Imaging Journal of Clinical and Medical Sciences
Image processing techniques for the detection of brain tumours
Barmak Honarvar Shakibaei Asli* and Anaëlle Jasmin
Cite this as
Shakibaei Asli BH, Jasmin A (2023) Image processing techniques for the detection of brain tumours. Imaging J Clin Medical Sci 10(1): 004-012. DOI: 10.17352/2455-8702.000140Copyright License
© 2023 Shakibaei Asli BH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: This paper is centered around advancing brain image analysis through the introduction and evaluation of advanced methods.
Methods: With the overarching goal of enhancing both image quality and disease classification accuracy, the paper sets out to address crucial aspects of modern medical imaging. The research's trajectory begins by laying a strong foundation through an in-depth exploration of the principles governing Magnetic Resonance Imaging (MRI) and Computed Tomography (CT). This understanding serves as a springboard for the subsequent phases, wherein image quality improvement takes center stage.
Results: By employing cutting-edge image processing techniques, the research aims to reduce noise and enhance image clarity, thereby setting the stage for more reliable and precise analysis. The second phase involves segmentation, a pivotal step in brain image analysis. Various segmentation methods will be assessed to determine their efficacy in accurately identifying distinct brain structures. Finally, the paper delves into the realm of deep learning, particularly leveraging CNN, to classify brain images based on disease types. This sophisticated approach holds promise for refining disease identification accuracy by identifying nuanced patterns within the images.
Conclusion: Overall, the research aspires to modernize and elevate the field of brain image analysis, ultimately contributing to improved medical diagnostics and insights.
Abbreviations
MRI: Magnetic Resonance Imaging; IQA: Image Quality Assessment; CNN: Convolutional Neural Network; SSIM: Structural Similarity Index; PSNR: Peak Signal-to-Noise Ratio; BRISQUE: Blind/Referenceless Image Spatial Quality Evaluator; PIQE: Perception-based Image Quality Evaluator; NIQE: Naturalness Image Quality Evaluator
Introduction
Brain tumours have become a major health concern in recent years, affecting people of all ages and nationalities. According to the Cancer Research UK charity, there are approximately 11,400 people diagnosed with a primary brain tumoureach year in the UK. This highlights the urgent need for advanced medical imaging techniques to improve the accuracy of diagnosis, prognosis, and treatment of brain tumours [1]. Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans are among the most commonly used medical imaging modalities for brain tumourdetection and diagnosis in the UK. However, the images produced by these modalities are often complex and require advanced image processing techniques to extract meaningful information. Medical image processing is an interdisciplinary field that combines computer science, physics, mathematics, and medicine to develop techniques to analyze, manipulate, and visualize medical images. These techniques are essential in enhancing the quality of medical images, reducing noise, and extracting features that are crucial for diagnosis and treatment [2].
Brain tumourdetection and classification can significantly benefit from image processing techniques. Image processing can help improve the accuracy of tumourdetection, identification of its location, and determination of its type. It can also provide valuable information for surgical planning and therapy selection. Image processing techniques can also help in the early detection of brain tumours, enabling earlier interventions and better patient outcomes [3].
In the realm of modern healthcare, medical imaging has revolutionized the diagnosis and treatment of various medical conditions. Among the most crucial applications of medical imaging techniques are brain imaging modalities such as MRI, CT, and X-rays. These modalities provide invaluable insights into the intricate workings of the human brain, aiding clinicians in making accurate diagnoses, planning surgeries, and monitoring treatment outcomes.
This section explores the definition, types, symptoms, detection, and treatment of brain tumours, offering insights into current research and advancements in the field.
Definition
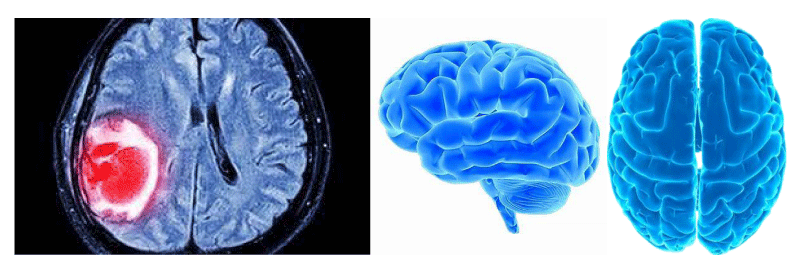
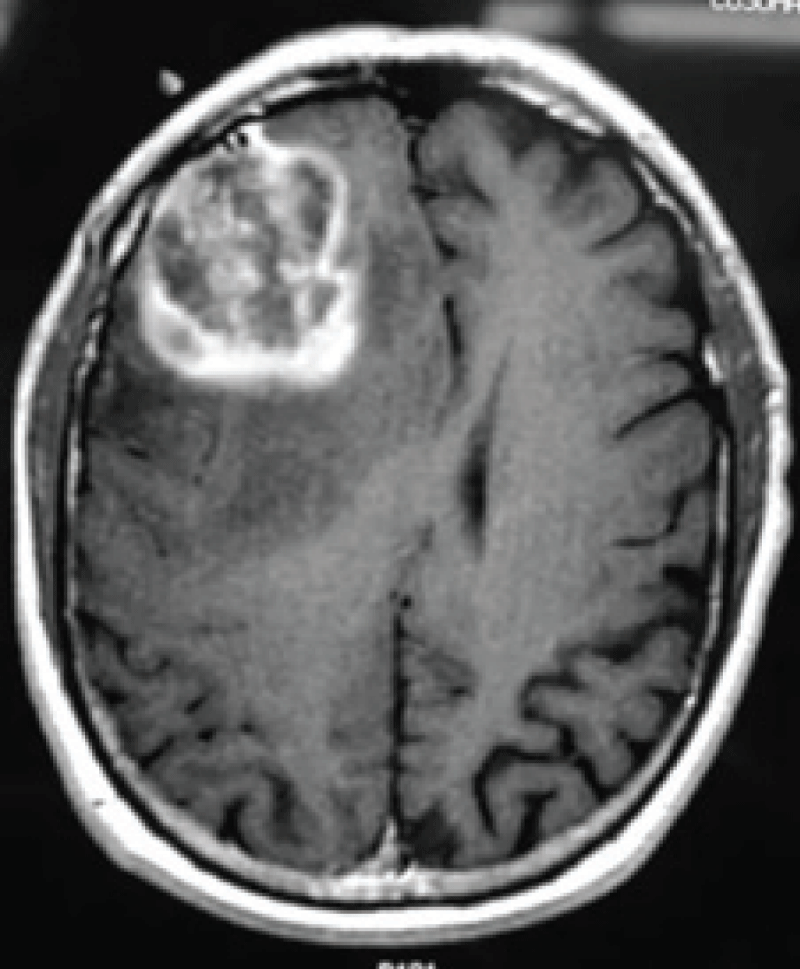
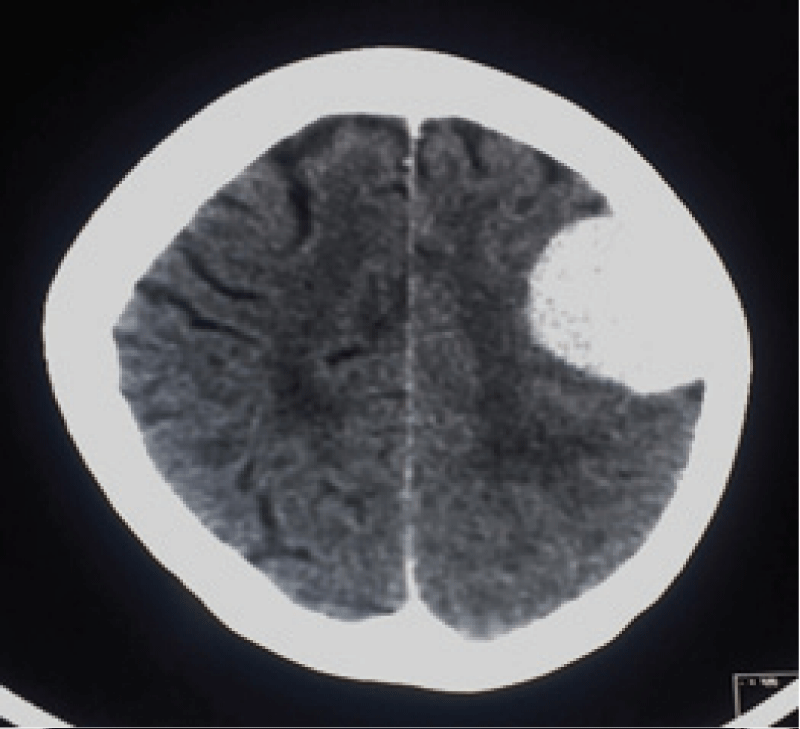
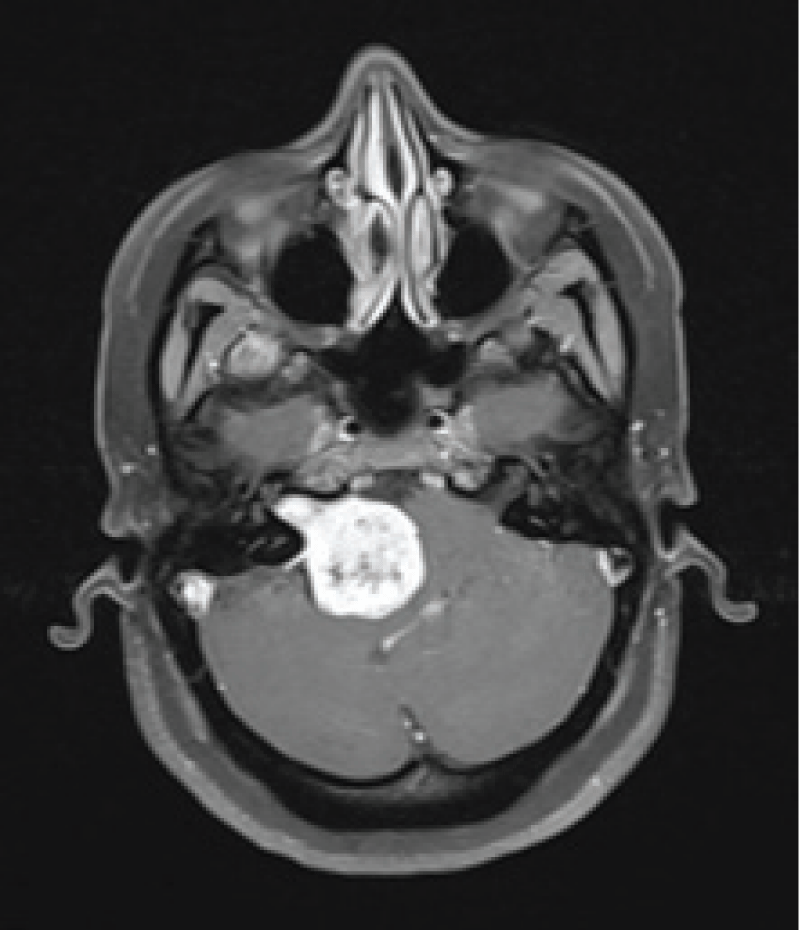
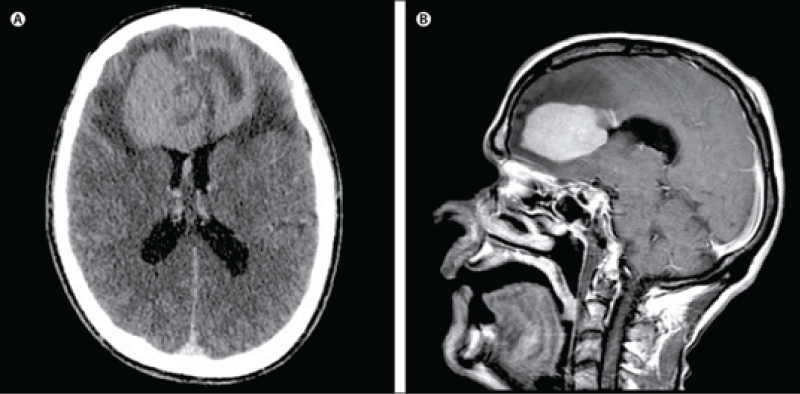
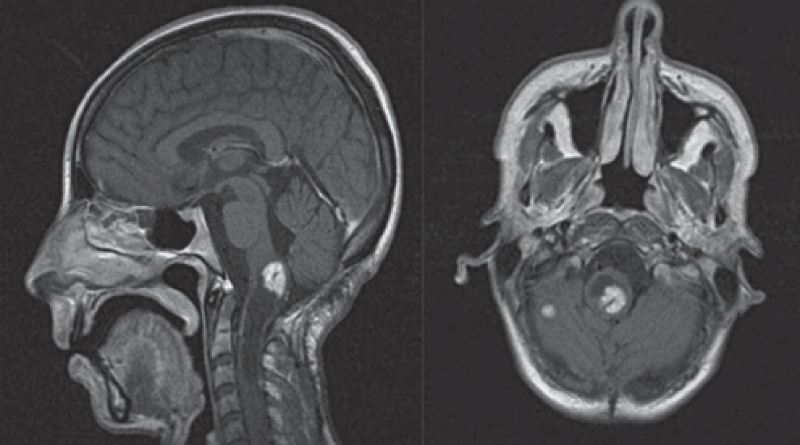
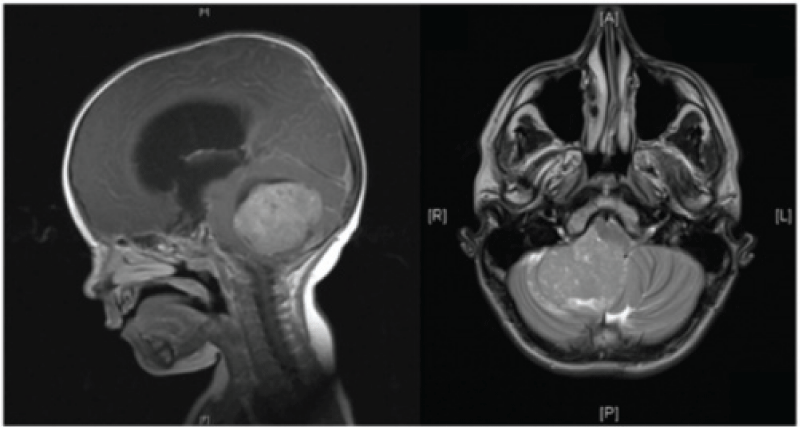
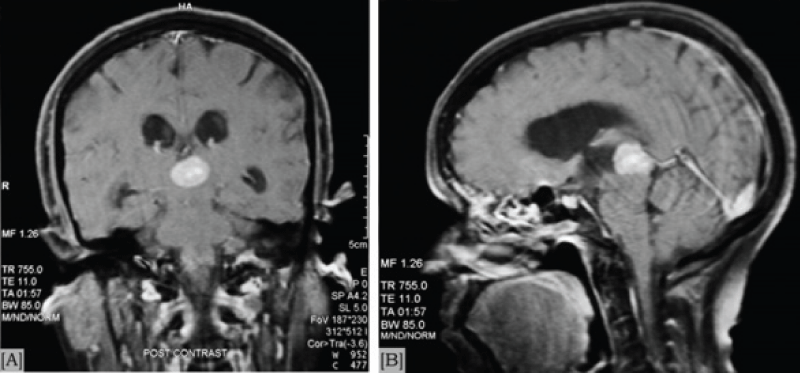
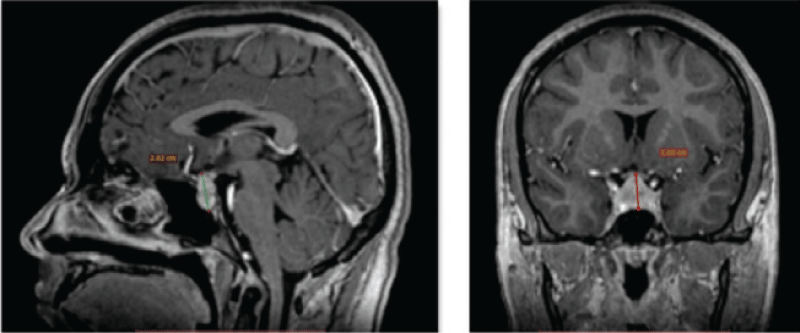
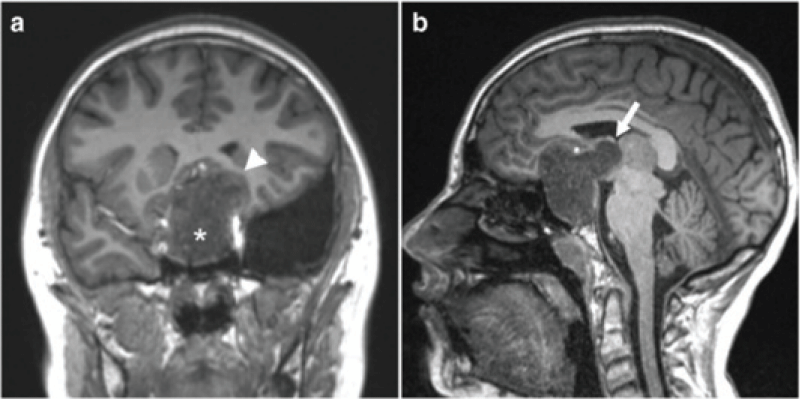
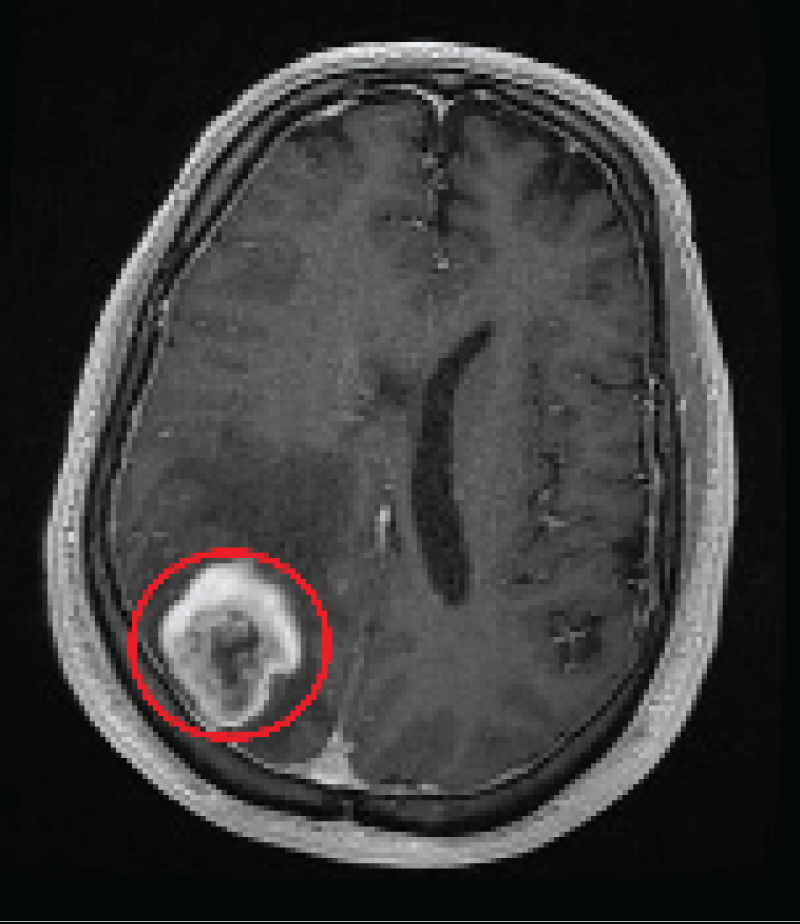
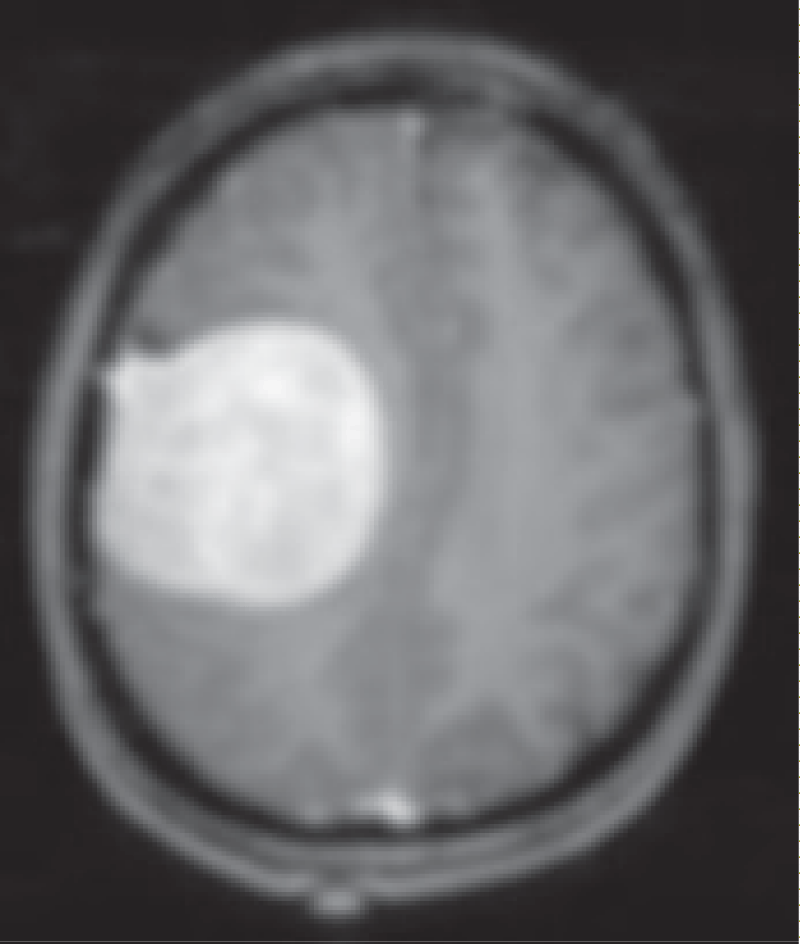
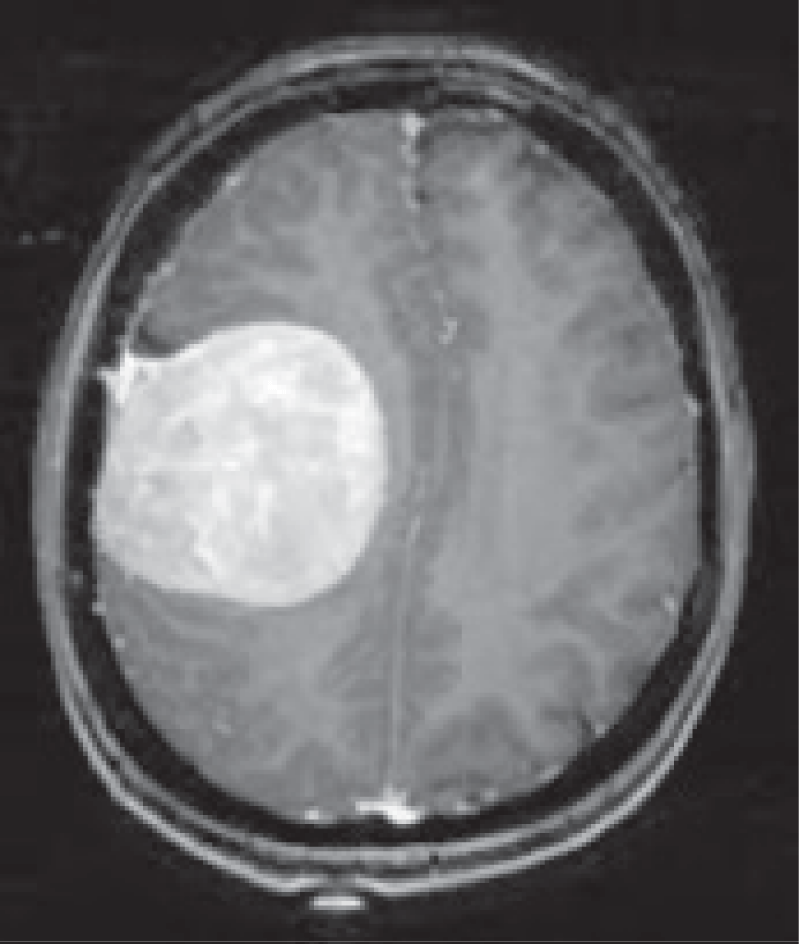
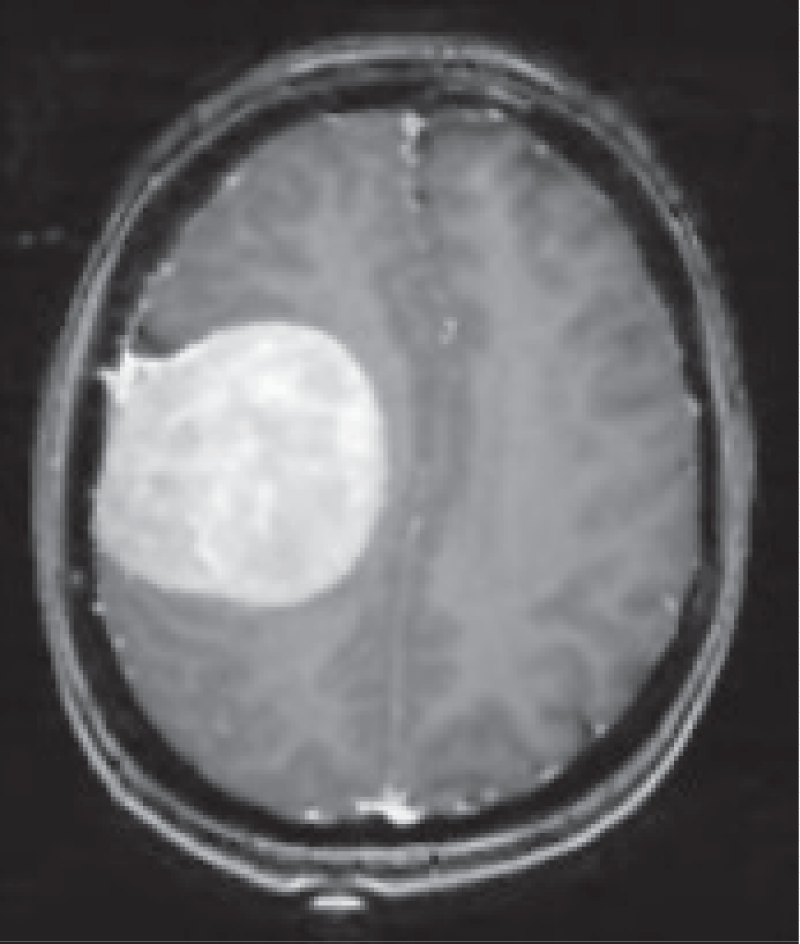
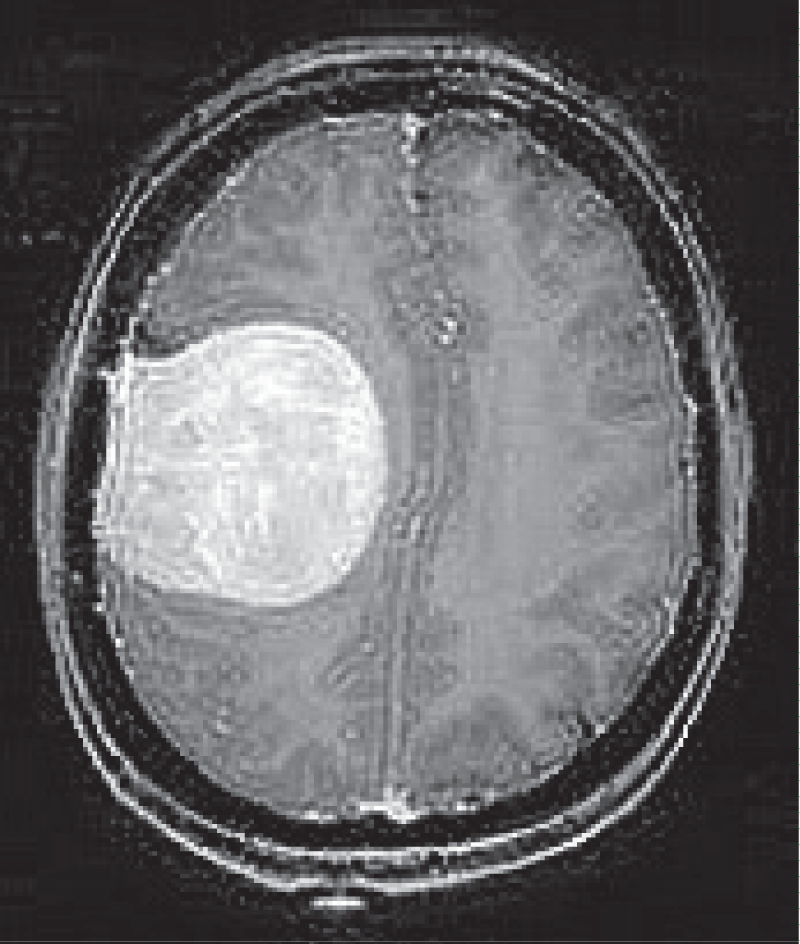
A brain tumourrefers to an abnormal growth of cells in the brain or its surrounding tissues, which can potentially disrupt the normal functioning of the central nervous system [4]. Figure 1 illustrates the different views of brain images including tumours.
A brain tumouroriginates when there is an uncontrolled multiplication of cells within the brain. While the exact causes of brain tumours are not always clear, certain factors such as exposure to ionizing radiation, genetic predisposition, environmental factors, and certain medical conditions may contribute to their development [8]. An overview of current advances in medical image processing and machine learning techniques for the detection and categorization of brain tumors is provided in [9]. Together with these topics, recent research in [10] describes component extraction, Machine Learning (ML), Transfer Learning (TL), morphology of brain tumors, and available data sets and augmentation techniques.
Types of brain tumours
Brain tumours can be classified into two main categories: primary and secondary. Primary brain tumours are those that begin within the brain itself, originating from the various cells and structures that make up the brain. There are many different types of primary brain tumours, as shown in Table 1. Secondary brain tumours, also known as metastatic brain tumours, are cancers that have spread from other parts of the body, such as the lungs, breast, or colon, and have migrated to the brain through the bloodstream [11].
Symptoms
The symptoms of brain tumours can vary depending on the location, size, and growth rate of the tumour. Common signs and symptoms may include persistent headaches, seizures, cognitive or memory problems, changes in mood or personality, visual or hearing disturbances, balance and coordination issues, nausea, vomiting, and progressive neurological deficits. However, it is essential to note that these symptoms are not exclusive to brain tumours and can be associated with various other conditions.
Detection
The diagnosis of a brain tumourtypically involves a combination of medical history evaluation, neurological examination, imaging techniques such as magnetic resonance imaging (MRI) or computed tomography (CT) scan, and, in some cases, a biopsy. Once a brain tumouris diagnosed, further tests may be conducted to determine the type, grade, and extent of the tumour, which help guide treatment decisions [21]. A proposed image moments technique applied in the health monitoring of brain tumours via image analysis procedures [22].
Treatment
Treatment options for brain tumours are multifaceted and depend on several factors, including the type, grade, location, and size of the tumour, as well as the patient's overall health. The primary treatment modalities include surgery, radiation therapy, and chemotherapy, often used in combination or sequentially. Surgery aims to remove as much of the tumouras safely possible, while radiation therapy and chemotherapy are employed to target and eliminate remaining cancer cells or slow down tumourgrowth.
In addition to conventional treatments, other therapeutic approaches may be utilized in the management of brain tumours. These can include targeted therapies, immunotherapy, hormone therapy, and supportive care measures to alleviate symptoms and enhance the patient's quality of life [23].
Methodology
Brain MRI plays a vital role in the detection of brain tumours. However, these images often suffer from blurring and noise, which can hinder accurate tumourdetection. To overcome this challenge, several techniques are commonly employed to deblur and denoise brain MRI images. Additionally, post-processing techniques are used to enhance the image quality, segment tumourregions, and classify the presence of tumours.
Image acquisition
In the pursuit of advancing medical imaging technology and improving the detection and diagnosis of brain tumours, researchers often rely on publicly available datasets for their studies.
The Kaggle Brain MRI Images dataset consists of a comprehensive collection of brain magnetic resonance imaging (MRI) scans, encompassing both tumour and non-tumor cases, as shown in Table 2 [24] (Note that this research complies with data protection regulations, ethical guidelines, and privacy concerns using Kaggle datasets). It provides a diverse range of brain images, allowing to explore various imaging characteristics associated with brain tumours.
This dataset includes a substantial number of MRI images, representing different slices and orientations of the brain. Each image is labeled to indicate the presence or absence of a tumour, and also the type of tumour, facilitating the development and evaluation of tumour detection algorithms.
Pre-processing techniques
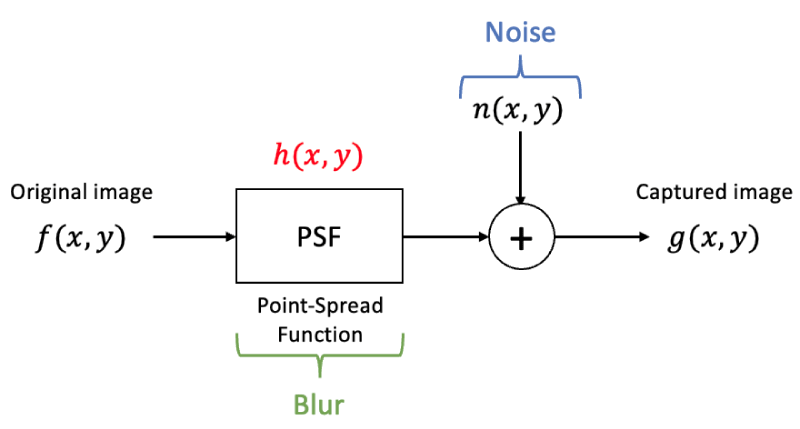
In the field of image processing and analysis, images are often regarded as 2D signals, subject to noise and blurring during the acquisition or transmission process. Figure 2 shows the imaging system which is modeled the acquired image in terms of the original image by convolving it with a point-spread function (PSF) and added noise. These inherent challenges can significantly impact the accuracy of classification tasks. Therefore, to improve the performance of image classification algorithms, it is essential to undertake pre-processing and processing techniques to mitigate noise and blur, enabling more robust and reliable image classification results [25-27].
Here is the equation of the captured image
x and Y refer to the image pixels. f is the original view, h refers to the blur, and n to the noise. G is the final 2D signal, the captured image.
The convolution of f by h corresponds to the following calculation:
To avoid this complex calculation, the Fourier transform can be applied:
Eq. (3) is the easiest way to understand the presence of noise and blur on a captured image.
Fourier transform is a mathematical tool that decomposes a signal or image into its constituent frequencies, represented as complex numbers, providing insights into the presence and strength of different frequency components for deblurring and image enhancement applications [28,29].
Results
The pre-processing stage focused on mitigating the effects of blurring and noise in brain MRI images. A combination of advanced image processing techniques was employed to achieve this goal. The pre-processing pipeline included Gaussian blur, total variation filtering, Wiener filtering, and Lucy-Richardson filtering. Each technique was applied to a sample of tumorous brain MRI images.
The Gaussian blur was employed to reduce image noise and create smoother image contours. The total variation filter, known for its ability to preserve edges while denoising images, helped enhance the contrast of the tumourregions. The Wiener filter, which aims to remove noise from images, contributed to further noise reduction in the pre-processed MRI scans. Lastly, the Lucy-Richardson filter, a popular deblurring technique, effectively restored lost details and improved the overall image quality as shown in Table 3.
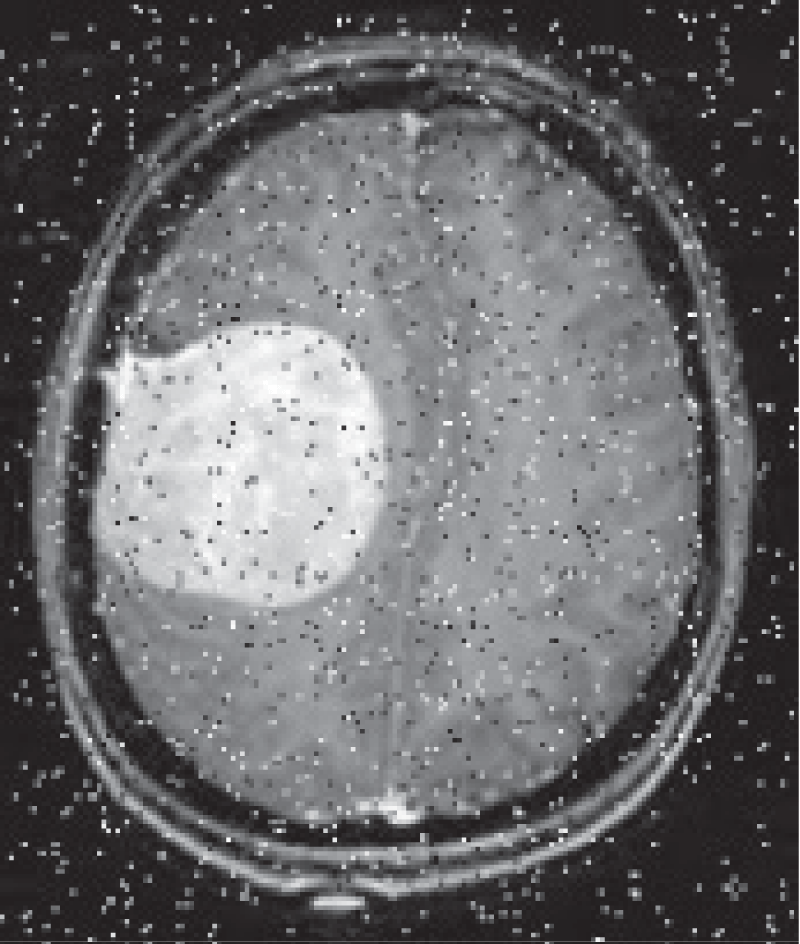
Poisson noise is commonly encountered in low-light imaging conditions, such as in certain types of medical imaging equipment. It appears as random variations in pixel intensities and can introduce unwanted artifacts into the image. By simulating Poisson noise and then applying Poisson noise removal techniques, the images were effectively restored to reduce the impact of noise on the accuracy of tumour detection.
Similarly, salt and pepper noise was artificially added to the images to simulate the presence of random black and white pixels that can occur due to various factors, such as data corruption during transmission or errors in image acquisition. Techniques for salt and pepper noise reduction were then applied to eliminate these noisy pixels, improving the clarity of the images and facilitating more precise tumour region identification.
The non-local mean filtering and wavelet thresholding techniques were also utilized in conjunction with Poisson noise and salt and pepper noise removal to further enhance the quality of the pre-processed brain MRI images. These combined algorithms effectively addressed noise and other artifacts while preserving critical image features, resulting in clearer and more reliable images for subsequent analysis and interpretation.
By carefully incorporating these pre-processing techniques, the brain MRI images were optimized for tumour detection and classification, providing a solid foundation for accurate diagnosis and treatment planning. The pre-processed images from Table 4, play an essential role in improving the performance and reliability of the subsequent image analysis and classification algorithms, enabling medical professionals to make more informed decisions in the context of brain tumour detection and treatment.
Image quality assessment results
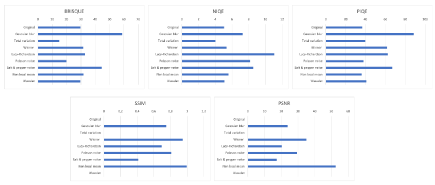
Several Image Quality Assessment (IQA) methods, including Blind/Referenceless Image Spatial Quality Evaluator (BRISQUE), Perception-based Image Quality Evaluator (PIQE), Naturalness Image Quality Evaluator (NIQE) [30], Structural Similarity Index (SSIM), and Peak Signal-to-Noise Ratio (PSNR) [31], were employed to evaluate the quality of the pre-processed brain MRI images, as shown in Figure 3. These IQA methods are essential for objectively measuring and comparing the performance of different pre-processing techniques, as well as for assessing the impact of various noise reduction and deblurring algorithms on image quality.
Figure 3 shows that, based on the BRISQUE metric as a no-reference method, the total variation filter and Poisson noise have a significantly good impact on the original image. However, Gaussian blur and Salt and pepper degrade the quality of the original image. On the other hand, as SSIM is based on a comparison and full-reference approach, the Non-local mean (NLM) image is the closest to the original one. Salt and pepper noise is the method that generates the furthest image from the original one.
Detection results
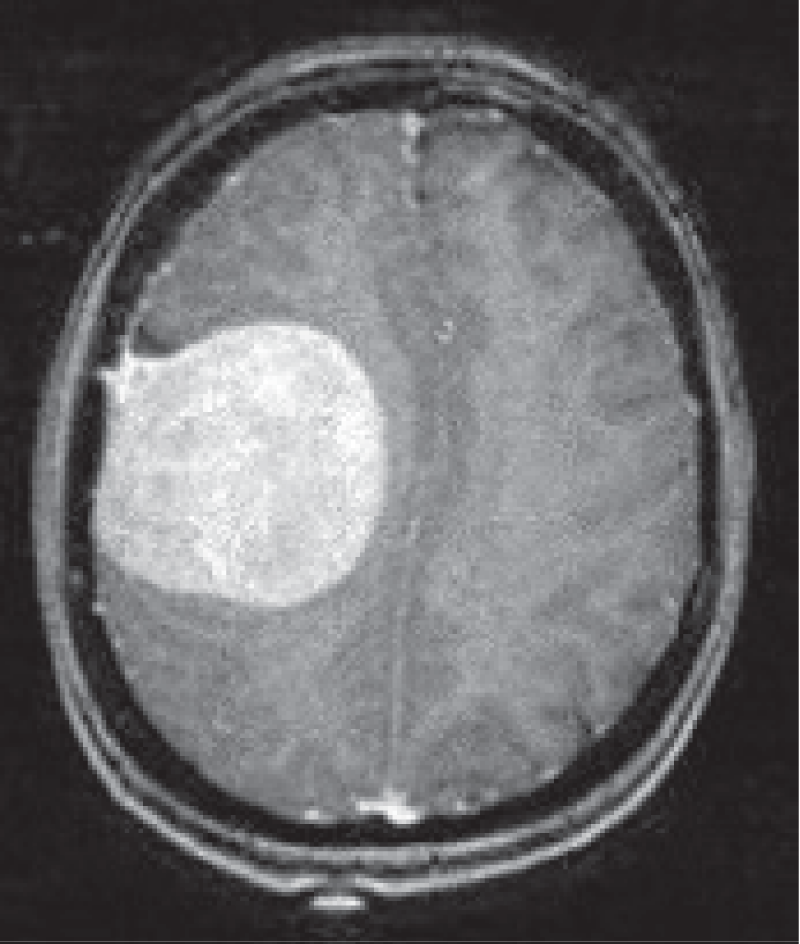
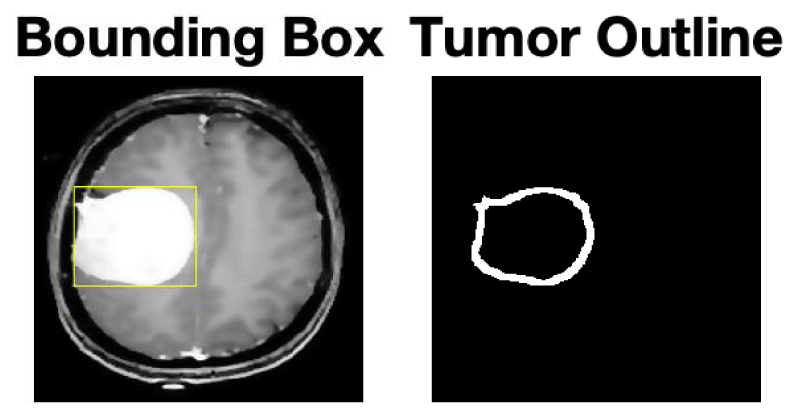
Building upon meticulous segmentation, the detection phase introduces refined visualizations that emphasize the tumour's localization and shape. The integration of a bounding box around the tumouroffers a precise spatial representation of the anomaly within the brain MRI image, as shown in Figure 4. This bounding box not only encapsulates the tumour's spatial extent but also enhances the visibility of its location. Further enhancing the detection process, the intricate outline of the tumouris crafted using techniques such as dilation and erosion. The result is a finely detailed representation of the tumour's contours, facilitating a deeper understanding of its characteristics and boundaries. Through this comprehensive detection process, medical professionals can accurately assess the tumour's presence and develop targeted approaches for further analysis.
Classification results
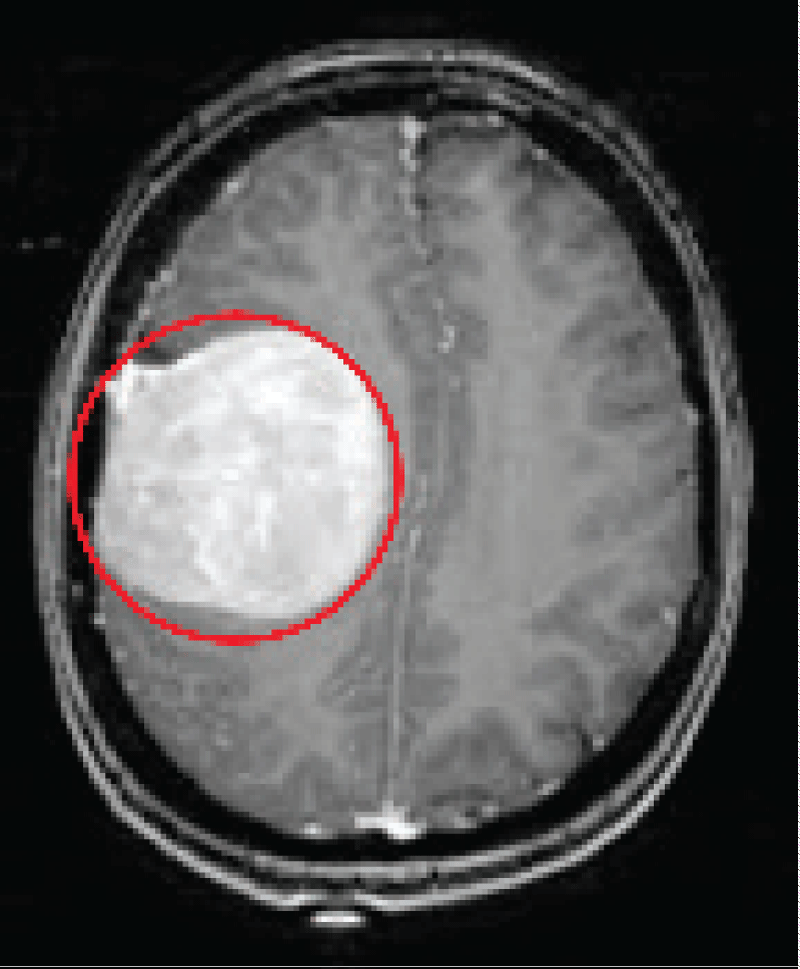
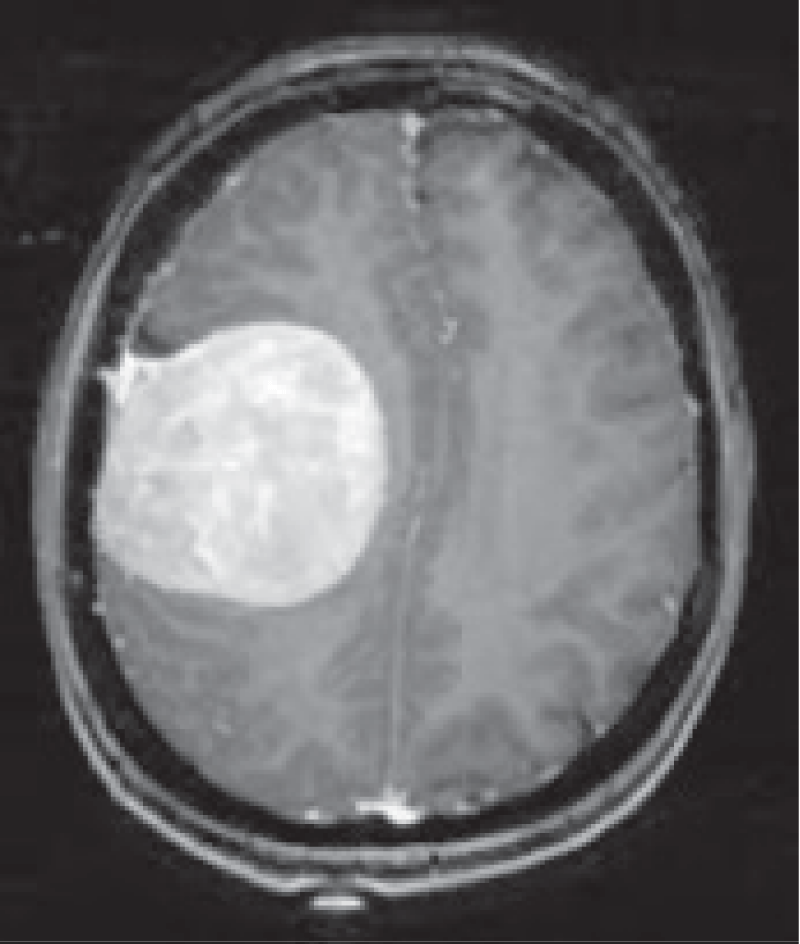
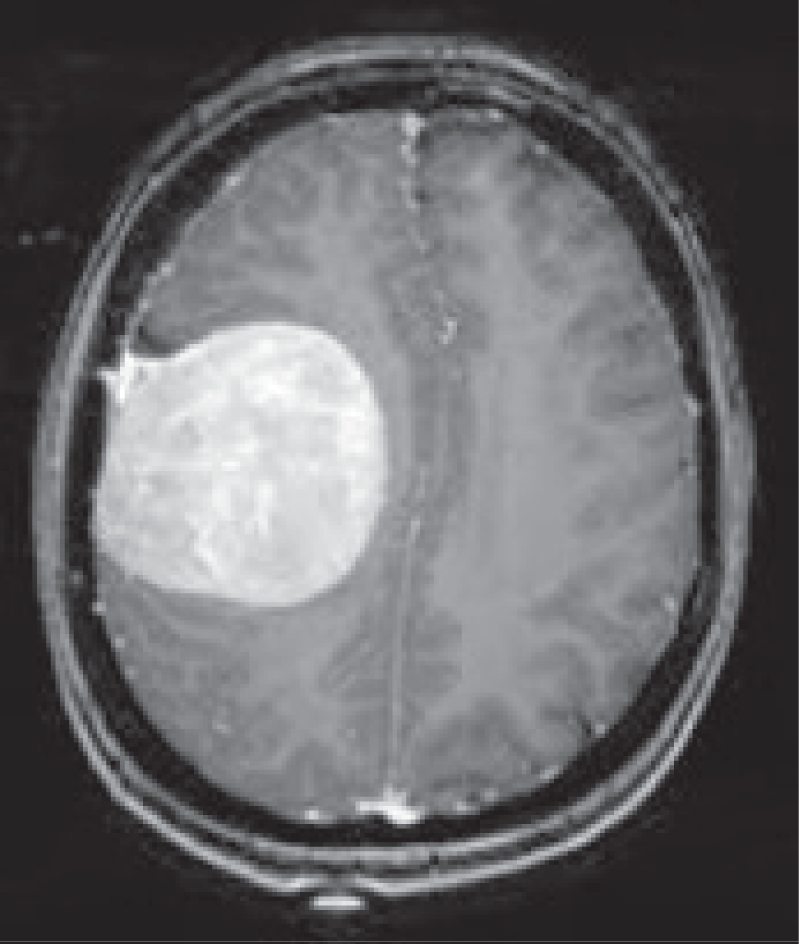
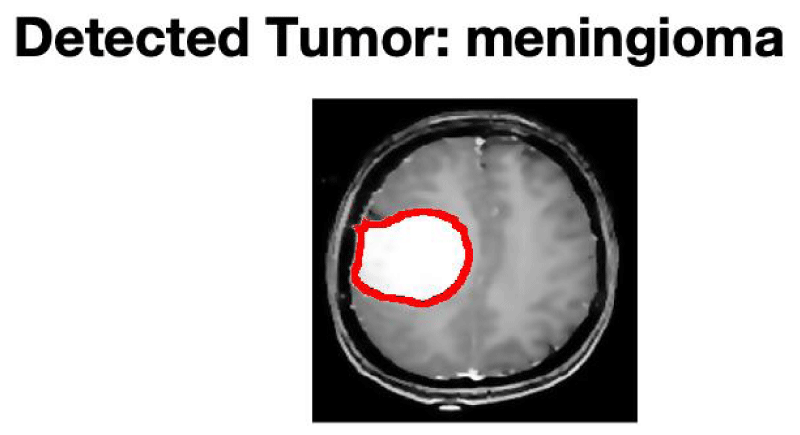
The integration of deep learning-based classification techniques adds a layer of sophistication to the analysis, allowing for the identification of the tumortype from the MRI data. This pivotal phase involves training a neural network to distinguish between different tumor categories, such as meningioma, glioma, or pituitary tumour. Convolutional Neural Network (CNN) provides a segmentation-free approach that does away with the requirement for labor-intensive feature extractor techniques. Because of this, we used various CNN designs for brain tumor detection and classification [32-34]. The classification output holds significant clinical value, as it provides crucial information about the tumour's underlying nature. By leveraging machine learning algorithms, medical practitioners can efficiently categorize tumours, enabling them to make timely and informed decisions about treatment strategies. This classification outcome augments the diagnostic process by offering insights into the tumour's potential behavior and guiding personalized medical care. Figure 5 shows the original image with the outline of the tumour, as well as the type of tumor which is displayed in the figure title.
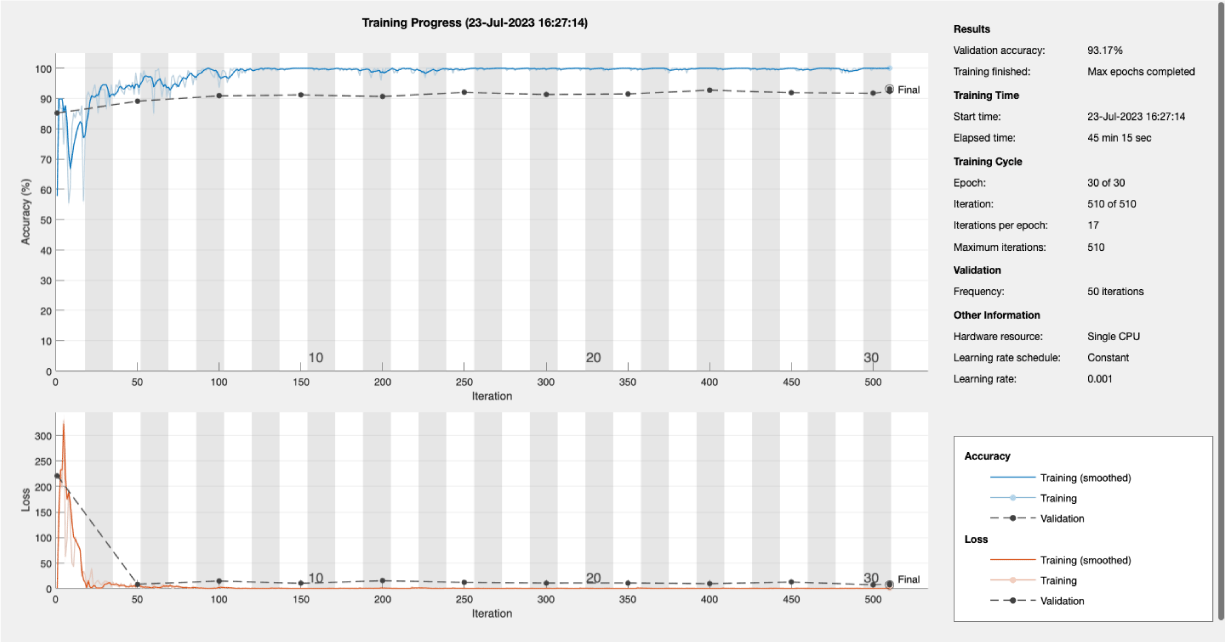
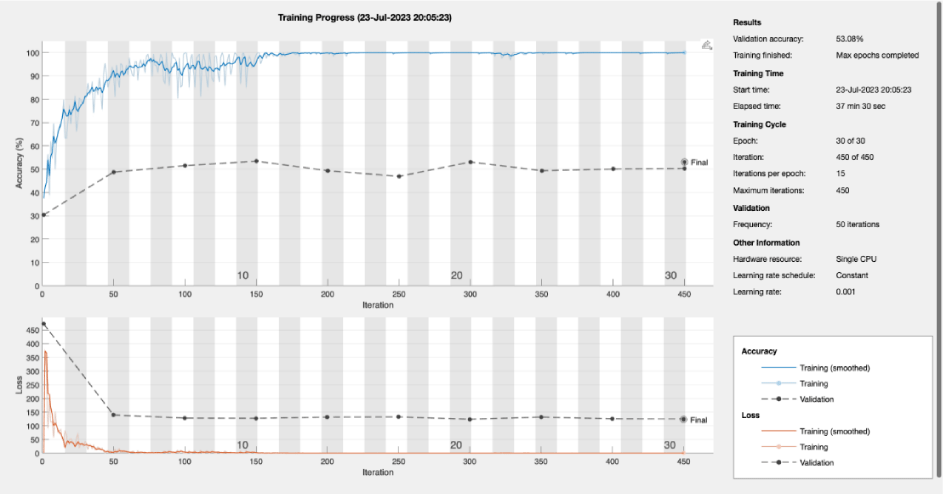
Figure 6 visually presents the training progress of a brain image classification model specifically designed to differentiate between images with tumours and those without. This figure displays key training metrics and their evolution over training epochs, reflecting how effectively the model is learning to make accurate classifications. The X-axis represents the training epochs, while the Y-axis shows the values of the training metrics, which may include accuracy, loss, and validation metrics. This graphical representation provides valuable insights into the model's learning curve and its performance in distinguishing between brain images with tumours and those deemed tumour-free.
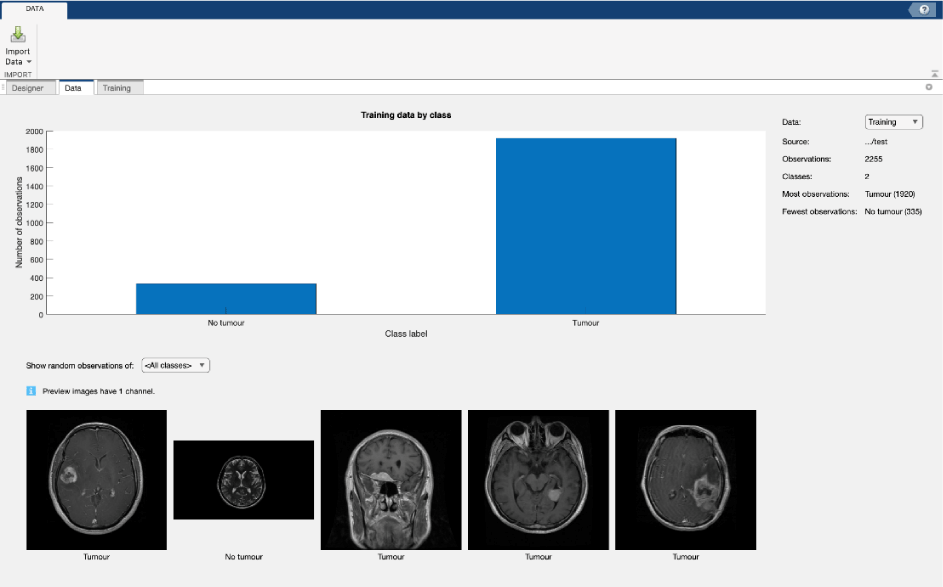
As shown in Figure 7, one of the datasets contained over 2200 figures of brain MRI, tumorous and non-tumorous. Figures 6 and 8 show the training progress of a brain image classification model specifically designed to differentiate between tumourtypes.
Discussion
The results presented encompass a comprehensive analysis of brain MRI images to detect and characterize tumorous regions. The analysis pipeline consists of four key phases: pre-processing, segmentation, detection, and classification. The pre-processing phase serves as the initial step, involving techniques such as contrast adjustment, anisotropic diffusion, and resizing. These techniques contribute to refining image quality, minimizing noise, and ensuring uniform dimensions across the dataset. In the segmentation phase, the generation of a tumoursegmentation mask effectively isolates the tumorous region from the brain's anatomical structures. This foundational step lays the groundwork for subsequent analyses by accurately delineating the region of interest. Moving to the detection phase, the process is evidenced through an image depicting the tumourarea with a bounding box and another image highlighting the defined tumourboundary. These visualizations intricately outline the localized tumourand precisely define its boundaries, emphasizing the efficacy of the detection process in identifying and visualizing the presence of tumours. In the classification phase, the tumouroutlined in the original image is showcased, demonstrating how a deep neural network trained to classify tumourtypes as meningioma, glioma, or pituitary tumourcan be applied. The amalgamation of these phases is illustrated by the interplay of various images, representing different stages of analysis and underlining the methodology's comprehensive nature. By seamlessly integrating techniques spanning pre-processing, image segmentation, and neural network-based classification, this approach offers a robust framework for accurate brain tumouranalysis. This approach not only advances diagnostic accuracy but also lays the groundwork for future advancements in medical image analysis and diagnosis.
There are many challenges in brain tuomour detection such as complexity and heterogeneity of tumours, small lesion detection, imaging modalities, and interpretability and explainability of neural networks and deep learning algorithms. Moreover, Limited Availability of Labeled Data and computational resources could be considered as limitations of this study.
Conclusion
The foundational understanding of MRI principles enabled a robust start to this research, allowing for informed decisions throughout the research. The pre-processing phase, involving noise reduction and image clarity enhancement, substantially improved image quality, making subsequent analysis more reliable. The exploration and comparison of different segmentation methods provided valuable insights into accurately identifying brain structures.
The primary highlight of this research lies in the successful implementation of CNNs for brain image classification. The utilization of CNN architecture showcased the potential of deep learning in extracting complex features and patterns from brain images. The results demonstrated improved accuracy in classifying brain images based on disease types, a significant achievement in medical image analysis.
While this research has paved the way for advanced brain image analysis, several avenues for future exploration remain open. Firstly, further investigation into incorporating more intricate deep learning architectures, such as ResNet-50 or more recent variations, could yield even higher classification accuracy. Exploring ensembles of CNN models and transfer learning from large datasets might contribute to enhancing the robustness and generalizability of the model.
Additionally, extending the research to a broader range of diseases and medical conditions could provide a more comprehensive evaluation of the model's applicability. Integrating additional data modalities, such as functional MRI or diffusion tensor imaging, could enrich the analysis and potentially lead to more holistic insights into brain health. Furthermore, the pre-processing phase could benefit from advancements in noise reduction techniques and more advanced image restoration methods, potentially enhancing image quality and subsequently improving downstream analysis.
- NHS. Brain tumours. https://www.nhs.uk/conditions/brain-tumours/.
- Beucler N, Dagain A, Sellier A. Duret Brainstem Hemorrhage Secondary to Large Supratentorial Meningioma. World Neurosurg. 2023 May 24;177:1-2. doi: 10.1016/j.wneu.2023.05.067. Epub ahead of print. PMID: 37236310.
- Solanki S, Singh UP, Chouhan SS, Jain S. Brain tumor detection and classification using intelligence techniques: An overview. IEEE Access. 2023.
- Kurdi SZ, Ali MH, Jaber MM, Saba T, Rehman A, Damaševičius R. Brain Tumor Classification Using Meta-Heuristic Optimized Convolutional Neural Networks. J Pers Med. 2023 Jan 20;13(2):181. doi: 10.3390/jpm13020181. PMID: 36836415; PMCID: PMC9965936.
- Li G. AI matches humans at diagnosing brain cancer from tumourbiopsy images. 2020. https://www.newscientist.com/article/ 2229306-ai-matches-humans-at-diagnosing-brain-cancer-from-tumour/ /-biopsy-images
- Profile view of a brain. https://pngimg.com/image/86579.
- Top view of a brain. https://pngimg.com/image/86597.
- DeAngelis LM. Brain tumors. N Engl J Med. 2001 Jan 11;344(2):114-23. doi: 10.1056/NEJM200101113440207. PMID: 11150363.
- Kandimalla SY, Vamsi DM, Bhavani S, VM M. Recent Methods and Challenges in Brain Tumor Detection Using Medical Image Processing. Recent Patents on Engineering. 2023; 17(5): pp.8-23.
- Solanki S, Singh UP, Chouhan SS, Jain S. Brain Tumor Detection and Classification using Intelligence Techniques: An Overview. IEEE Access. 2023.
- Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors. J Magn Reson Imaging. 2018 May 7;48(3):571–89. doi: 10.1002/jmri.26171. Epub ahead of print. PMID: 29734497; PMCID: PMC6175399.
- Nelson SJ, Cha S. Imaging glioblastoma multiforme. Cancer J. 2003 Mar-Apr;9(2):134-45. doi: 10.1097/00130404-200303000-00009. PMID: 12784879.
- Maggio I, Franceschi E, Tosoni A, Nunno VD, Gatto L, Lodi R, Brandes AA. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021 Jun 1;10(2):CNS72. doi: 10.2217/cns-2021-0003. Epub 2021 May 21. PMID: 34015955; PMCID: PMC8162186.
- Dr Louis Hofmeyr. Acoustic neuroma (vestibular schwannoma).2023. https://lmhofmeyr.co.za/conditions/conditions-we-specialise-in/ acoustic-neuroma/
- Karia SJ, McArdle DJT. AIDS-related primary CNS lymphoma. Lancet. 2017 Jun 3;389(10085):2238. doi: 10.1016/S0140-6736(17)30056-9. Epub 2017 Apr 6. PMID: 28392104.
- Prontera A, Puzzolante A, Carpeggiani P, Pavesi G. Symptomatic anterior cerebral artery vasospasm after brainstem hemangioblastoma resection. A case report. Neuroradiol J. 2014 Apr;27(2):186-90. doi: 10.15274/NRJ-2014-10019. Epub 2014 Apr 18. PMID: 24750707; PMCID: PMC4202852.
- Trieu EQ, Huang SY, Yang JY. Medulloblastoma: Clinical challenges and emerging molecular discoveries,” in Neurooncology-Newer Developments. IntechOpen. 2016.
- Rastogi M, Gupta MK, Revannasiddaiah S, Seam RK. The lack of sleep, the pineal gland and breast cancer. BMJ Case Rep. 2012 Sep 17;2012:bcr2012006763. doi: 10.1136/bcr-2012-006763. PMID: 22987906; PMCID: PMC4543568.
- Raeesa F, Mahale A, Vinay B S. A curious case of vanishing pituitary adenoma. Radiol Case Rep. 2020 May 19;15(7):1050-1053. doi: 10.1016/j.radcr.2020.04.021. PMID: 32461776; PMCID: PMC7240058.
- Drapeau A, Walz PC, Eide JG, Rugino AJ, Shaikhouni A, Mohyeldin A, Carrau RL, Prevedello DM. Pediatric craniopharyngioma. Childs Nerv Syst. 2019 Nov;35(11):2133-2145. doi: 10.1007/s00381-019-04300-2. Epub 2019 Aug 5. PMID: 31385085.
- Butowski NA. Epidemiology and diagnosis of brain tumors. Continuum (Minneap Minn). 2015 Apr;21(2 Neuro-oncology):301-13. doi: 10.1212/01.CON.0000464171.50638.fa. PMID: 25837897.
- Honarvar Shakibaei Asli B, Wang Y. Moment-based image enhancement for brain tumor health monitoring. In: 11th International Conference on Through-life Engineering Services - TESConf2022, 8-9 November 2022, Cranfield, UK, Paper number 3405. 2022.
- Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, Ryken T, Moore MK, Egan KM, Olson JJ. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014 Apr;11(4):203-22. doi: 10.1038/nrclinonc.2014.25. Epub 2014 Feb 25. PMID: 24569448; PMCID: PMC4041037.
- Chakrabarty N. Brain MRI images for Brain tumor detection, Kaggle. 2019. https://www.kaggle.com/datasets/navoneel/brain-mri-images-for-brain-tumor-detection (Accessed: 10 November 2023).
- Honarvar Shakibaei B, Jahanshahi P. Image deconvolution by means of frequency blur invariant concept. ScientificWorldJournal. 2014;2014:951842. doi: 10.1155/2014/951842. Epub 2014 Aug 12. PMID: 25202743; PMCID: PMC4147381.
- Shakibaei BH, Flusser J. Image deconvolution in the moment domain. Moments and Moment Invariants—Theory and Applications. 2014; 1:111-125.
- Kumar A, Paramesran R, Shakibaei BH. Moment domain representation of nonblind image deblurring. Appl Opt. 2014 Apr 1;53(10):B167-71. doi: 10.1364/AO.53.00B167. PMID: 24787200.
- Asli BHS, Flusser J, Zhao Y, Erkoyuncu JA, Krishnan KB, Farrokhi Y, Roy R. Ultrasound image filtering and reconstruction using DCT/IDCT filter structure. IEEE Access. 2020; 8:141342-141357.
- Honarvar Shakibaei Asli B, Zhao Y, Erkoyuncu JA. Motion blur invariant for estimating motion parameters of medical ultrasound images. Sci Rep. 2021 Jul 12;11(1):14312. doi: 10.1038/s41598-021-93636-4. PMID: 34253807; PMCID: PMC8275601.
- Mittal A, Moorthy AK, Bovik AC. No-reference image quality assessment in the spatial domain. IEEE Trans Image Process. 2012 Dec;21(12):4695-708. doi: 10.1109/TIP.2012.2214050. Epub 2012 Aug 17. PMID: 22910118.
- Chen MJ, Bovik AC. Fast structural similarity index algorithm.Journal of Real-Time Image Processing. 2011; 6:281-287.
- Aggarwal M, Khullar V, Goyal N, Singh A, Tolba A, Thompson EB, Kumar S. Pre-Trained Deep Neural Network-Based Features Selection Supported Machine Learning for Rice Leaf Disease Classification. Agriculture. 2023; 13(5):936.
- Sharma N, Gupta S, Mohamed HG, Anand D, Mazón JLV, Gupta D, Goyal N. Siamese convolutional neural network-based twin structure model for independent offline signature verification. Sustainability. 2022; 14(18):11484.
- Singh TP, Gupta S, Garg M, Gupta D, Alharbi A, Alyami H, Anand D, Ortega-Mansilla A, Goyal N. Visualization of Customized Convolutional Neural Network for Natural Language Recognition. Sensors (Basel). 2022 Apr 8;22(8):2881. doi: 10.3390/s22082881. PMID: 35458866; PMCID: PMC9026827.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
