (26%), 6 lymphomas (12%) and 5 medulloblastoma (10%) and 12 other types (24%). Artifacts and brain lesions, prevent to determine the DN/P SI ratio and GP/TH SI ratio between the first and last MR examination in all patients, and the DN/P SI ratio difference was determined in 47 patients and the GP/TH SI ratio was calculated in 36 patients.
Signal intensity analysis
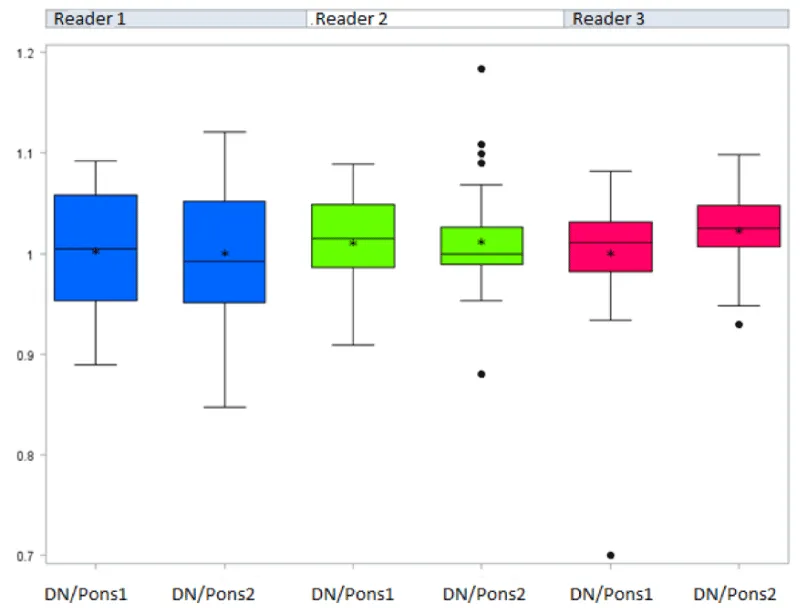
Differences in median values of the measurements and GP/TH and DN/P signal intensity ratios, at baseline and at the last MRI were identified according to the readers.
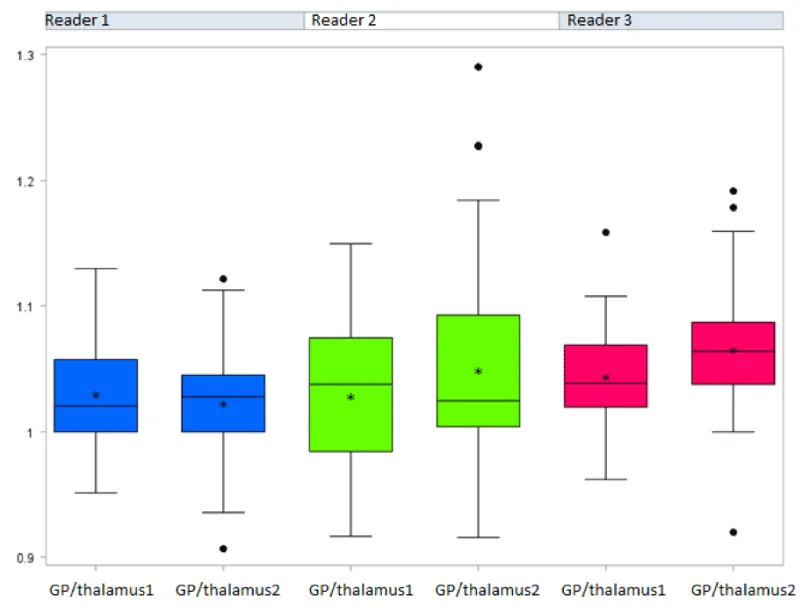
The general linear model considering the potential impact of the three readers showed a significantly increased GP/TH signal intensity ratio between the first and the last MRI (p =0.0305) (Figure 3a) [Table 2a]. Conversely, no significant difference between the first and the last MRI was evidenced for the DN/P SI ratios (p=0.2668) (Figure 3b) [Table 2b and 3] (Figure 4). No significant difference between reader measures was found for both ratios.
Impact of some potential factors on the value of the last MRI
We tested the impact of different potential factors on the values of the last MRI and identified significant differences in the value of the first MRI, the reader, the total number of MRIs, and whether or not the patient had received brain radiotherapy [Table 4].
Discussion
Our study showed a significant increase in the signal intensity ratio of the GP/TH on unenhanced T1-weighted MRI in children after serial intravenous injection of macrocyclic GBCA. The study did not show a significant increase in signal intensity of the DN/P ratio. Those results are contradictory to most previous studies reporting no increased signal intensity in the brain after serial injections of macrocyclic GBCAs [34,35] but increased signal intensity when linear GBCAs were used [36-38].
The limitations of the use of linear GBCAs are different in Europe and America. The European Medicines Agency (EMA) recommends restricting the use of linear GBCAs considering the potential associated risk of gadolinium deposition in the brain and limits the use of gadobenate dimeglumine (MultiHance®) to hepatic uptake to visualize poorly vascularized hepatic lesions [39]. The Food and Drug Administration (FDA) does not restrict the use of linear GBCAs but requests health care professionals to consider the retention characteristics of each agent when selecting a GBCA for patients who may be at higher risk for gadolinium retention [40]. Rahalti et al. have shown gadolinium deposition could be avoided in patients with meningioma by using unenhanced brain MRI for follow-up scans [41].
However, Rossi Espagnet, et al. have shown an increased signal intensity ratio of GP/TH and DN/P after serial administrations of macrocyclic GBCA [42] and gadolinium deposits in the brain, bone, skin, and liver have been demonstrated with both linear and macrocyclic agents in autopsy studies [43] and in rodent necropsy studies [44]. The issue of gadolinium deposits in the brain has to be considered with GBCA either used as macrocyclic GBCAs or linear, even if macrocyclic GBCAs seem to be more stable than linear GBCA [45,46].
Murata, et al. identify deposits of gadolinium during autopsies in the brain of five patients without known blood-brain barrier abnormalities after multiple injections of macrocyclic GBCA, with concentrations detected in the globus pallidus higher than those measured in the dentate nuclei [30]. Stanescu et al. also demonstrated pediatric patients exposed to GBCAs including both macrocyclic and linear ionic agents had higher gadolinium retention in the globus pallidus [47]. Those findings may explain the increased signal intensities of GP/TH ratio in our study whereas no increase in DN/P ratio was reported.
In the current study, only children with cancer disease or hematologic malignancy were included. Patients had a brain tumor or leukemia with neurologic disorders but the region of interest was drawn on non-pathologic areas in order to avoid the blood-brain barrier rupture areas.
Patients at risk of spontaneously increased signal intensity were excluded. Indeed, T1 hyperintensity in the globus pallidus on T1-weighted images has been described in many diseases including hepatic dysfunction, neurofibromatosis type 1, hemodialysis, and abnormal renal function [47-49]. High signal intensity on T1-weighted images in the dentate nucleus can be seen in patients with Langerhans cell histiocytosis [51], multiple sclerosis [52,53] and hepatic encephalopathy [54], hemodialysis patients [55].
Clinical consequences of gadolinium retention in the brain remain unknown. No specific symptoms related to gadolinium deposition have been reported [46-57].
The dentate nucleus is predominantly used during complex motor functions, sensory functions, and cognitive tasks [58], and GP lesions may be associated with dystonia and parkinsonism [59]. Since most of our patients had significant neurological symptoms related to their pathology, the clinical consequences of gadolinium deposition were not easily assessable. Long-term longitudinal studies are required to describe the clinical significance of gadolinium deposition in the brain, particularly in the pediatric population.
The limits of our study include its retrospective design, its monocentric data collection, the reduced sample size, and the absence of a control group. Furthermore, MR imaging was acquired using various systems and scan protocols. The presence of possible confounding factors such as the history of brain radiation [60,61], or history of chemotherapy and different treatment such as surgery has to be considered. Also, we don’t know how many patients received total parenteral nutrition, and parenteral nutrition can increase the signal intensity of the globus pallidus. Then four patients aged less than two years were included despite incomplete myelinization [62].
Indeed, this study showed that radiation therapy, the value of the first MRI, the reader, and the total number of MRI scans were significantly associated with higher signal intensity on unenhanced T1 images on the last MRI.
Conclusion
Our study showed increased signal intensity, suggesting gadolinium deposit, in globus pallidus of children after multiple intravenous administration of macrocyclic GBCAs, as previously reported with linear GBCA. Despite no evidence of clinical adverse effects related to brain gadolinium deposits, such results have to be carefully considered especially in children for whom neurodevelopment may be impeded.
- Runge VM. Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus. Invest Radiol. 2016 May;51(5):273-9. doi: 10.1097/RLI.0000000000000273. PMID: 26945278.
- Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017 Jul;16(7):564-570. doi: 10.1016/S1474-4422(17)30158-8. Epub 2017 Jun 13. PMID: 28653648.
- Costa AF, van der Pol CB, Maralani PJ, McInnes MDF, Shewchuk JR, Verma R, Hurrell C, Schieda N. Gadolinium Deposition in the Brain: A Systematic Review of Existing Guidelines and Policy Statement Issued by the Canadian Association of Radiologists. Can Assoc Radiol J. 2018 Nov;69(4):373-382. doi: 10.1016/j.carj.2018.04.002. Epub 2018 Sep 22. PMID: 30249408.
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999 Sep 8;99(9):2293-352. doi: 10.1021/cr980440x. PMID: 11749483.
- Pałasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47(4):1107-14. PMID: 11996100.
- Matsumura T, Hayakawa M, Shimada F, Yabuki M, Dohanish S, Palkowitsch P, Yoshikawa K. Safety of gadopentetate dimeglumine after 120 million administrations over 25 years of clinical use. Magn Reson Med Sci. 2013 Dec 25;12(4):297-304. doi: 10.2463/mrms.2013-0020. Epub 2013 Oct 29. PMID: 24172794.
- Ramalho J, Ramalho M. Gadolinium Deposition and Chronic Toxicity. Magn Reson Imaging Clin N Am. 2017 Nov;25(4):765-778. doi: 10.1016/j.mric.2017.06.007. Epub 2017 Sep 8. PMID: 28964466.
- Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008 May;66(2):175-9. doi: 10.1016/j.ejrad.2008.01.025. Epub 2008 Mar 14. PMID: 18343072.
- Granata V, Cascella M, Fusco R, dell'Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo A, Petrillo A. Immediate Adverse Reactions to Gadolinium-Based MR Contrast Media: A Retrospective Analysis on 10,608 Examinations. Biomed Res Int. 2016;2016:3918292. doi: 10.1155/2016/3918292. Epub 2016 Aug 29. PMID: 27652261; PMCID: PMC5019936.
- Neuberger J, Madden S, Collett D. Review of methods for measuring and comparing center performance after organ transplantation. Liver Transpl. 2010 Oct;16(10):1119-28. doi: 10.1002/lt.22131. PMID: 20879010.
- Niendorf HP, Dinger JC, Haustein J, Cornelius I, Alhassan A, Clauss W. Tolerance data of Gd-DTPA: a review. Eur J Radiol. 1991 Jul-Aug;13(1):15-20. doi: 10.1016/0720-048x(91)90049-2. PMID: 1889423.
- Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol. 2010 Feb;194(2):430-4. doi: 10.2214/AJR.09.3099. PMID: 20093606.
- Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012 Aug;264(2):414-22. doi: 10.1148/radiol.12112025. Epub 2012 May 1. PMID: 22550309.
- Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011 Feb;196(2):W138-43. doi: 10.2214/AJR.10.4885. PMID: 21257854.
- Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000 Aug;12(2):205-13. doi: 10.1002/1522-2586(200008)12:2<205::aid-jmri1>3.0.co;2-p. PMID: 10931582.
- Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology. 2018 Feb;286(2):731. doi: 10.1148/radiol.2017174037. Erratum for: Radiology. 2018 Feb;286(2):471-482. PMID: 29356629.
- Beckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics. 2015 Oct;35(6):1738-50. doi: 10.1148/rg.2015150033. PMID: 26466182.
- Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol. 2007 Dec;189(6):1533-8. doi: 10.2214/AJR.07.2554. PMID: 18029897.
- Sherry AD, Caravan P, Lenkinski RE. Primer on gadolinium chemistry. J Magn Reson Imaging. 2009 Dec;30(6):1240-8. doi: 10.1002/jmri.21966. PMID: 19938036; PMCID: PMC2853020.
- Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009 Dec;30(6):1249-58. doi: 10.1002/jmri.21967. PMID: 19938037.
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014 Mar;270(3):834-41. doi: 10.1148/radiol.13131669. Epub 2013 Dec 7. PMID: 24475844.
- Quattrocchi CC, Ramalho J, van der Molen AJ, Rovira À, Radbruch A; GREC, European Gadolinium Retention Evaluation Consortium and the ESNR, European Society of Neuroradiology. Standardized assessment of the signal intensity increase on unenhanced T1-weighted images in the brain: the European Gadolinium Retention Evaluation Consortium (GREC) Task Force position statement. Eur Radiol. 2019 Aug;29(8):3959-3967. doi: 10.1007/s00330-018-5803-6. Epub 2018 Nov 9. PMID: 30413951.
- McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015 Jun;275(3):772-82. doi: 10.1148/radiol.15150025. Epub 2015 Mar 5. PMID: 25742194.
- Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014 Oct;49(10):685-90. doi: 10.1097/RLI.0000000000000072. PMID: 24872007.
- Hu HH, Pokorney A, Towbin RB, Miller JH. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016 Oct;46(11):1590-8. doi: 10.1007/s00247-016-3646-3. Epub 2016 Jun 9. PMID: 27282825.
- Roberts DR, Chatterjee AR, Yazdani M, Marebwa B, Brown T, Collins H, Bolles G, Jenrette JM, Nietert PJ, Zhu X. Pediatric Patients Demonstrate Progressive T1-Weighted Hyperintensity in the Dentate Nucleus following Multiple Doses of Gadolinium-Based Contrast Agent. AJNR Am J Neuroradiol. 2016 Dec;37(12):2340-2347. doi: 10.3174/ajnr.A4891. Epub 2016 Jul 28. PMID: 27469211; PMCID: PMC5161565.
- Ryu YJ, Choi YH, Cheon JE, Lee WJ, Park S, Park JE, Kim WS, Kim IO. Pediatric Brain: Gadolinium Deposition in Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted Images Is Dependent on the Type of Contrast Agent. Invest Radiol. 2018 Apr;53(4):246-255. doi: 10.1097/RLI.0000000000000436. PMID: 29300210.
- Young JR, Qiao J, Orosz I, Salamon N, Franke MA, Kim HJ, Pope WB. Gadolinium deposition within the paediatric brain: no increased intrinsic T1-weighted signal intensity within the dentate nucleus following the administration of a minimum of four doses of the macrocyclic agent gadobutrol. Eur Radiol. 2018 Nov;28(11):4882-4889. doi: 10.1007/s00330-018-5464-5. Epub 2018 May 9. PMID: 29744642; PMCID: PMC6226378.
- Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015 Jul;276(1):228-32. doi: 10.1148/radiol.2015142690. Epub 2015 May 5. PMID: 25942417.
- Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Invest Radiol. 2016 Jul;51(7):447-53. doi: 10.1097/RLI.0000000000000252. PMID: 26863577.
- Kanda T, Matsuda M, Oba H, Toyoda K, Furui S. Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015 Dec;277(3):924-5. doi: 10.1148/radiol.2015150697. PMID: 26599932.
- Bjørnerud A, Vatnehol SAS, Larsson C, Due-Tønnessen P, Hol PK, Groote IR. Signal Enhancement of the Dentate Nucleus at Unenhanced MR Imaging after Very High Cumulative Doses of the Macrocyclic Gadolinium-based Contrast Agent Gadobutrol: An Observational Study. Radiology. 2017 Nov;285(2):434-444. doi: 10.1148/radiol.2017170391. Epub 2017 Sep 8. PMID: 28885891.
- Kang KM, Choi SH, Hwang M, Yun TJ, Kim JH, Sohn CH. T1 Shortening in the Globus Pallidus after Multiple Administrations of Gadobutrol: Assessment with a Multidynamic Multiecho Sequence. Radiology. 2018 Apr;287(1):258-266. doi: 10.1148/radiol.2017162852. Epub 2017 Nov 1. PMID: 29091750.
- Tibussek D, Rademacher C, Caspers J, Turowski B, Schaper J, Antoch G, Klee D. Gadolinium Brain Deposition after Macrocyclic Gadolinium Administration: A Pediatric Case-Control Study. Radiology. 2017 Oct;285(1):223-230. doi: 10.1148/radiol.2017161151. Epub 2017 Jun 21. PMID: 28640695.
- Radbruch A, Haase R, Kickingereder P, Bäumer P, Bickelhaupt S, Paech D, Wick W, Schlemmer HP, Seitz A, Bendszus M. Pediatric Brain: No Increased Signal Intensity in the Dentate Nucleus on Unenhanced T1-weighted MR Images after Consecutive Exposure to a Macrocyclic Gadolinium-based Contrast Agent. Radiology. 2017 Jun;283(3):828-836. doi: 10.1148/radiol.2017162980. Epub 2017 Mar 8. PMID: 28273007.
- Flood TF, Stence NV, Maloney JA, Mirsky DM. Pediatric Brain: Repeated Exposure to Linear Gadolinium-based Contrast Material Is Associated with Increased Signal Intensity at Unenhanced T1-weighted MR Imaging. Radiology. 2017 Jan;282(1):222-228. doi: 10.1148/radiol.2016160356. Epub 2016 Jul 28. PMID: 27467467.
- Pullicino R, Radon M, Biswas S, Bhojak M, Das K. A Review of the Current Evidence on Gadolinium Deposition in the Brain. Clin Neuroradiol. 2018 Jun;28(2):159-169. doi: 10.1007/s00062-018-0678-0. Epub 2018 Mar 9. PMID: 29523896.
- Cao Y, Huang DQ, Shih G, Prince MR. Signal Change in the Dentate Nucleus on T1-Weighted MR Images After Multiple Administrations of Gadopentetate Dimeglumine Versus Gadobutrol. AJR Am J Roentgenol. 2016 Feb;206(2):414-9. doi: 10.2214/AJR.15.15327. Epub 2015 Dec 23. PMID: 26700156.
- Gadolinium-containing contrast agents. London (GB): European Medicines Agency; 2017: https://www.ema.europa.eu/en/medicines/human/referrals/gadolinium-containing-contrast-agents. Accessed 2019 March 29
- FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings (FDA Drug Safety Communication). Silver Spring (MD): U.S. Food and Drug Administration (FDA); 2018: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Accessed 2019 March 29.
- Rahatli FK, Donmez FY, Kibaroglu S, Kesim C, Haberal KM, Turnaoglu H, Agildere AM. Does renal function affect gadolinium deposition in the brain? Eur J Radiol. 2018 Jul;104:33-37. doi: 10.1016/j.ejrad.2018.04.017. Epub 2018 Apr 20. PMID: 29857863.
- Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017 Sep;47(10):1345-1352. doi: 10.1007/s00247-017-3874-1. Epub 2017 May 19. Erratum in: Pediatr Radiol. 2017 Sep;47(10 ):1366. PMID: 28526896.
- Roberts DR, Welsh CA, LeBel DP 2nd, Davis WC. Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017 Mar 21;88(12):1206-1208. doi: 10.1212/WNL.0000000000003735. Epub 2017 Feb 15. PMID: 28202695.
- Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Invest Radiol. 2016 Jul;51(7):447-53. doi: 10.1097/RLI.0000000000000252. PMID: 26863577.
- McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, Williamson EE, Eckel LJ. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology. 2017 Nov;285(2):546-554. doi: 10.1148/radiol.2017161595. Epub 2017 Jun 27. PMID: 28653860.
- Radbruch A. Are some agents less likely to deposit gadolinium in the brain? Magn Reson Imaging. 2016 Dec;34(10):1351-1354. doi: 10.1016/j.mri.2016.09.001. Epub 2016 Sep 11. PMID: 27629022.
- Stanescu AL, Shaw DW, Murata N, Murata K, Rutledge JC, Maloney E, Maravilla KR. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol. 2020 Mar;50(3):388-396. doi: 10.1007/s00247-019-04535-w. Epub 2020 Jan 27. PMID: 31989188.
- Lai PH, Chen C, Liang HL, Pan HB. Hyperintense basal ganglia on T1-weighted MR imaging. AJR Am J Roentgenol. 1999 Apr;172(4):1109-15. doi: 10.2214/ajr.172.4.10587157. PMID: 10587157.
- Rahatli FK, Donmez FY, Kesim C, Haberal KM, Turnaoglu H, Agildere AM. Can unenhanced brain magnetic resonance imaging be used in routine follow up of meningiomas to avoid gadolinium deposition in brain? Clin Imaging. 2019 Jan-Feb;53:155-161. doi: 10.1016/j.clinimag.2018.10.014. Epub 2018 Oct 13. PMID: 30343167.
- da Silva CJ, da Rocha AJ, Jeronymo S, Mendes MF, Milani FT, Maia AC Jr, Braga FT, Sens YA, Miorin LA. A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol. 2007 Sep;28(8):1474-9. doi: 10.3174/ajnr.A0600. PMID: 17846194; PMCID: PMC8134392.
- Prayer D, Grois N, Prosch H, Gadner H, Barkovich AJ. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol. 2004 May;25(5):880-91. PMID: 15140741; PMCID: PMC7974468.
- Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology. 2009 May;251(2):503-10. doi: 10.1148/radiol.2511081269. PMID: 19401576.
- Absinta M, Rocca MA, Filippi M. Dentate nucleus T1 hyperintensity in multiple sclerosis. AJNR Am J Neuroradiol. 2011 Jun-Jul;32(6):E120-1. doi: 10.3174/ajnr.A2536. Epub 2011 Apr 14. PMID: 21493765; PMCID: PMC8013147.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008 Oct;29(9):1612-21. doi: 10.3174/ajnr.A1139. Epub 2008 Jun 26. PMID: 18583413; PMCID: PMC8118773.
- Cao Y, Zhang Y, Shih G, Zhang Y, Bohmart A, Hecht EM, Prince MR. Effect of Renal Function on Gadolinium-Related Signal Increases on Unenhanced T1-Weighted Brain Magnetic Resonance Imaging. Invest Radiol. 2016 Nov;51(11):677-682. doi: 10.1097/RLI.0000000000000294. PMID: 27272543.
- Bauer K, Lathrum A, Raslan O, Kelly PV, Zhou Y, Hewing D, Botkin C, Turner JA, Osman M. Do Gadolinium-Based Contrast Agents Affect 18F-FDG PET/CT Uptake in the Dentate Nucleus and the Globus Pallidus? A Pilot Study. J Nucl Med Technol. 2017 Mar;45(1):30-33. doi: 10.2967/jnmt.116.180844. Epub 2016 Nov 10. PMID: 27834725.
- Welk B, McArthur E, Morrow SA, MacDonald P, Hayward J, Leung A, Lum A. Association Between Gadolinium Contrast Exposure and the Risk of Parkinsonism. JAMA. 2016 Jul 5;316(1):96-8. doi: 10.1001/jama.2016.8096. PMID: 27380348.
- Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2010 Mar;9(1):22-8. doi: 10.1007/s12311-009-0119-3. Epub 2009 Jun 10. PMID: 19513801.
- Münchau A, Mathen D, Cox T, Quinn NP, Marsden CD, Bhatia KP. Unilateral lesions of the globus pallidus: report of four patients presenting with focal or segmental dystonia. J Neurol Neurosurg Psychiatry. 2000 Oct;69(4):494-8. doi: 10.1136/jnnp.69.4.494. PMID: 10990510; PMCID: PMC1737132.
- Tamrazi B, Nguyen B, Liu CJ, Azen CG, Nelson MB, Dhall G, Nelson MD. Changes in Signal Intensity of the Dentate Nucleus and Globus Pallidus in Pediatric Patients: Impact of Brain Irradiation and Presence of Primary Brain Tumors Independent of Linear Gadolinium-based Contrast Agent Administration. Radiology. 2018 May;287(2):452-460. doi: 10.1148/radiol.2017171850. Epub 2017 Nov 30. PMID: 29189102; PMCID: PMC5929364.
- Kasahara S, Miki Y, Kanagaki M, Yamamoto A, Mori N, Sawada T, Taoka T, Okada T, Togashi K. Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology. 2011 Jan;258(1):222-8. doi: 10.1148/radiol.10100508. Epub 2010 Nov 2. PMID: 21045180.
- Branson HM. Normal myelination: a practical pictorial review. Neuroimaging Clin N Am. 2013 May;23(2):183-95. doi: 10.1016/j.nic.2012.12.001. Epub 2013 Feb 15. PMID: 23608684.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
