Imaging Journal of Clinical and Medical Sciences
That’s not Metastatic Thyroid Cancer: Patterns of Non-malignant Radioiodine Uptake
Daniella F Pinho*, Andrew Ceranske, Anish Patel, Fangyu Peng and Jason Wayne Wachsmann
Cite this as
Pinho DF, Ceranske A, Patel A, Peng F, Wachsmann JW (2017) That’s not Metastatic Thyroid Cancer: Patterns of Non-malignant Radioiodine Uptake. Imaging J Clin Medical Sci 4(1): 001-0005. DOI: 10.17352/2455-8702.000033Radioactive Iodine (RAI) has been an integral part of the identification, surveillance, and treatment of the most common types of metastatic thyroid carcinoma since its first use in 1941. However, false positive uptake of RAI on whole body scintigraphy scans in tissues other than in metastatic disease is frequently observed. This article presents a case series of relatively uncommon situations of RAI uptake which could be mistaken for malignant uptake. Cases were divided into categories based on uptake mechanism: metabolic, physiologic, inflammatory, and contamination.
Introduction
Radioactive iodine (RAI) has played a key role in the identification and treatment of the most common types of thyroid cancer since its inception in 1936, with the first recorded treatment of hyperthyroidism in 1941 [1]. Use of iodine-131 (I 131) for treatment of thyroid cancer was soon to follow with the first demonstrated uptake from thyroid adenocarcinoma by Seidlin in 1943 [2]. In the years that followed, these first experiences allowed RAI to become a critical radiopharmaceutical in the treatment and surveillance of papillary and follicular thyroid carcinoma.
RAI is produced in a nuclear reactor by the neutron bombardment of Tellurium 127 and has a half-life of 8.02 days. Once ingested, nowadays usually in pill form, about 90% of the dosed RAI is rapidly absorbed and secreted into the extracellular iodine pool [3]. Part of this absorbed RAI is renally excreted, but given the usually high TSH expression in thyroid carcinomas and its effect to increase the expression of the sodium-iodine symporter (NIS), much is taken up into by follicular cells into tissue by the NIS receptors. Once trapped in the follicular cell, it rapidly undergoes organification. Organification is defined as oxidation and eventual binding of the RAI to tyrosine residues on thyroglobulin [4,5]. During this process and for substantial time after, RAI undergoes Beta decay and with consequent damage of the surrounding tissue. RAI predominantly decays by Beta emission to xenon-133, with a gamma emission of 364 keV [3]. Importantly, when I-131 decays, 10% of this decay is expressed as gamma rays, which can be detected by standard gamma cameras thereby allowing for surveillance of disease response and localization of potential metastatic lesions.
Whole body scintigraphy is used in patients with differentiated thyroid carcinomas, for assessment after treatment with RAI, and may also be used for localization of metastatic disease in patients with increase in thyroglobulin levels. However, it is by no means a perfect method. Whereas I-131 is a sensitive marker for the detection of thyroid cancer, it is not specific to thyroid tissue. Physiologic uptake is commonly observed in the salivary glands, thymus, and GI tissue. Thyroid tissue, either residual cells in the thyroidectomy bed or in ectopic location will also have increased RAI uptake [6]. This is commonly seen and well understood. However, RAI uptake has been observed in several other circumstances, which can make the surveillance and treatment of metastatic thyroid cancers extremely difficult. Inflammation, contamination, and importantly metabolism are all conditions in which RAI uptake has been observed [7-10]. It becomes difficult and extraordinarily important in the treatment of patients with metastatic thyroid cancers to be able to determine whether the uptake seen on whole body scintigraphy is representative of metastatic disease or not. Therefore, precise interpretation by a trained radiologist or nuclear medicine physician is critical in optimally managing this high-risk disease.
Based on that, we have highlighted a series of cases which illustrate unique examples of thyroid uptake which would appear to represent metastatic thyroid disease, but in fact do not. This was correlated with the mechanism of localization to further aid understanding and clinical recall. By presenting these cases that illustrate rare, but important instances, in which metastatic disease can be mistaken for other types of uptake, our goal is to prevent patient worry, misdiagnosis, and potential unnecessary treatments.
Materials and Methods
Clinical cases were retrieved in a retrospective manner from the nuclear medicine database and cases were reviewed and selected. From this extensive dataset, cases which illustrated unique examples of non-malignant and/or non-thyroidal uptake on I-131 whole body scintigraphy scans were thoroughly reviewed. Protected patient information was systematically removed from all images. The uptake on these cases were categorized as metabolic, physiologic, inflammation and contamination. After case selection, a review of the literature was performed to elucidate different physiological mechanisms of iodine localization and to correlate imaging findings.
Cases
Case 1: Metabolic uptake of RAI
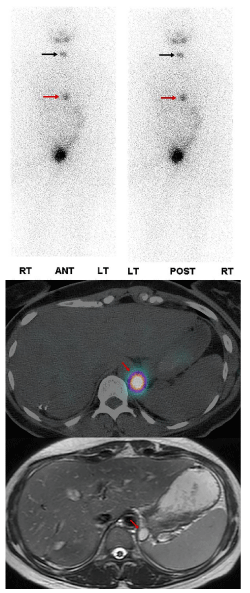
Hepatic uptake seen on thyroid scintigraphy was originally thought to be secondary to residual metastatic thyroid cells. Later studies were not able to show its correlation with serum thyroglobulin or distant metastatic disease [3]. Currently, RAI uptake in the liver is attributed to the metabolism of thyroglobulin from either residual normal and/or malignant thyroid cells. There is also evidence for normal hepatic uptake based on findings of functional sodium-iodine symporters (NIS) expressed in the intrahepatic bile ducts which is correlated with the administered dose of I-131. The higher the dose, usually the higher the uptake seen in the liver. Patients with hepatic steatosis can have increased RAI uptake related to delayed excretion secondary to the delayed action of deiodinase in the liver cells (Figure 1).
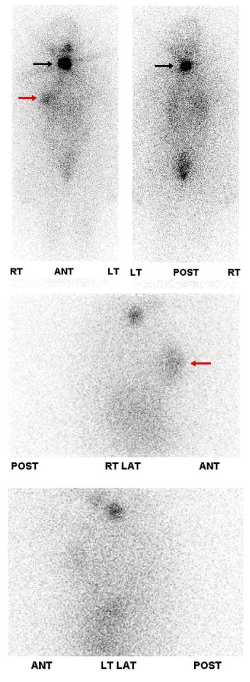
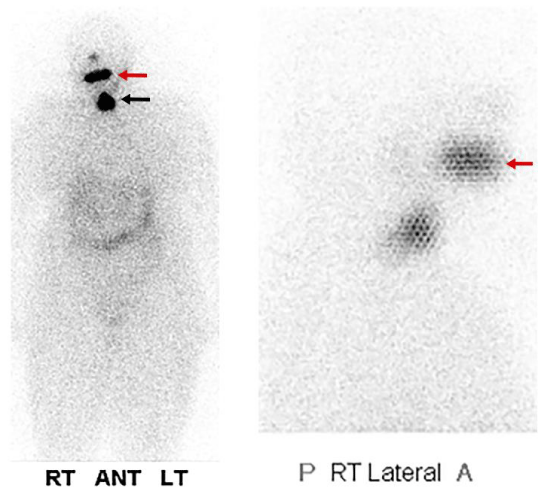
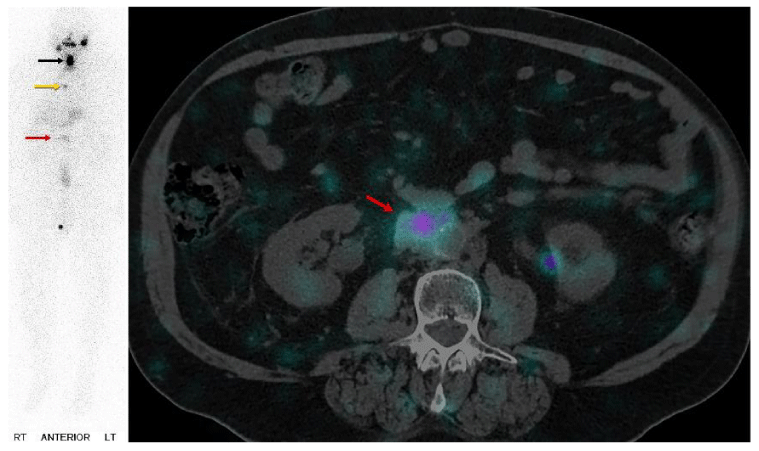
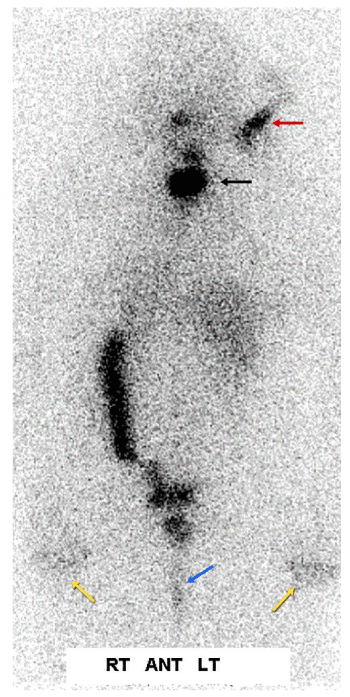
Case 2: Physiologic uptake of RAI
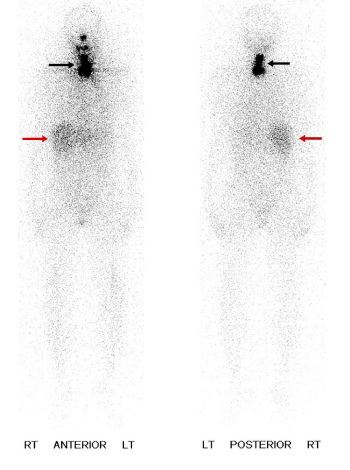
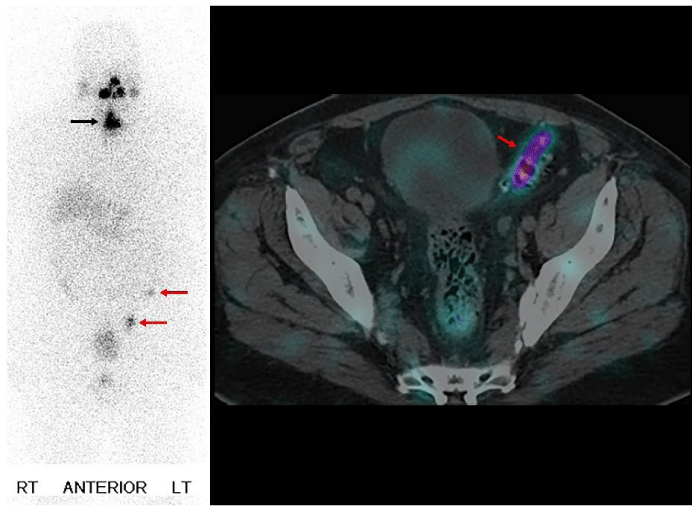
The next cases represent physiologic uptake of RAI (Figures 2-5). This will be reviewed through cases showing colon, thymic, breast, and gastric mucosa (in a gastric diverticulum) uptake.
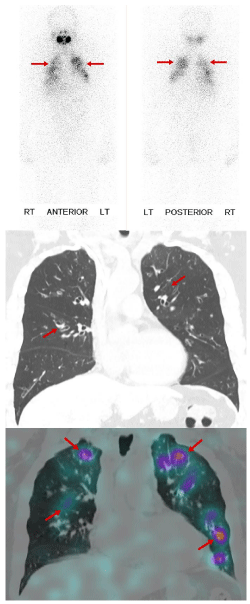
Case 3: Inflammatory uptake of RAI
Uptake of RAI related to inflammatory tissues is secondary to increased perfusion and vasodilatation. Additionally, enhanced capillary permeability can result in stasis of RAI, with retention in leukocytes in posttraumatic clots or tissues (leukocytes induce iodide organification by myeloperoxidase). Examples of inflammatory disease that can have RAI uptake are illustrated in figures 6 -8.
Case 4: External contamination by RAI
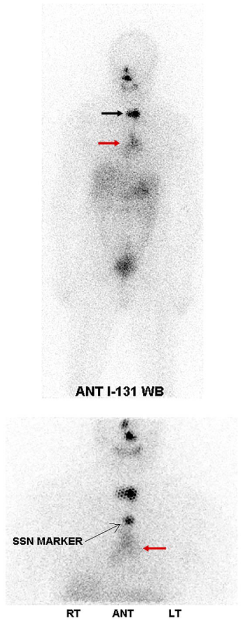
RAI is excreted by all body fluids including sweat, breast milk, urine, vomitus, nasal, tracheobronchial, lacrimal, salivary secretions and feces. Contamination is usually easily recognized by its pattern and location of uptake. Two examples are shown in figures 9,10.
Discussion
I-131 has good sensitivity for the detection of thyroid tissue and is an important lifesaving theranostic agent that can detect and treat thyroid cancer. As detailed above, given that there are other causes of uptake including physiological, metabolic, inflammatory and contamination, RAI is not specific to metastatic thyroid cancer. It is important to recognize the causes and distribution of non-malignant uptake to decrease the number of false positive studies, resulting in a specificity of over 90% as reported on literature [11,12].
The ultimate goal is to improve patient care through more comprehensive radiographic understanding of these studies. If doubt remains, SPECT/CT should be used for further delineation of areas of uptake that cannot be explained on the planar images.
- Sawin CT, Becker DV (1997) Radioiodine and the treatment of hyperthyroidism: the early history. Thyroid 2: 163-176. Link: https://goo.gl/T98cmh
- Sieigel E (1999) The beginnings of radioiodine therapy of metastatic thyroid carcinoma: a memoir of Samuel M. Seidlin, M. D. (1895-1955) and his celebrated patient. Cancer Biotherapy and Radiopharmaceuticals 14: 71-79. Link: https://goo.gl/cLGEug
- Jong-Ryool Oh, Byeong-Cheol Ahn (2012) False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologicvariants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging 2: 362-385. Link: https://goo.gl/cjxdT6
- Byeong-Cheol Ahn (2012) Sodium iodide symporter for nuclear molecular imaging and gene therapy: from bedside to bench and back 2: 392-402. Link: https://goo.gl/ioFq4L
- Chung JK (2002) Sodium iodide symporter: its role in nuclear medicine. J Nucl Med 43: 1188-1200. Link: https://goo.gl/0dFpo8
- Lind P, Kohlfurst S (2006) Respective roles of thyroglobulin, radioiodine imaging, and positron emission tomography in the assessment of thyroid cancer. Semin Nucl Med 36: 194-205. Link: https://goo.gl/BN1892
- Carlisle MR, Lu C, McDougall IR (2003) The interpretation of I-131 scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun 24: 715-735. Link: https://goo.gl/C1xLRi
- Chung JK, Lee YJ, Jeong JM, Lee DS, Lee MC, et al. (1997) Clinical significance of hepatic visualization on iodine-131 whole-body scan in patients with thyroid carcinoma. J Nucl Med 38: 1191-1195. Link: https://goo.gl/OXv2Wc
- Vermiglio F, Baudin E, Travagli JP, Caillou B, Fragu P et al. (1996) Iodine concentration by the thymus in thyroid carcinoma. M. J Nucl Med 37: 1830-1831. Link: https://goo.gl/bErBko
- Hammami MM, Bakheet S (1996) Radioiodine breast uptake in non-breastfeeding women: clinical and scintigraphic characteristics. J Nucl Med 37: 26-31. Link: https://goo.gl/jWduIe
- Ibis E, Wilson CR, Collier BD, Akansel G, Isitman AT, et al. (1992) Iodine-131 contamination from thyroid cancer patients. J Nucl Med 33: 2110-2115. Link: https://goo.gl/H2fXCw
- McDougall IR (1995) Whole-body scintigraphy with radioioidine-131: a comprehensive list of false-positives with some examples. Clin Nucl Med 20: 869–875. Link: https://goo.gl/7U0p0e
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
