Global Journal of Anesthesiology
The optimal timing of Ambu® Aura onceTM Laryngeal Mask Airway insertion with propofol induction
Wei Chen1, Rebecca Downey1, Richard Sheu1, Hui Qu2, Iwona Bonney1 and Peishan Zhao1*
2Laboratory of Epidemiology and Public Health, Yale University, 60 College St, New Haven, CT 06510, USA
Cite this as
Chen W, Downey R, Sheu R, Qu H, Bonney I, et al. (2021) The optimal timing of Ambu® Aura onceTM Laryngeal Mask Airway insertion with propofol induction. Glob J Anesth 8(1): 001-007. DOI: 10.17352/2455-3476.000052Background: Laryngeal Mask Airway (LMA) is usually inserted without muscle relaxants, which requires good jaw relaxation. Previous studies have focused on creating the optimal condition for LMA insertion with different anesthetic adjuncts. This study is to determine whether the time interval between induction and insertion influences placement conditions. Insertion of LMA at the best time interval may decrease the complications associated with LMA placement.
Methods: This is a prospective randomized study with a total of 198 ASA I or II patients assigned to three groups: Group 0”, Group 60” and Group 90”, with number representing the seconds from loss of eye lash reflex (ELR) to LMA insertion. All patients were pretreated with intravenous midazolam 2 mg and fentanyl 1 mcg/kg at a given time. Induction was achieved with 2.5 mg/kg propofol. Ambu® AuraOnceTM LMA was placed by a blinded anesthesiologist who also assessed the condition for LMA insertion based on a score system. The primary outcome is to find the optimal condition for LMA insertion in each group. The conditions were defined as optimal or non-optimal based on the total score of 6 or > 6, respectively.
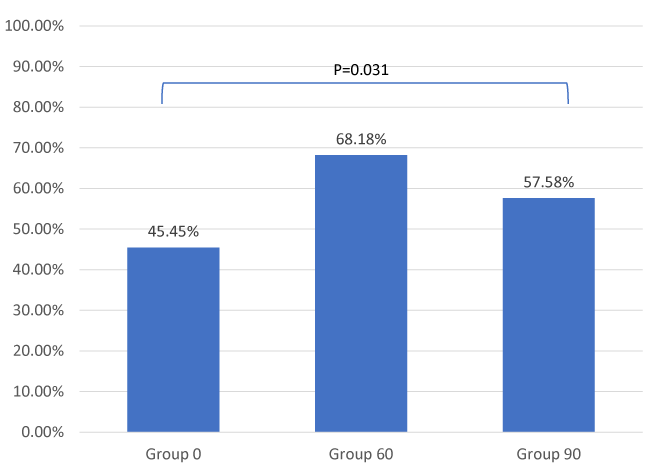
Results: The percentage of the optimal condition was significantly different amongst the three groups (p = 0.031). The optimal condition was 68% in Group 60”, that was significantly higher than 45% in Group 0” (p = 0.008), but not than 58% in Group 90” (p=0.207).
Conclusions: Induction of general anesthesia with 2.5 mg/kg of propofol, with pre-administration of midazolam and fentanyl, provided the highest percentage of optimal condition of LMA insertion at 60 seconds after loss of ELR.
Introduction
Since its prototype introduction in 1983, Laryngeal Mask Airway (LMA) has been used world-wide for General Anesthesia (GA) in properly selected patients, as an alternative to the Endotracheal Tube (ETT). By 2011, an estimated 56% of GA was managed by some form of supraglottic airway in the United Kingdom [1]. Compared to ETT, the use of LMA results in lower incidence of laryngospasm, postoperative hoarse voice, and coughing [2]. Similar to ETT placement, the insertion of LMA requires a certain depth of anesthesia to achieve adequate jaw-opening and to avoid undesirable events. These undesirable events include coughing, gagging, patient movements, and laryngospasm. Prior studies have focused primarily on the comparisons of various anesthetic agents and anesthetic adjuncts to create an optimally induced patient to aid easy insertion of LMA. Several anesthetic adjuncts such as midazolam [3], opioids [4,5], lidocaine [6] and ketamine [7] have shown to reduce the dose of propofol and create satisfactory LMA insertion conditions. LMA placement conditions were also enhanced with muscle relaxants [8,9]. However, the time frames at which the LMAs were inserted after induction in all of these studies were either subjective (determined by the inserter) or inconsistent, thus making it unclear whether the time interval between induction and insertion influences placement conditions.
This pilot study sought to investigate the optimal timing of Ambu® AuraOnceTM LMA (Ambu Inc. Glen Burnie, MD, USA) insertion with the most commonly used IV anesthetic induction agent, propofol, without co-administration of muscle relaxant. 0 seconds, 60 seconds, and 90 seconds were chosen as the intervals in the study, because the argument about the timing of LMA placement usually falls immediately after or sometime after the induction. We hypothesized that immediate placement after propofol induction, defined by loss of eye lash reflex (ELR), would provide the best LMA insertion conditions based on empirical experience.
Material and methods
This study was approved by the Institutional Review Board (IRB) at Tufts Medical Center (TMC, IRB # 8910), and written informed consent was obtained from all subjects. The trial was registered at clinicaltrials.gov before patient enrolment (NCT 00972491, Principal investigator: Peishan Zhao, date of registration: September 7, 2009) and this manuscript followed the applicable Equator guidelines.
Sample size estimation
With limited literature on the research topic of this study, the sample size calculation was based on a study by Wong, et al., that showed a 65% rate of “optimal condition” following induction of subjects at 90 seconds [10]. For the purpose of our power and sample size estimation, we took this 65% rate to be true for 60 seconds as well. To achieve 90% power to detect a linear trend in the optimal condition of LMA insertion, while using a two-sided Chi-squared test for trends and a significant p-value of 0.05, a sample size of 66 per group was needed. The simple size estimation and patient randomization scheme were generated by statistician in the research institute at TMC.
Patient selection and randomization
Between October 2009 and December 2013, 250 eligible subjects were selected to be recruited to make sure the sample size was met for proper analysis. Regardless of gender or ethnicity, patients 18 years or older with an American Society of Anesthesiologists (ASA) physical status I-II and scheduled to undergo a surgical procedure at TMC for which LMA was an appropriate choice were recruited. Exclusion criteria included: patients with risk of aspiration; mouth opening < 2.5 cm; limitation of neck movement; anticipated difficult airway; obstructive sleep apnea; morbid obese patients defined as Body Mass Index (BMI) ≥ 40 or 35 with one serious obesity-related condition; pregnant women; prisoners; use of sedative or recreational drugs; patient refusal, known allergic reaction to the drugs used and operative procedure for which use of an LMA was not deemed safe. We planned to recruit ASA III patients when registered at clinicaltrials.gov, then excluded these patients due to concerns of dramatic hemodynamic instability with 2.5 mg/kg propofol induction.
After informed consents were obtained, the enrolled subjects were randomly assigned to one of the three study groups: Group 1: 0 seconds, Group 2: 60 seconds, and Group 3: 90 seconds, which respectively represented the time-lapse in seconds after loss of ELR. The randomization scheme was developed using SAS 9.1.3 (SAS Institute Inc., Cary, NC). Randomization envelopes were opened only when the subject agreed to participate in the study.
Study protocol
A Peripheral Intravenous (IV) line and infusion of lactated ringer’s solution were started in the holding area prior to surgery. In addition, routine 2 mg of IV midazolam was given to all participants right before being taken to the OR. Upon arrival to the OR, IV fentanyl 1.0 mcg/kg was given before standard ASA monitors were placed. Vital signs were taken as a pre-induction baseline and then taken immediately 1 minute, 2 minutes and 3 minutes after LMA insertion. Participants were preoxygenated for 3 minutes and pre-treated with 2 ml of 2% lidocaine IV to decrease pain upon injection [11]. Induction was performed immediately after lidocaine injection by investigator 1 with IV propofol 2.5 mg/kg injected over a 15 second span and was concluded after loss of LER determined by investigator 1. After investigator 1 tracked 0 seconds, 60 seconds, or 90 seconds of time-lapsed, a blinded investigator 2 (LMA inserter) was summoned to insert size 4 LMAs for females and size 5 for males. Investigator 2 was present in the OR and blinded to randomized groups by facing the OR wall opposite to the OR table. The LMAs were lubricated with a water soluble gel and inserted with a deflated cuff according to the technique described by Brain [12]. The cuff was then inflated with 20-30 mL of air. The correct position of the LMA was confirmed by observing adequate chest rise, auscultating leakage in the neck, observing the capnogram and tidal volume, and absence of audible air leak. After LMA placement, anesthesia management was transferred to the primary team. Five attending anesthesiologists acted as investigator 2 and were trained by the primary investigator prior to the study regarding the score system used. The LMA insertion condition was graded by investigator 2 based on 6 categories used in previous studies [10,13]. The primary outcome was the optimal condition defined as a total score of 6 (see below) in each group. Non-optimal condition was based on a total score > 6. Secondary outcomes include: jaw opening (full 1, partial 2, and nil 3); insertion of LMA (easy 1, difficult 2, and impossible 3); coughing or gagging (none 1, some 2, and significant 3); hiccups (none 1, some 2); head or body movement (none 1, some 2, and significant 3) and laryngospasm or airway obstruction (none 1, partial 2, total 3). Laryngospasm is defined as airway obstruction with assumption that LMA is correctly placed. The numbers represent scores.
The apneic patient was mask ventilated with 100% oxygen only if O2 saturation decreased to less than 92% between loss of LER and LMA placement. If the LMA insertion was not successful on the first attempt, another dose of propofol 1 mg/kg was given with a second attempt 30 seconds later. If the second attempt was unsuccessful, propofol 0.5 mg/kg was administered and a third attempt was performed 30 seconds later. If the third attempt was unsuccessful, ETT intubation was initiated.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation, and categorical variables were summarized as frequency and percentages. The dependent variable/primary outcome (optimal conditions) and the independent variable (time of LMA insertion) represent ordinal variables with logical ordering i.e. (yes, no) and (0, 60 and 90 seconds). These variables were tested with a chi-squared test for association. Other categorical outcomes such as jaw opening, ease of LMA insertion, presence of coughing/gagging/hiccups, head and body movement, and occurrence of laryngospasm or airway obstruction, were analyzed in the same way. We used ANOVA to test the relationship between randomized groups and hemodynamic responses to LMA insertion (continuous variables). When data was skewed (number of attempts, total score, before and after insertion), we used a non-parametric test for association. When there were significant differences among groups, we used a non-parametric test to show differences between each of the two groups. Statistical analysis was performed using SPSS (version 13.0; SPSS Inc., Chicago, IL, USA). The level of significance was set at a p value < 0.05.
Results
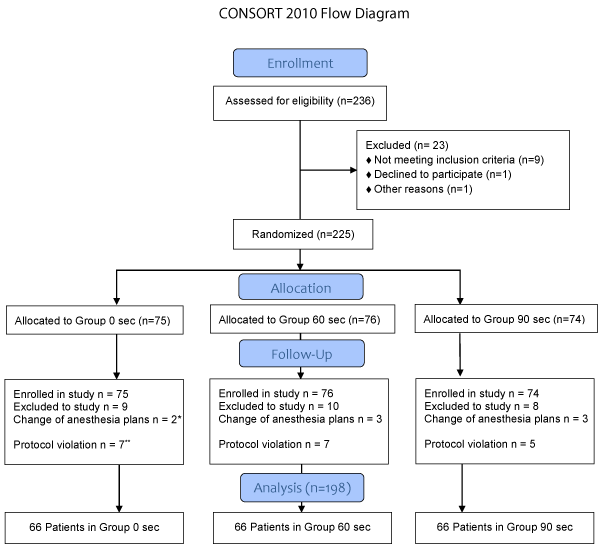
We planned to recruit 250 subjects to make sure we had 66 subjects in each group. In reality, we approached 236 subjects after meeting the goal of 198 subjects in total for statistical analysis. Detailed inclusion and exclusion are in Figure 1. There were no significant differences between groups regarding age, gender, height, weight, BMI, ASA physical status, history of asthma or drinking/smoking (Table 1).
We found that Group 2 had the highest percentage of optimal condition for LMA insertion at 68%, followed by 58% in Group 3. The least percentage of optimal condition was 45% in Group 1 (Figure 2). Although there were no significant differences in the individual score amongst the three groups, there was significant difference in the number of optimal conditions (score = 6) amongst these groups (p = 0.031). The percentage of optimal conditions was significantly higher in Group 2, compared with Group 1 (p = 0.008). However, there was no significant difference of number of optimal conditions between Group 1 and Group 3 (p=0.164) or Group 2 and Group 3 (p=0.207). The greatest contribution of this difference came from jaw opening where 80.3% in Group 2 were able to have full jaw opening, which was significantly higher than 65.2% in Group 1 (p=0.051) (Table 2).
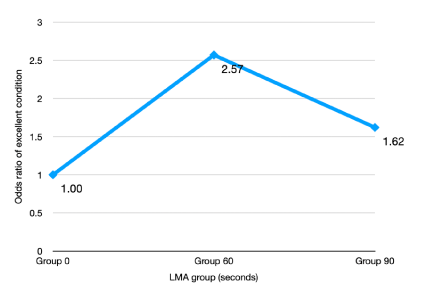
Multivariable ordered logistic regression models were developed and used to scan for potential confounding variables. Adjustments were made to age, height, weight, BMI, history of asthma, smoking and drinking status, ASA physical status and time to insertion in the full model. Using a backward selection procedure, findings that only “time to insertion” was significant (p = 0.0328), so it remained in the reduced model. It showed that compared to Group 1, the odds of getting an optimal condition was 2.57 times significantly higher in Group 2 [95% CI (1.265 – 5.226)] and 1.62 times higher in Group 3 [95% CI (0.819 – 3.236)] (Figure 3).
There was no difference in pre-induction systolic and diastolic blood pressures (BP), and heart rate (HR) amongst the three groups. However, the immediate post-insertion systolic BP of Group 1 was significantly higher than that of Group 2 (p = 0.0004) and Group 3 (p = 0.0014). In addition, the immediate post-insertion HR of Group 1 was significantly higher than that of Group 2 (p = 0.028) and Group 3 (p = 0.0184). (Table 3). When compared, the hemodynamic changes between 1 min post LMA insertion and pre-induction showed that there were no significant changes in HR and systolic BP amongst the three groups. In regard to diastolic BP changes, that of Group 1 was not significantly higher than that of Group 2 (p = 0.054) but was significantly higher than that of Group 3 (p = 0.021).
There were 7 patients in Group 1, 5 patients in Group 2 and 2 patients in Group 3 that required second attempts to place LMA. However, no patients required intubation throughout the entirety of the study.Discussion
Propofol has rapid onset of action with a peak effect of 1.6 minutes and a decline of effects within minutes due to redistribution from Central Nervous System (CNS) to muscles and adipose tissues [14]. Moreover, propofol possesses muscle relaxing properties with central mechanisms [15]. The onset time of muscle relaxation is 10-20 seconds, with effect lasts 4-6 min. [16] IV propofol induction has been shown to be superior to thiopental due to its lower incidence of postoperative complications [8,17]. These properties make propofol a popular induction agent for GA with LMA. A 2.5 mg/kg dose was recommended for IV bolus induction of GA [18]. However, when 2.5 mg/kg propofol is used alone in un-premedicated patients it may provide less satisfactory conditions of LMA insertion, giving rise to patient gagging, coughing, body movement and even laryngospasm [19]. We co-administered 1 mcg/kg fentanyl with 2.5 mg/kg propofol because this dose, in a previous study, provided optimal conditions for LMA insertion in most studied subjects (65% of cases) [10]. Lower dose (0.5 mcg/kg) of fentanyl or placebo had higher incidence of unsatisfied mouth opening and LMA insertion, more swallowing and patient movement, while higher doses (1.5 and 2.0 mcg/kg) of fentanyl resulted in higher incidence of coughing/gagging and laryngospasm [10]. In addition, 1 mcg/kg fentanyl provided similar conditions for LMA insertion and better hemodynamic parameters than 2 mcg/kg fentanyl [20]. We injected fentanyl when the patient was brought to the OR because the time from entering OR to induction of GA was approximately 5 min, which is the optimal onset time of fentanyl before tracheal intubation [21]. During the study period, we routinely gave each patient midazolam as an anxiolytic before transferring to OR.
Contrary to our hypothesis, we found that the best time to insert LMA is 60 seconds after loss of ELR, since 68% patients had the optimal condition for LMA insertion at this time point. Compared with 0 seconds, the odds ratio of optimal condition is 2.57 at 60 seconds and 1.62 at 90 seconds. No study was found comparing time-lapse between induction and LMA insertion. Previous studies that focused on the insertion condition and complications of LMA placement used 0 seconds - 3 min intervals from propofol induction to insertion of LMA [20,22]. However, in a dose–response study to determine an optimal dosage of fentanyl used with propofol for LMA insertion, Wong, et al. pre-administered fentanyl 1mcg/kg (injected over 10 seconds) followed immediately by 2.5 mg/kg propofol (injected over 10 seconds) as an induction agent and achieved optimal condition for LMA insertion in 65% of subjects [10]. The authors inserted LMA 90 seconds after patient received the first medication, fentanyl. Considering patient loss of ELR within 10 seconds after propofol injection [13], we calculated insertion time in Wong’s study that is very close to 60 seconds after patient loss of ELR. Bapat, et al. compared condition for LMA insertion after induction with 1 mcg/kg Fentanyl, followed 60 seconds later by 2.5 mg/kg propofol (Group P), or 1.5 mg/kg lidocaine followed 30 seconds later by 5 mg/kg thiopentone (Group LT), or 0.1 mg/kg midazolam followed 3 minutes later by 5 mg/kg thiopentone (Group MT). LMA was inserted 60 seconds after induction. They found 66% (33/50) of patients in propofol group had excellent conditions for LMA insertion. In addition, there was no laryngospasm in propofol group compared to 28% and 6% in LT group and MT group, respectively [6]. No midazolam was given to the patients in these 2 comparable studies. In this study, 2 mg of IV midazolam (average 0.03 mg/kg) was given to all patients. Midazolam at 0.04 mg/kg reduced the propofol induction dose of GA for LMA insertion [3], which might explain the better result we found. It should be noticed that induction with only 2.5 mg/kg propofol does not seem to provide an optimal condition for LMA insertion at 60 seconds after loss of ELR. When compared to induction with 8% sevoflurane in 50% nitrous oxide and oxygen, induction with 2.5 mg/kg propofol without pre induction opioids or benzodiazepines led to 11% (5/44) laryngospasm and higher incidences of coughing, gaging and body movement [23].
LMA is usually placed without muscle relaxants. Full jaw opening shows reduced muscular tone and increased success rate of LMA insertion. Limited jaw opening is acknowledged as a cause of difficult placement of LMA. Ganatra, et al. used maximum jaw relaxation as the time point to insert LMA. They used fentanyl 1 mcg/kg followed 3 minutes later with 2.5 mg/kg propofol injected over 45 seconds. The mean time taken from induction to successful LMA insertion was 73 seconds [13]. We injected propofol over 15 seconds and had the most optimal jaw opening condition at 60 seconds (80.3%). The total time from start of induction to LMA insertion was 75 seconds, which is very close to the 73 seconds in Ganatra’s study. The maximum muscle relaxing property of propofol seemed to be well correlated with its peak effect at 1.6 minutes.
Early studies indicated that in adults, the incidence of laryngospasm was 1-4% during LMA placement [24]. One patient in study Group 1 had a laryngospasm. This patient was a 41 year old otherwise healthy non-obese female who presented for excision of left breast mass. She had no history of tobacco or chronic alcohol use. After LMA insertion, the patient had typical inspiratory stridor, then paradoxical respiratory effort and tracheal tug, but no end tidal CO2 was seen on the monitor. The laryngospasm was broken by injection of more propofol, removing of LMA and positive pressure ventilation. Among the risk factors for laryngospasm during anesthesia [25], we found the only possible provoker of laryngospasm in our patient was light level of anesthesia. In comparison to pre-induction vital signs, the systolic BP and HR of Group 1 immediately after post-insertion, maintained significantly better than that of Group 2 and Group 3 (Table 3). This may suggest depth of anesthesia in Group 1 was not as deep as the other 2 groups. Our result clearly indicated that the time of 0 seconds was not the best time to place LMA. Previous studies also indicated that in most instances, the conditions were less than optimal when insertion of LMA was attempted immediately after the loss of verbal contact [26].
Anesthesia providers usually place LMA even without optimal condition. In our study, 32-55% of patients did not have an optimal condition for insertion. Only 3% (2/66 in Group 3) – 11% (7/66 in Group 1) had LMA inserted with second attempt. Similarly, 7% - 20% of patients required more than one attempt for LMA insertion in Wong’s study although less than 65% subjects had optimal conditions [10]. With current advances in medication such as propofol and anesthesia providers’ techniques, laryngospasms during LMA insertion does not happen as often as it used to [24,27]. However, to avoid potential complications, one should wait for the optimal condition achieved before LMA insertion, especially in current busy practice.
Body/head movement occurred in some patients in each group with similar incidence. Previous study showed that the dose of propofol required to produce loss of motor response to jaw thrusting varies considerably (95% reference interval: 1.7-3.6 mg/kg) [26]. This wide variability implies that a fixed dose of propofol, 2.5 mg/kg may be more than needed for some patients, while not enough for others. The wide inter-individual variability may be the reason why similar numbers of patients in each group had movement.
Propofol is known to cause hiccups [28,29]. The incidence of propofol hiccups is unknown. Hiccups occurred in 1 patient in Group 1 and 2 patients in Group 2 and Group 3 each. Hiccups spontaneously resolved in each of the patients. However, hiccups could result in laryngospasm [29] and more attention should be paid to patient with this side effect. Lidocaine 1 mg/kg IV was successfully used to relieve hiccups [29].
This is a prospective, double-blind, randomized controlled study. However, there were several limitations noticed during the study. First, time intervals of 30 seconds, 45 seconds, 120 seconds, etc., were not included due to the nature of a pilot study. The time points within 120 seconds is worthy of further study, since most anesthesia providers do not wait 2 minutes after disappearance of ELR to insert LMA. Secondly, previous study showed that males were more sensitive to propofol [30] and sensitivity to propofol also increased with age [31]. However, patients’ demographics were evenly distributed in the three groups. We do not believe that this gender and age difference in propofol pharmacology affected the outcome of our study. Thirdly, to make sure the accurate insertion time occurred, the LMA inserter (anesthesiologist 2) needed to be present in the OR. Although one may argue that this anesthesiologist may not be totally blind by guessing the study time point, we asked LMA inserter not to look at the induction of GA and inducing anesthesiologist kept talking to the patient even in sleep.
In conclusion, under our study condition, we found that the best time to insert Ambu® AuraOnceTM LMA was 60 seconds after loss of ELR, since 68% patients had the optimal condition for LMA insertion at this time point. The odd ratio of optimal condition is 2.6 at 60 seconds and 1.6 at 90 seconds compared to 0 seconds.
The authors thank Ms. Nichole Formicola for proofreading.
- van Zundert TC, Brimacombe JR, Ferson DZ, Bacon DR, Wilkinson DJ, et al. (2012) Archie Brain: celebrating 30 years of development in laryngeal mask airways. Anaesthesia 67: 1375-1385. Link: https://bit.ly/2UBKikF
- Yu SH, Beirne R (2010) Laryngeal mask airways have a lower risk of airway complications compared with endotracheal intubation: a systematic review. J Oral Maxillofac Surg 68: 2359-2376. Link: https://bit.ly/3kpMTJx
- Gill PS, Shah J, Ogilvy A (2001) Midazolam reduces the dose of propofol required for induction of anaesthesia and laryngeal mask airway insertion. Eur J Anaesth 18: 166-170. Link: https://bit.ly/3xDlnfg
- Hui JK, Critchley LA, Karmakar MK, Lam PK (2002) Co-administration of alfentanil-proofol improves laryngeal mask airway insertion compared to fentanyl-propofol. Can J Anaesth 49: 508-512. Link: https://bit.ly/36y3P8o
- Bouvet L, Da-Col X, Rimmelé T, Allaouchiche B, Chassard D, et al. (2010) Optimal remifentanil dose for laryngeal mask airway insertion when co-administered with a single standard dose of propofol. Can J Anaesth 57: 222-229. Link: https://bit.ly/3r3VHpw
- Bapat P, Joshi RN, Young E, Jago RH (1996) Comparison of propofol versus thiopentone with midazolam or lidocaine to facilitate laryngeal mask insertion. Can J Anaesth 43: 564-568. Link: https://bit.ly/3yLmbyG
- Goh PK, Chiu CL, Wang CY, Chan YK, Loo PL (2005) Randomized Double-blind Comparison of Ketamine-Propofol, Fentanyl-Propofol and Propofol-Saline on Haemodynamics and Laryngeal Mask Airway Insertion Conditions. Anaesth Intensive Care 33: 223-228. Link: https://bit.ly/3i0qVKg
- Eftekhari J, Kazemi Haki B, Tizro P, Alizadeh V (2015) A comparison to facilitate insertion of the laryngeal mask: term of recovery and postoperative nausea and vomiting after anesthesia with propofol- atracurium and thiopental-atracurium. Acta Med Iran 53: 117-121. Link: https://bit.ly/3xOwDoU
- Koh KF, Chen FG, Cheong KF, Esuvaranathan V (1999) Laryngeal mask insertion using thiopental and low dose atracurium: a comparison with propofol. Can J Anaesth 46: 670-674. Link: https://bit.ly/3xDAJ3p
- Wong CM, Critchley LA, Lee A, Khaw KS, Ngan Kee WD (2007) Fentanyl dose–response curves when inserting the LMA ClassicTM laryngeal mask airway. Anaesthesia 62: 654-660. Link: https://bit.ly/2UKkHG5
- Johnson RA, Harper NJN, Chadwick S, Vohra A (1990) Pain on injection of propofol Methods of alleviation Anaesthesia. 45: 439-442. Link: https://bit.ly/3AZpaFy
- Brain AI, McGhee TD, McAteer EJ, Thomas A, Abu-Saad MA, et al. (1985) The laryngeal mask airway. Development and preliminary trials of a new type of airway. Anaesthesia 40: 356-361. Link: https://bit.ly/2UBKXTb
- Ganatra SB, D'Mello J, Butani M, Jhamnani P (2002) Conditions for insertion of the laryngeal mask airway: comparisons between sevoflurane and propofol using fentanyl as a co-induction agent. A pilot study. Eur J Anaesthesiol 19: 371-375. Link: https://bit.ly/3r6kZTR
- Struys MM, De Smet T, Depoorter B, Versichelen LF, Mortier EP, et al. (2000) Comparison of plasma compartment versus two methods for effect compartment-controlled-target-ontrolled infusion for propofol. Anesthesiology 92: 399-406. Link: https://bit.ly/3e8D8Lt
- Dueck MH, Oberthuer A, Wedekind C, Paul M, Boerner U (2003) Propofol Impairs the Central but Not the Peripheral Part of the Motor System. Anesth Analg 96: 449–455. Link: https://bit.ly/3ww3Rbv
- Borgeat A, Dessibourg C, Rochani M, Suter PM (1991) Sedation by propofol in tetanus--is it a muscular relaxant? Intensive Care Med 17: 427-429. Link: https://bit.ly/3i5GLmG
- Chia YY, Lee SW, Liu K (2008) Propofol causes less postoperative pharyngeal morbidity than thiopental after the use of a laryngeal mask airway. Anesth Analg 106: 123-126. Link: https://bit.ly/3i7S6mt
- Cummings GC, Dixon J, Kay NH, Windsor JP, Major E, et al. (1984) Dose requirements of ICI 35,868 (propofol, 'Diprivan') in a new formulation for induction of anaesthesia. Anaesthesia 39: 1168-1171. Link: https://bit.ly/3rd51rd
- Scanlon P, Carey M, Power M, Kirby F (1993) Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth 40: 816-868. Link: https://bit.ly/3i5uZsE
- Dutt A, Joad AK, Sharma M (2012) Induction for classic laryngeal mask airway insertion: Does low-dose fentanyl work? J Anaesthesiol Clin Pharmacol 28: 210-213. Link: https://bit.ly/3B5ZVSn
- Ko SH, Kim DC, Han YJ, Song HS (1998) Small-dose fentanyl: optimal time of injection for blunting the circulatory responses to tracheal intubation. Anesth Analg 86: 658-661. Link: https://bit.ly/2UGD27h
- Brown GW, Patel N, Ellis FR (1991) Comparison of propofol and thiopentone for laryngeal mask insertion. Anaesthesia 46: 771-772. Link: https://bit.ly/3kpO2AP
- Molloy ME, Buggy DJ, Scanlon P (1999) Propofol or sevoflurane for laryngeal mask airway insertion. Can J Anaesth 46: 322-326.
- Asai T, Morris S (1994) The laryngeal mask airway: its features, effects and role. Can J Anaesth 41: 930-960. Link: https://bit.ly/2UJYicg
- Gavel G, Walker RWM (2014) Laryngospasm in anesthesia. Continuing Education in Anaesthesia Critical Care & Pain 14: 47-51. Link: https://bit.ly/2U1NPIY
- Drage MP, Nunez J, Vaughan RS, Asai T (1996) Jaw thrusting as a clinical test to assess the adequate depth of anaesthesia for insertion of the laryngeal mask. Anaesthesia 51: 1167–1170. Link: https://bit.ly/3hBYVxo
- Olsson GL, Hallen B (1984) Laryngospasm During Anaesthesia. A Computer-Aided Incidence Study in 136 929 Patients. Acta Anaesth Scand 28: 567-575. Link: https://bit.ly/3xxBfjg
- Khan K. (2011) Muscle twitching and hiccups with propofol. J Anaesthesiol Clin Pharmacol 27: 418. Link: https://bit.ly/3wzhvKH
- Landers C, Turner D, Makin C, Zaglul H, Brown R (2008) Propofol associated hiccups and treatment with lidocaine. Anesth Analg 107: 1757-1758. Link: https://bit.ly/36z6deV
- Pleym H, Spigset O, Kharasch ED, Dale O (2003) Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand 47: 241-259. Link: https://bit.ly/2VAAPL0
- Schnider TW, Minto CF, Shafer SL, Gambus PL, Andresen C, et al. (1999) The influence of age on propofol pharmacodynamics. Anesthesiology 90: 1502–1516. Link: https://bit.ly/3r5mVMs

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
