Imaging Journal of Clinical and Medical Sciences
Influence of contrast administration side and communication with patient on motion artefact size on breast MRI
Damir Štimac1,2*, Matea Bogović2, Ana Božanić3,4 and Petra Valković Zujić5,6
2Department of Radiology, National Memorial Hospital Vukovar, Županijska ulica 35, Vukovar, Croatia
3Medical Physics and Biophysics Department, Faculty of Medicine, University of Rijeka, Braće Branchetta 42, Rijeka, Croatia
4Medical Physics and Radiation Protection Department, University Hospital Rijeka, Krešimirova 42, Rijeka, Croatia
5Department of Radiology, Faculty of Medicine, University of Rijeka, Braće Branchetta 42, Rijeka, Croatia
6Department of Radiology, University Hospital Rijeka, Krešimirova 42, Rijeka, Croatia
Cite this as
Štimac D, Bogović M, Božanić A, Zujić PV (2023) Influence of contrast administration side and communication with patient on motion artefact size on breast MRI. Imaging J Clin Medical Sci 10(1): 013-019. DOI: 10.17352/2455-8702.000141Copyright License
© 2023 Štimac D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Magnetic resonance imaging is recognized as a useful modality in breast imaging. Motion is considered the most relevant cause of artefacts in breast MRI leading to degraded image quality or rendering studies non-diagnostic. The important reason for patient motion is contrast administration. Contrast administration may lead to discomfort, feeling of tingling and warmth, and pain in the arm contrast was administered to. The aim of our study was to determine the influence of contrast agent injection side and improve communication with patients on motion artifacts on subtraction reconstructions in order to prove that unexpected events may cause non-physiological movement resulting in motion artifacts, and that well-timed warning may decrease such artifacts. 146 patients with breast MRI from July 2019 to May 2020 were included. 71 of them were warned before the dynamic sequence started, and 75 of them didn't receive any warning for contrast application. The pectoral shift, in millimeters in the anteroposterior and lateral-lateral directions, was measured. Pectoral shift showed to be larger in unwarned patients when compared to warned ones with high significance (p=0.001) -no artifacts were observed in 361 warned, and 267 in unwarned women. Furthermore, artifacts were significantly larger on the side contralateral to contrast administration (median value of 2mm for the same side, and 1mm on the opposite side). In conclusion, our study showed that if patients knew the exact time of contrast application, the motion artifacts would be less pronounced; and that we should apply the contrast agent on the side opposite to the breast pathology is expected in.
Abbreviations
MRI: Magnetic Resonance Imaging; MR: Magnetic Resonance; CA: Contrast Agent; PACS: Picture Archiving and Communication System; TIRM: Turbo Inversion Recovery Magnitude; SPAIR: SPectral Attenuated Inversion Recovery; WP: Warned Patients; UWP: Unwarned Patients; AP: Antero-Posterior; LL: Latero-Lateral
Introduction
Magnetic resonance imaging (MRI) is widely recognized as a useful imaging modality for high-risk women screening, for preoperative and post-treatment evaluation, to investigate carcinoma of unknown primary tumor or syndrome, and patients without conclusive mammographic or sonographic imaging findings [1,2].
Breast MRI identifies early signs of cancer, detecting its neoangiogenesis, increased vessel permeability, and permeability of the ductal basal membrane. Cancer detection and visualization are based on pathophysiologic changes, such as cancer proliferation, infiltrative growth, and metastasis [3,4]. MRI is a functional imaging tool with proven benefits in breast cancer screening and diagnosis along with mammography, ultrasound (US), and image-guided needle biopsy. Breast MRI studies should be interpreted by radiologists with expertise in breast imaging since it's often being used as a supplemental modality for equivocal findings on mammography or ultrasound [5]. There are no studies regarding the learning curve in MRI interpretation, but the knowledge of conventional breast imaging studies and the principle of MRI interpretation along with drawbacks and pitfalls are crucial in adequate breast MRI assessment.
MRI showed higher accuracy compared to mammography and ultrasound in detecting and characterizing breast cancers as well as differentiation of malignant from benign lesions. In order to diagnose or exclude cancer, MRI scanning yields mandatory intravenous administration of a gadolinium-containing contrast agent (CA) [6,7]. Even though Breast MRI has evolved from contrast-enhanced to multiparametric imaging technique, the basic framework for any MRI protocol is dynamic T1-weighted contrast-enhanced sequence, and subtraction reconstructions.
Subtraction of unenhanced images from the corresponding contrast-enhanced images is crucial for lesion detection and characterization, mostly when T1-weighted (T1W) non-fat-saturated or fat-saturated technical protocols are utilized [8]. The dynamic analysis investigates the permeability of the vessels that supply a lesion [9]. This is done by obtaining a series of T1- T1-weighted acquisition between 5 and 7 minutes after contrast material administration [10,11]. In the case of leaky vessels of invasive malignant lesions, rapid enhancement in the early phase and fast contrast washout are observed [12]. In benign lesions with less-permeable vessels, the contrast gradient over the vessel wall will still be positive, and therefore the enhancement of the lesion still increases in the later contrast-enhanced scans. This is reflected in the shape of the time–signal intensity curves. A persistent increase (type 1 kinetic curve) refers to progressive enhancement and is commonly associated with benign lesions in 83 % of cases. Type 2 curve is a plateau pattern (6 – 64 % association with malignancy) whereas a washout curve demonstrating initial increase and rapid washout is characteristic of a malignant lesion (33 – 85 % association with malignancy). Finally, the pre-and postcontrast axial T1 images are reviewed. Subtraction-reconstructed images are based on the same principles as digital subtraction angiography. Fat does not enhance post-contrast sequences and using subtraction essentially cancels out all the fat signals on the T1 images leaving behind the enhancing areas, i.e., masses, lymph nodes, and blood vessels. The T1 images are also excellent for evaluating intraductal blood and lymph node morphology, identifying the benign fatty hilum of normal lymph nodes [13].
The sensitivity and specificity of all existing imaging in breast cancer detection is limited to around 70 %, due to both clinical and technical factors [14-16].
Although breast MRI has an established role in the surveillance, workup, staging, and follow-up of breast cancer, there are some difficulties in breast MRI interpretation, such as background parenchymal enhancement due to hormonal stimulation or proliferative fibrocystic disease and lesion characteristics such as small lesion size and non-mass type [17,18], as well as acquisition-related motion artifacts, which may significantly hamper image quality. While patient movement during image acquisition may have several consequences,1 such as blurring and ghosting [19,20], motion between dynamic acquisitions is most relevant in breast MRI. An anatomical shift between pre- and post-contrast acquisitions leads to typical artifacts on subtracted images: either subtraction of parenchymal (isointense on T1-weighted [T1W] images) from neighboring fatty (bright on T1W) tissue or vice versa. As a result, pseudo-enhancement and signal blackout occur that may simulate or mask lesions [19]. These artefacts might either hide suspicious findings or mimic them, leading to misdiagnosis or unnecessary follow-up or complementary examinations (e.g., second-look ultrasound, MRI rescanning, or short-term follow-up).
While image subtraction and dynamic measurements are the main requirements for optimized breast MRI, these images are susceptible to motion artefacts, partial volume effects, and low-fat suppression [21]. Motion is considered the most relevant cause of artefacts in breast MRI potentially leading to degraded image quality or rendering studies completely non-diagnostic, even if the protocol itself is technically optimal [22]. Effects on signal intensity, conspicuity of moving structures, as well as general blurring, can obscure real lesions or create fabricated findings - pseudo lesions. Both physiologic and non-physiologic movement can cause artefacts in the phase-encoding direction. Physiologic motion can be caused by fluid, including pleural fluid or blood in the vessels: this type of motion-related artefact is challenging to eliminate, but its effects can be minimized if the phase-encoding direction is in the left-to-right direction [23]. Physiologic motion, typically caused by respiration and cardiac motion, and non-physiologic motion due to anxiety and discomfort [24] also results in unsatisfactory image quality due to breast displacement between the acquisition of unenhanced and contrast-enhanced images, generating misregistration artefacts on subtracted images. Non-physiological artefacts can be influenced by calming patients, giving them a sense of confidence, and informing them of all the next steps to achieve artefact-free images [25].
It is rather important to inform patients how the breast MRI examination is being done, in order to prepare them, improve patients' compliance, and decrease patients' discomfort. Explaining the importance of lying still during the examination is very important in the preparation routine. Radiology staff should use blankets and earmuffs to improve patients' comfort further.
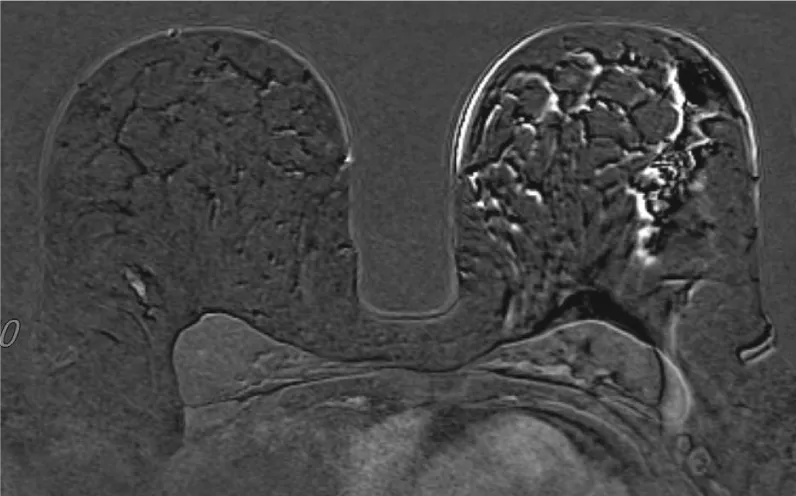
An important reason for patient motion is contrast administration. Contrast administration may lead to discomfort, feeling of tingling and warmth, and even some pain in the arm the contrast was administered to. If it appears suddenly, with no verbal warning, it may cause arm yanking, with all the muscle movements that go along, including pectoral muscle shift. Pectoral muscle shift may move breast tissue, resulting in motion artefacts on the subtraction reconstructed images, as explained before. We presumed that gentle verbal warning of patients before starting and right after finishing the nonenhanced sequence, which precedes contrast enhancement, may prevent hand yank, and decrease motion artefacts. Furthermore, if patients would have had expected pathology in the single breast (e.g. in case of the pre-surgical staging of the breast cancer) we might administer a contrast agent in the contra-lateral arm in order to decrease movement artefact in the breast of interest.
The aim of the study was to determine the influence of the contrast agent injection side and improve communication with patients to motion artefacts on subtraction reconstructions in order to prove that unexpected events may cause non-physiological movement resulting the motion artefacts, and that well-timed warning may decrease such artefacts.
For the implementation of this study, each patient signed a handwritten informed consent for breast MRI in which she agreed to the examination and the application of gadolinium contrast. The Ethics Committee of Dr. Juraj Njavro Vukovar National Memorial Hospital gave written approval for conducting research in that institution.
Materials and methods
A prospective cohort analysis of contrast-enhanced breast MRI examinations carried out between July 2019 and May 2020 was performed in the Department of Radiology, National Memorial Hospital Vukovar. All breast MRI examinations were performed on a 1.5T magnetic field strength system (Avanto by Siemens Healtheneers, Erlangen, Germany), with a dedicated breast array coil system (8 channel coil by Siemens Heatheneers, Erlangen; Germany) with an image matrix of 384x288. MRI protocol included: axial T1 spin-echo, axial T2 TIRM, and axial T1 SPAIR dynamic study. T1-weighted sequences are useful for detecting the presence of a fatty component within a lesion, which is also a significant aspect in predicting its benign nature [26]. T2-weighted sequences with fat saturation are useful for detecting mass lesions and cysts. In the T1 SPAIR dynamic study, an unenhanced image was obtained during 60-second scanning. After an unenhanced image, contrast media (gadoterate meglumine) intravenous injection was applied during a 30-second pause through an automatic injector (Accutron MR by Medtron AG, Germany). After that pause 5 subsequential one-minute long contrast-enhanced T1 SPAIR dynamic scans were obtained. Standard subtraction images were reconstructed by subtracting the precontrast images from the following postcontrast images on a pixel-by-pixel basis [27]. No breast compression was used during the examination as recommended by the European Society of Breast Imaging (EUSOBI) [28].
The study included 156 patients who were regularly scheduled for an MRI examination and had standard examination consent filled in prior to the examination. All patients were routinely informed of the examination and were told that the technical quality of breast MRI depends on their compliance. Men were excluded from this study. Our patients were between 27 and 87 years old.
They were advised to breathe normally during the examination, stay still, and remain calm upon contrast administration which can be felt as a warm, sometimes slightly burning sensation in their arm. Cannula for contrast application was introduced to the cubital vein and contrast application arm was noted for each patient. The first group of patients didn't receive any further information regarding the exam, and they weren't communicated with before or during contrast administration (unwarned patients group). The second group of patients (the warned patients group) received a contrast application warning through noise-canceling headsets that enabled one-way communication from the radiology technician to the patient. Accordingly, they were verbally alerted before the unenhanced dynamic sequence would start, as well as upon its ending, i.e. right before intravenous contrast application. They were explained this protocol before the examination started.
A clinical radiology specialist with 17 years of specialist experience in breast imaging measured movement artefacts at workstations with minimally two 3MP medical graded monitors in hospital picture archiving and communication system (Infinitt PACS, Infinitt Healthcare, South Korea). Only patients with whole five dynamic subtraction sequences were included in the study. Displacement of the edge of the pectoral muscle was considered to be a constant parameter that shows the intensity of movement artefacts: the larger the displacement, the lower the diagnostic quality of the image. The displacement of the edge of the pectoral muscle, or the pectoral shift, in millimeters in the anteroposterior and lateral-lateral directions, were measured presuming that anteroposterior shift is harder to cancel since breathing movement considerably contributes to it. The pectoral shift was measured on the most orthogonal slice to the pectoral muscle. We considered only one reader to be enough since the measurement of pectoral muscle shift is a simple operation demanding no special training, and is expected to be uniform.
Statistical analysis was performed using Statistica software (v13.5, 2018) by TIBCO. Mann-Whitney test was used to test the differences with the p<0.05 considered statistically significant.
Results
156 patients underwent breast MRI in one year period from July 2019 to May 2020. Patients were scheduled due to pre-surgical staging, evaluation of neo-adjuvant therapy, nipple discharge, problem-solving, high-risk patient screening, and occult carcinoma search. 10 of them were ruled out of the study regarding short protocol with only two post-contrast scans. 71 of them were warned before the dynamic sequence started (before non-enhanced scanning), as well as right before injecting contrast agents (warned patients - WP), and 75 of them didn't receive any warning for contrast application (unwarned patients - UWP).
Women included in the study were aged 27 to 87 (WP 27 to 80 years old, UWP 30 to 87), mean age of 52 (WP 51.1, UWP 52.8 years old). There is no statistically significant difference in age between warned and unwarned groups of patients (p=0.35) (Table 1).
If patients are divided into two age groups (younger than 50, and 50 and older), pectoral shift showed to be larger for patients older than 50 in both warned (p=0) and unwarned groups (p=0).
Mann Whitney U Test showed a statistically significant difference between warned and not warned women - the motion artefact proved to be smaller in warned women (p=0.001) with no artefacts observed in 361 warned, and only 267 in unwarned women (Table 2).
There is no statistically significant difference in motion artefact size between AP and LL for unwarned patients (p=0.064), but there is one for warned patients. Motion artefact is larger in AP projection than in LL projection for warned patients (p=0.002) (Table 3).
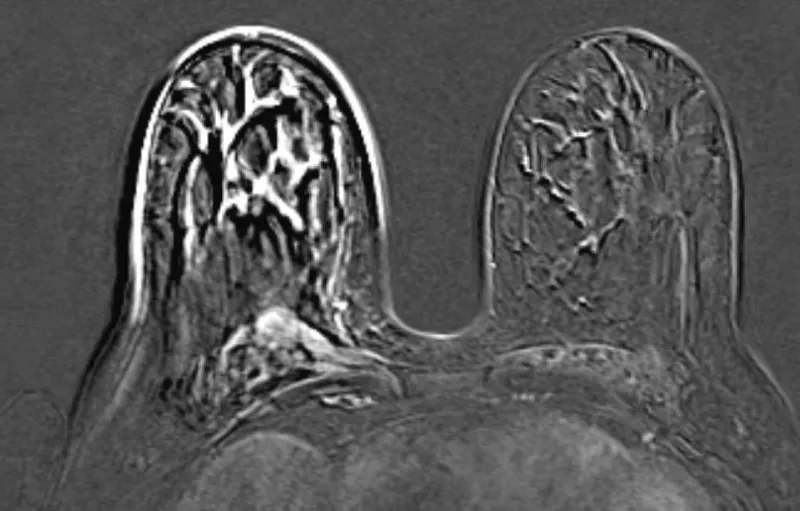
70 women received contrast agents in their left arm (30 warned women, 40 unwarned ones), and 77 of them in the right arm (42 warned women, 35 unwarned ones). Artefacts proved to be smaller on the contra-lateral side of the arm the contrast was injected into (Table 4).
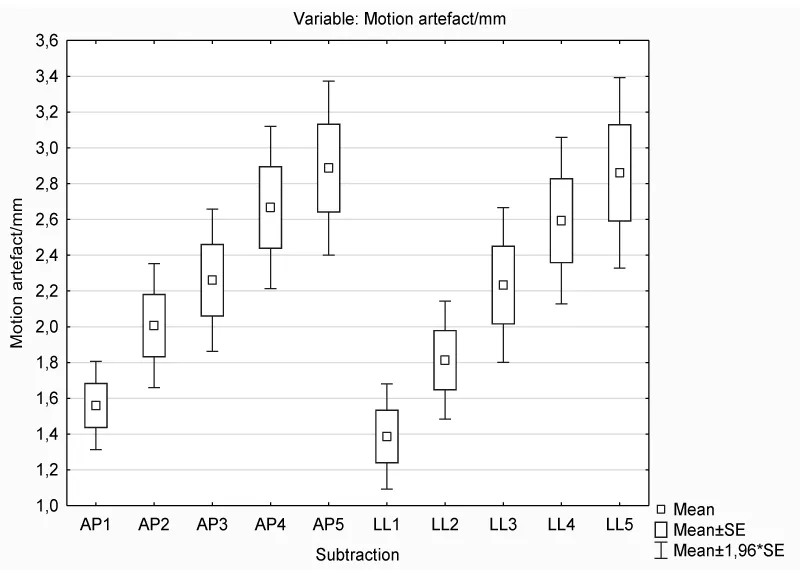
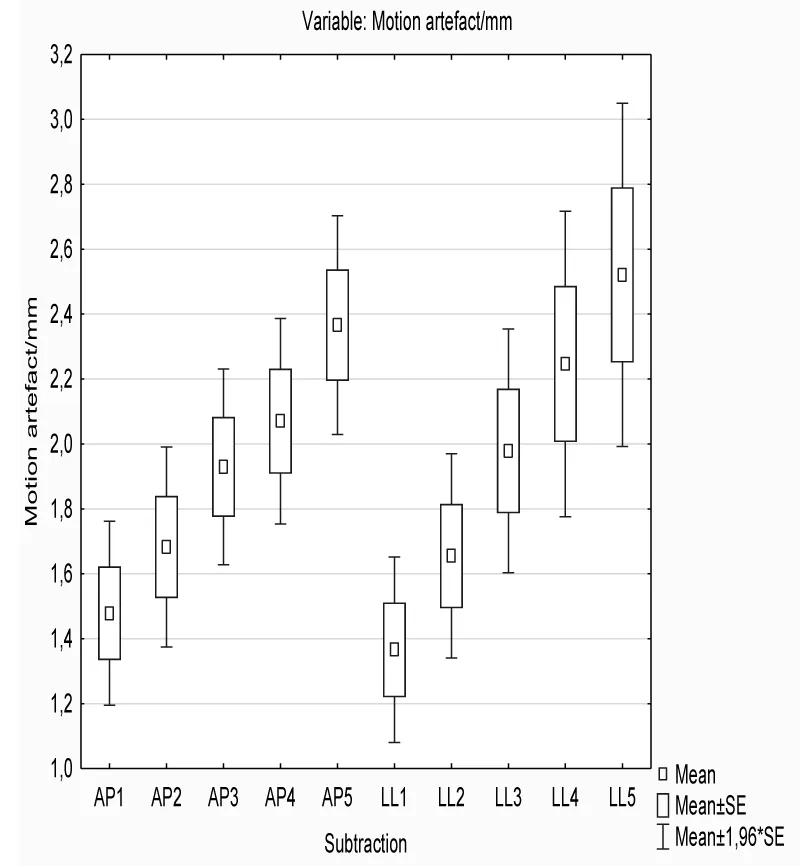
Artefacts showed to be larger on the later dynamic scans in both directions in the unwarned women group (Figure 1), and the same trend was observed in the warned group as well (Figure 2).
Unwarned patients showed similar artefact size in millimeters in both directions - no statistically significant difference was found in the antero-posterior and latero-lateral direction in this group (p=0.064). On the other hand, the pectoral shift was larger in the antero-posterior direction in comparison with the latero-lateral movement of the pectoral muscle in warned women (p=0.002).
Discussion
Previous studies showed that motion artefacts are the most frequent and maybe the most important artefacts affecting breast MR image quality [29]. In our study, 628 scans showed no pectoral muscle shift in any direction, 1553 scans showed minimal shift (1 to 2mm) probably due to breathing, while 739 showed larger pectoral movement (Table 2).
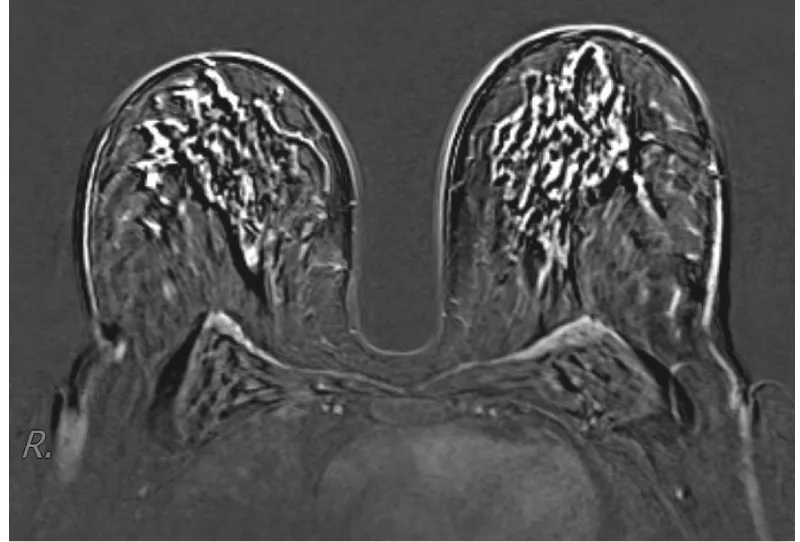
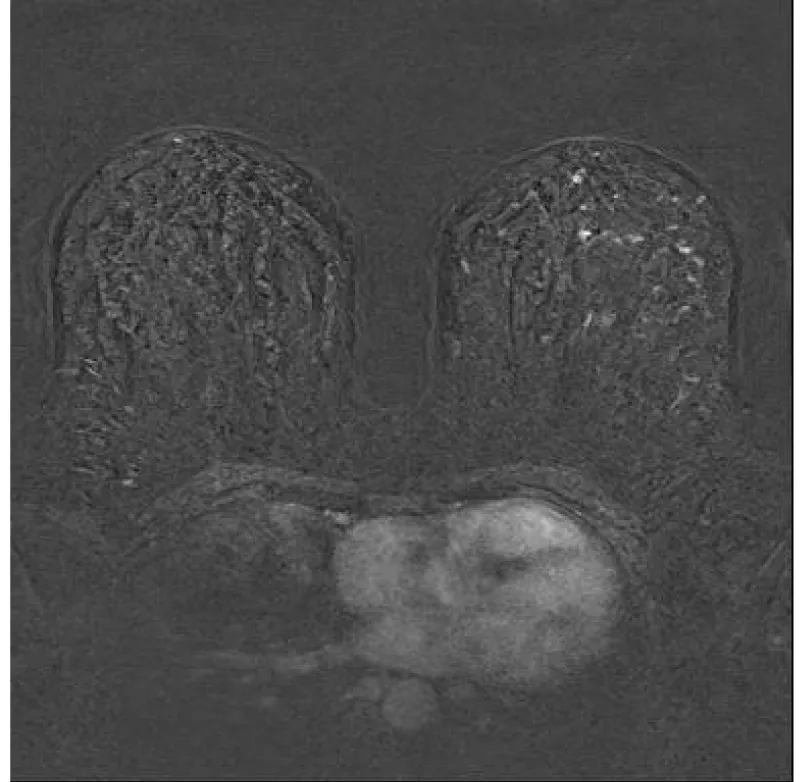
Our study showed that verbal warning before starting and upon ending the first, unenhanced, dynamic sequences, significantly decreased movement artefacts (Table 2). Larger motion artefacts in unwarned women are probably due to the unexpected discomfort of contrast application (Figure 3). A surprising discomfort induces avoidance and escapes instinct through thoracic movement in between two scans, which causes motion artefact on subtraction reconstruction images. Warned women expected the discomfort in advance and readily put up with it with less body movement (Figure 4). There are no papers quantifying the importance of additional patient contrast administration warnings in order to decrease these unfavorable events published to our knowledge.
An interesting fact is that unwarned women showed the same amount of pectoral shift in both antero-posterior and latero-lateral directions. However, warned women showed a greater amount of pectoral shift in the antero-posterior direction than in the latero-lateral (Table 3). Breathing contributes significantly to antero-posterior pectoral shift, it cannot be avoided, therefore antero-posterior motion artefacts decrease showed to be less susceptible to a verbal warning as opposed to a latero-lateral shift.
Furthermore, we have proved that motion artefact are significantly larger on the breast ipsi-lateral to contrast agent administration arm (Table 4). The reason may be the same: instinctive avoidance of the discomfort is more prominent on the side of the body the contrast is administered to, thus the artefacts are larger on that side (Figure 5,6).
This concurs with one previously published study [30] which reviewed contrast administration laterality with breast MR motion artefacts relationship. Motion artefacts were studied in three subjective categories (optimal, no reduction of diagnostic power, and reduction of diagnostic power), while we quantified motion artefacts measuring the post-contrast shift of pectoral muscle on the subtraction reconstructed images in millimeters, which we found a more unbiased indicator.
Motion artefacts were larger in women older than 50 than in the younger group which shows that physical fitness plays an important role in keeping still for the time of scanning. The next argument supporting this fact is that artefact would increase in almost all patients in the later dynamic sequences during the scanning. This is in line with a study advocating shorter protocols in order to reduce motion artefacts [31].
This research was conducted on a single MR machine and we've found that a major limitation of our study. The multicentric fashion of the study would be preferred in order to exclude particular MR scanner influence on results.
Regarding the listed data and the results of this study, we can safely conclude that warning patients of the contrast administration at the right time reduces motion artefacts significantly. These results made us implement the described warning in the standard breast MR protocol in our hospital. However, bear in mind how important is to properly explain breast MR protocol before placing a patient into the MR gantry, and to let the patients know why and what are we warning them about. It does take a few minutes more per examination, but it showed to be worth it due to much better breast MR image quality.
The other repercussion of this study is wiser contrast administration laterality selection. If we expect pathology in one breast (e.g. left one), we should choose to apply contrast agent to the contra-lateral arm (right one in the previous example), since patients showed greater pectoral muscle movement on the contrast agent administration side.
Conclusion
The motion artifact on the breast MRI is smaller on the contralateral side in the arm that has the canulla attached. The shift is smaller in the earlier sequences, which confirms the theory of implementing shorter protocols. Good communication and detailed explanation of the examination process reassures the patient, reduces movement artifacts and ultimately brings better quality images.
Take home message
Place the canulla in the contralateral arm on the side of the breast in which the lesion is of interest. Explain to the patient the course of the examination and announce when the contrast will be applied in order to reduce motion artifacts and improve the quality of the images.
- Spick C, Szolar DHM, Preidler KW, Tillich M, Reittner P, Baltzer PA. Breast MRI used as a problem-solving tool reliably excludes malignancy. Eur J Radiol. 2015 Jan;84(1):61-64. doi: 10.1016/j.ejrad.2014.10.005. Epub 2014 Oct 19. PMID: 25454098.
- Leung JW. MR imaging in the evaluation of equivocal clinical and imaging findings of the breast. Magn Reson Imaging Clin N Am. 2010 May;18(2):295-308, ix-x. doi: 10.1016/j.mric.2010.02.012. PMID: 20494313.
- Kuhl CK. The Changing World of Breast Cancer: A Radiologist's Perspective. Invest Radiol. 2015 Sep;50(9):615-28. doi: 10.1097/RLI.0000000000000166. PMID: 26083829; PMCID: PMC4623842.
- Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology. 2009 Nov;253(2):281-3. doi: 10.1148/radiol.2532091401. PMID: 19864520.
- Warner E, Causer PA, Wong JW, Wright FC, Jong RA, Hill KA, Messner SJ, Yaffe MJ, Narod SA, Plewes DB. Improvement in DCIS detection rates by MRI over time in a high-risk breast screening study. Breast J. 2011 Jan-Feb;17(1):9-17. doi: 10.1111/j.1524-4741.2010.01018.x. PMID: 21251121.
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007; 244(3):672-91. doi: 10.1148/radiol.2443051661.
- Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 2009 Jan;22(1):28-39. doi: 10.1002/nbm.1273. PMID: 18654999.
- Rausch DR, Hendrick RE. How to optimize clinical breast MR imaging practices and techniques on Your 1.5-T system. Radiographics. 2006 Sep-Oct;26(5):1469-84. doi: 10.1148/rg.265055176. PMID: 16973776.
- Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology. 1989 Mar;170(3 Pt 1):681-6. doi: 10.1148/radiology.170.3.2916021. PMID: 2916021.
- Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, Schild HH. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999 Apr;211(1):101-10. doi: 10.1148/radiology.211.1.r99ap38101. PMID: 10189459.
- Daniel BL, Yen YF, Glover GH, Ikeda DM, Birdwell RL, Sawyer-Glover AM, Black JW, Plevritis SK, Jeffrey SS, Herfkens RJ. Breast disease: dynamic spiral MR imaging. Radiology. 1998 Nov;209(2):499-509. doi: 10.1148/radiology.209.2.9807580. PMID: 9807580.
- Partridge SC, Stone KM, Strigel RM, DeMartini WB, Peacock S, Lehman CD. Breast DCE-MRI: influence of postcontrast timing on automated lesion kinetics assessments and discrimination of benign and malignant lesions. Acad Radiol. 2014 Sep;21(9):1195-203. doi: 10.1016/j.acra.2014.04.013. Epub 2014 Jul 4. PMID: 24998690; PMCID: PMC4166542.
- James F, Wiedenhoefer MD, FACOG DABR. Pamela O, Kenneth A. Kist An overview of breast MRI By Hassan Shahid, Carol Dornbluth.
- Alnafea MA. Detection and diagnosis of breast diseases. Breast Imaging. 2017. https://doi.org/10.5772/intechopen.69898.
- Badu-Peprah A, Adu-Sarkodie Y. Accuracy of clinical diagnosis, mammography and ultrasonography in preoperative assessment of breast cancer. Ghana Med J. 2018 Sep;52(3):133-139. doi: 10.4314/gmj.v52i3.5. PMID: 30602798; PMCID: PMC6303551.
- Weledji EP, Tambe J. Breast Cancer Detection and Screening, Medical & Clinical Reviews. 2018; 4. https://doi.org/10.21767/2471-299X.1000071.)
- Uematsu T, Kasami M, Watanabe J. Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol. 2011 Nov;21(11):2261-7. doi: 10.1007/s00330-011-2175-6. Epub 2011 Jun 18. PMID: 21688006.
- Baltzer PAT, Kaiser WA, Dietzel M. Lesion type and reader experience affect the diagnostic accuracy of breast MRI: a multiple reader ROC study. Eur J Radiol. 2015 Jan;84(1):86-91. doi: 10.1016/j.ejrad.2014.10.023. Epub 2014 Nov 14. PMID: 25466772.
- Ojeda-Fournier H, Choe KA, Mahoney MC. Recognizing and interpreting artifacts and pitfalls in MR imaging of the breast. Radiographics. 2007 Oct;27 Suppl 1:S147-64. doi: 10.1148/rg.27si075516. PMID: 18180224.
- Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging. 2015 Oct;42(4):887-901. doi: 10.1002/jmri.24850. Epub 2015 Jan 28. PMID: 25630632; PMCID: PMC4517972.
- Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, Evans A, Fallenberg E, Forrai G, Fuchsjäger MH, Gilbert FJ, Helbich TH, Heywang-Köbrunner SH, Camps-Herrero J, Kuhl CK, Martincich L, Pediconi F, Panizza P, Pina LJ, Pijnappel RM, Pinker-Domenig K, Skaane P, Sardanelli F; European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition. Breast MRI: EUSOBI recommendations for women's information. Eur Radiol. 2015 Dec;25(12):3669-78. doi: 10.1007/s00330-015-3807-z. Epub 2015 May 23. PMID: 26002130; PMCID: PMC4636525.
- Taourel P, Thomassin I, Tardivon A. Indications actualisees de l’IRM du sein: synthese du referentiel edite par The European Society of Breast Cancer Specialist (EUSOMA). Imag la Femme. 2011; 21:154-159.
- Ojeda-Fournier H, Choe KA, Mahoney MC. Recognizing and interpreting artifacts and pitfalls in MR imaging of the breast. Radiographics. 2007 Oct;27 Suppl 1:S147-64. doi: 10.1148/rg.27si075516. PMID: 18180224.
- Rausch DR, Hendrick RE. How to optimize clinical breast MR imaging practices and techniques on Your 1.5-T system. Radiographics. 2006 Sep-Oct;26(5):1469-84. doi: 10.1148/rg.265055176. PMID: 16973776.
- Szumowski J, Coshow W, Li F, Coombs B, Quinn SF. Double-echo three-point-Dixon method for fat suppression MRI. Magn Reson Med. 1995 Jul;34(1):120-4. doi: 10.1002/mrm.1910340118. PMID: 7674890.
- Choi EJ, Youk JH, Choi H, Song JS. Dynamic contrast-enhanced and diffusion-weighted MRI of invasive breast cancer for the prediction of sentinel lymph node status. J Magn Reson Imaging. 2020 Feb;51(2):615-626. doi: 10.1002/jmri.26865. Epub 2019 Jul 16. PMID: 31313393.
- Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008 Jul;18(7):1307-18. doi: 10.1007/s00330-008-0863-7. Epub 2008 Apr 4. PMID: 18389253; PMCID: PMC2441490.
- Clauser P, Dietzel M, Weber M, Kaiser CG, Baltzer PA. Motion artifacts, lesion type, and parenchymal enhancement in breast MRI: what does really influence diagnostic accuracy? Acta Radiol. 2019 Jan;60(1):19-27. doi: 10.1177/0284185118770918. Epub 2018 Apr 18. PMID: 29667880.
- Fiaschetti V, Pistolese CA, Funel V, Rascioni M, Claroni G, Della Gatta F, Cossu E, Perretta T, Simonetti G. Breast MRI artefacts: evaluation and solutions in 630 consecutive patients. Clin Radiol. 2013 Nov;68(11):e601-8. doi: 10.1016/j.crad.2013.05.103. Epub 2013 Aug 3. PMID: 23916550.
- Carbonaro LA, Schiaffino S, Clauser P, Tomkova L, Iodice M, Zuiani C, Sardanelli F. Side of contrast injection and breast size correlate with motion artifacts grade and image quality on breast MRI. Acta Radiol. 2021 Jan;62(1):19-26. doi: 10.1177/0284185120912408. Epub 2020 Mar 30. PMID: 32228030.
- Dogan BE, Scoggins ME, Son JB, Wei W, Candelaria R, Yang WT, Ma J. American College of Radiology-Compliant Short Protocol Breast MRI for High-Risk Breast Cancer Screening: A Prospective Feasibility Study. AJR Am J Roentgenol. 2018 Jan;210(1):214-221. doi: 10.2214/AJR.17.18267. Epub 2017 Nov 1. PMID: 29091003.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
