Global Journal of Anesthesiology
Evaluation of Dopamine Receptor Integrity after Sevoflurane Exposure in Neonatal Rat Brain Using Positron Emission Tomography
Qi Yin1, Merle G. Paule1, Tucker A. Patterson2, Fang Liu1, Charles M. Fogle1, Scott M. Apana3, Marc Berridge3, William Slikker Jr2, Cheng Wang1 and Xuan Zhang1*
2Office of the Director, National Center for Toxicological Research (NCTR), U.S. Food and Drug Administration (FDA), Jefferson, AR, USA
33D Imaging, LLC, Little Rock, AR, USA
Cite this as
Yin Q, Paule MG, Patterson TA, Liu F, Fogle CM, et al. (2018) Evaluation of Dopamine Receptor Integrity after Sevoflurane Exposure in Neonatal Rat Brain Using Positron Emission Tomography. Glob J Anesth 5(1): 001-006. DOI: 10.17352/2455-3476.000040Aim: The volatile general anesthetic sevoflurane is commonly used across all ages in the clinic. Sevoflurane-induced neurotoxic effects on the developing dopaminergic system are still unclear. The aim of this study was to evaluate the integrity of the D2/D3 receptor in developing rat brain utilizing molecular imaging techniques.
Method: Positron Emission Tomography (PET) coupled with Computerized Tomography (CT) approaches were used to evaluate the effects of developmental sevoflurane exposure on D2/D3 receptors in rat pups. The PET radiotracer, [18F]-fallypride, was used to examine whether dopamine receptors were affected by prolonged sevoflurane-exposure during the brain growth spurt. Six postnatal day (PND) 7 rats in the experimental group were exposed to sevoflurane at the clinically-relevant concentration of 2.5% (v/v) for 8 hours, and an equal number of control animals were exposed to room air for 8 hours. MicroPET/CT scans were sequentially collected on PNDs 14, 21, 28, and 35.
Results: No significant differences in the retention of [18F]-fallypride in striatum were detected between sevoflurane-exposed and control animals at any of the scan times. D2/D3 receptors are not affected by prolonged sevoflurane-induced general anesthesia during development.
Conclusion: Sevoflurane-induced neurotoxicity in the developing rodent brain was not associated with apparent derangements in dopamine D2/D3 receptors.
Introduction
Sevoflurane (1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy) propane) is the most commonly used general anesthetic across all ages in the United States. Less airway stimulation, lower blood solubility, minimal cardiovascular and respiratory side effects and other characteristics have made sevoflurane a more popular volatile anesthetic than isoflurane in clinical practice [1,2]. The precise mechanism of how sevoflurane could induce general anesthetics action is still not fully understood. The gamma-aminobutyric acid receptors (GABARs), N-methyl-D-aspartate receptors (NMDARs), glycine receptor, nicotinic acetylcholine receptors (nAChRs), 5-hydroxytryptamine type 3 (5-HT3) receptor, and dopamine receptor are six main regulators interacting with neurotransmitters in the brain. All of them have been reported as potential drug targets of sevoflurane [3-12].
There are substantial data indicating that prolonged exposure to sevoflurane can induce neurotoxicity in both rodents and nonhuman primates developing brains [13,14]. Significant behavioral research has also shown that exposure of neonatal mammalian animals to sevoflurane for a long period and/or multiple times will impair performance in the Morris water maze (MWM), a novel-object recognition test (NOR), social behaviors, fear conditioning and recognition memory at all ages [15-17]. Since there are many different kinds of neurons in the developing brain which may be affected by sevoflurane, the role dopaminergic neurons play in this complicated procedure need to be addressed. The function and expression levels of the dopamine D2/D3 receptor in the developing rodent brain exposed to a neurotoxic regimen of sevoflurane, has not been fully investigated.
Like glutamate and gamma aminobutyric acid, dopamine is an important neurotransmitter in the brain. It acts on dopamine receptors, which have five different subtypes (from D1 to D5), to regulate brain reward and pleasure, and many other physiological and pharmacological behaviors. Many diseases have been linked to dysfunction of loss of dopamine neurons, including Parkinson’s disease (PD), attention deficit hyperactivity disorder (ADHD), and others [18]. The five dopamine receptor subtypes can be divided into two subfamilies according to which type of downstream Gα protein they couple. The D2-like receptor subfamily includes D2, D3, and D4 subtype members, and they usually couple to Gαi/Gαo proteins, which may decrease the production of cAMP and the activation of protein kinase A (PKA) in the cytoplasm [18,19]. D2 receptors are highly expressed in the caudate, putamen (basal ganglia), and nucleus accumbens. D3 receptors have a more limited pattern of anatomical distribution such as in the nucleus accumbens [20,21]. The caudate, putamen and ventral striatum are three main nuclei of the striatum. The nucleus accumbens was included in the ventral striatum area [22], so the present research focused on evaluating the integrity of D2 and D3 receptors in this area.
Fallypride is a high-affinity dopamine D2/D3 receptor antagonist. [18F]-fallypride, a conjugation of fallypride and the short-lived radionuclide fluorine-18, was synthesized as a selective dopamine D2/D3 receptor radiotracer for PET scanning in vivo. Imaging studies utilizing [18F]-fallypride have been performed in rodents, non-human primates and humans [23-25]. As a minimally invasive, dynamic and leading approach, PET imaging can acquire quantitative images from living animal organs such as the brain [26,27]. Since PET imaging data can be collected from the same subject at multiple time points, it provides the possibility of continuously examining physiological and pharmacological processes of interesting targets in vivo.
The present study was designed to determine whether exposure to sevoflurane during a sensitive period of brain development in the rat alters specific aspects of dopaminergic systems, especially for D2/D3 receptors, which can be efficiently measured using a PET radiotracer.
Methods
Animals
The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the National Center for Toxicological Research (NCTR). All animal procedures were conducted in full accordance with the public health service (PHS) Policy on Humane Care and Use of Laboratory Animals.
Postnatal day (PND) 3 Sprague-Dawley rat pups with dams were purchased from Charles River Laboratories International, INC (Wilmington, MA) and were randomly assigned to control or sevoflurane exposure groups (n=6)/group. On PND 7 (16.6±5.4 g), rat pups in the treatment group were exposed to 2.5% (v/v) sevoflurane delivered in medical-grade air at a rate of 0.5-1.0 l/min for 8 hours. Animals in the control group stayed with a dam in room air for 8 hours. Sevoflurane (Webster Veterinary Supply, Sterling, MA) was delivered using an agent-specific vaporizer (Euthanex Corp, Palmer, PA, USA) attached to the anesthetic machine. Throughout the 8 hours of exposure the rat pups in the sevoflurane treatment group were kept in the induction chamber on an electronic heating pad to maintain body temperature at approximately 37°C. A charcoal filter canister was used to absorb the extra vaporized anesthetic from the chamber. Non-invasive pulse oximetry (MouseOX Plus Vital Sign Monitor, StarrTM Life Sciences, Oakmont, PA) was used to verify the physiological status of subjects. Heart and respiration rates and arterial blood O2 were recorded every one hour in anesthetized animals.
After treatment, rat pups were returned to the animal facility until the microPET/CT scans. Each rat was weighed before each scan. The microPET/CT scans were scheduled at multiple time points (PNDs 14, 21, 28 and 35). During these scans anesthesia was induced and maintained with isoflurane (1.5-2%) mixed with oxygen.
Radiotracer preparation
[18F]-fallypride (or (S)-N[1-allyl-2-pyrrolidinyl)methyl]-5-(3[18F]fluoropropyl-2,3-dimethoxy-benzamide) was prepared by 3D Imaging LLC (Little Rock, USA) following published procedures [28,29].
MicroPET/CT image acquisition
All microPET/CT image acquisitions were performed with an Inveon small animal PET/CT scanner (Siemens Preclinical Solutions, Washington, DC, USA). For a 90-min static PET scan a single dose of [18F]-fallypride (20.35±1.85 MBq) was injected into animals via tail vein under isoflurane anesthesia. Thirty mins after tracer injection a 90-min static PET scan was performed. CT scans were immediately initiated following the PET scan. All images were reconstructed using a two-dimensional ordered-subset expectation maximum algorithm (2D OSEM). MicroPET images were attenuated and scatter corrected by CT image. Image pixel size was 0.77 mm and the slice thickness was 0.796 mm.
Statistical analysis
Image analysis was performed using Inveon Research Workplace (Siemens Preclinical Solution, Washington, DC, USA). With fused PET/CT images as guidance, 1.9 mm3 three-dimensional regions of interest (ROIs) were manually defined in the striatum region of the left brain hemisphere. The intensity of tracer accumulations in the ROIs in the left striatum was converted to standard uptake values (SUVs).
GraphPad Prism version 6 (GraphPad Software, La Jolla, CA. USA) was used for data analyses and to produce graphs. All assays were analyzed using unpaired student t-tests. All analyses were two-tailed and considered to be statistically different at p-values less than 0.05.
Results
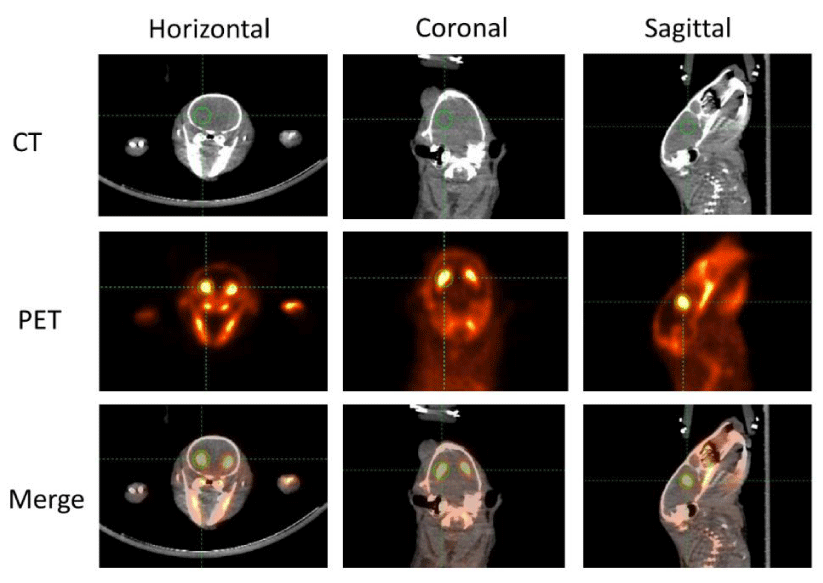
In order to improve the location accuracy of microPET imaging, a CT scan was scheduled immediately after the 90-min microPET scan. A representative picture is shown in figure 1. The image of a rat brain is presented in three different kinds of anatomical panels including the horizontal, coronal, and sagittal plane. CT images that were used as anatomical references are presented in the top row. PET images are presented in the middle row. The merged images of PET and CT were automatically given by the IRW software co-registration function. The combination of both structure and functional images were presented in the bottom row. The striatum is indicated by the green cross circle. As mentioned, D2 and D3 receptors are predominantly distributed in the striatum, so the strongest [18F]-fallypride retention area coincides with the striatum. In order to compare the radioactivity intensity of each subject a three-dimensional region of ROIs with the same volume was manually and randomly drawn in the left striatum. Since 18F is a sensitive bone-seeking isotope and much of the [18F]-fallypride is retained in bone during the scan procedure [31], high radioactive areas outside the brain were primarily observed in bones and teeth. Meanwhile, ROIs in the left frontal cortex were analyzed and compared. No apparent differences were found (data not shown).
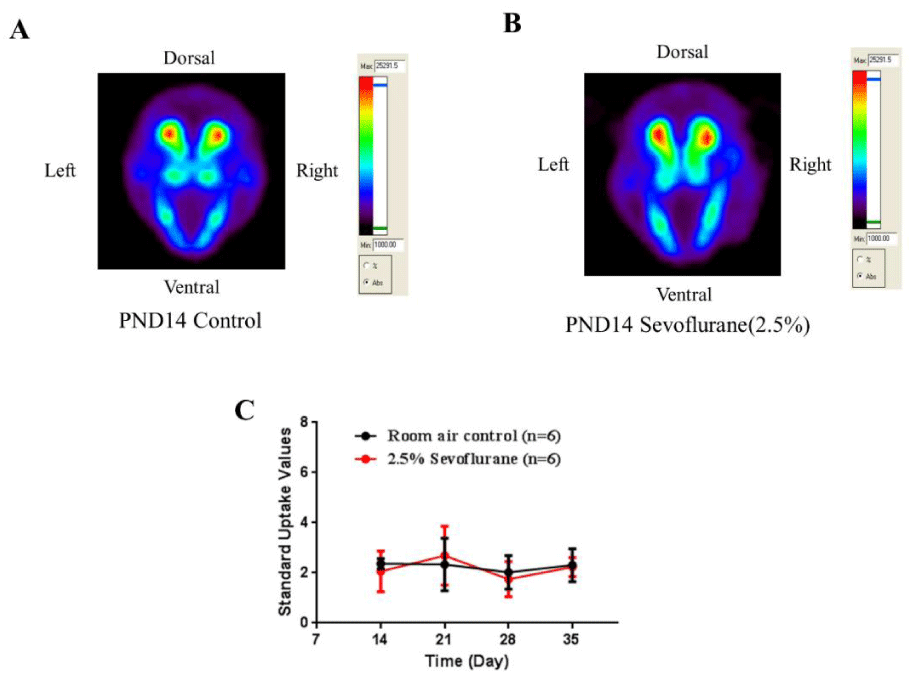
On PND 14 (one week following exposure), all animals were weighed and injected intravenously with [18F]-fallypride. The decay-corrected amount of injected [18F]-fallypride was calculated by subtracting the activity of the empty syringe from the activity of the full syringe. The radioactivity concentrations of ROIs were measured by the IRW. SUVs were calculated by average concentration of radioactivity in the ROIs multiplied by body weight and then divided by injected dose [32,33]. No statistically significant differences of [18F]-fallypride SUVs in the striatum were detected between exposure and control groups on PND 14. The uptake of radioactivity in control (Figure 2A) and sevoflurane-exposed rats (Figure 2B) were similar.
Serial microPET scans were performed on PND 21, 28, and 35 in order to monitor the dynamic development of the brain after sevoflurane exposure following the same procedure as described on PND 14. The SUVs of each time point were analyzed and no changes in D2/D3 receptor expression levels were found in the striatum of the sevoflurane-exposed and control rats (Figure 2C). The results indicate that an 8-hour sevoflurane exposure does not impair D2/D3 dopaminergic receptor function in the striatum.
Discussion
MicroPET/CT is a minimally invasive molecular imaging multimodality that was broadly used in both preclinical and clinical research [34]. To determine the expression levels, distribution pattern and function of dopamine receptors in both physiological and pathological conditions, many different kinds of dopamine receptor binding radiotracers have been developed [35-38]. Among them, [18F]-fallypride, a D2/D3 selective antagonist, is the radiotracer which has been well characterized and widely utilized in microPET studies. [18F]-fallypride can be used as radiotracer to evaluate dopamine receptor expression levels because of the following factors. First, the affinity of [18F]-fallypride for D2/D3 receptors can reach a very low concentration (0.03 nM) in vitro at room temperature and the in vivo affinity of [18F]-fallypride can be detected between 0.17±0.05 nM and 0.22±0.05 nM in baboon brains [39]. Also, the high affinity is not affected by the density of the D2/D3 receptor and the concentration of endogenous dopamine in different brain areas [39]. Second, the high-affinity gives [18F]-fallypride a high signal-to-noise ratio in PET scans. Compared to the first generation D2 radiotracers, which can only obtain images of the striatum that have high expression levels of dopamine D2 receptors, [18F]-fallypride provides an invaluable tool for whole brain D2/D3 receptor investigation [40]. To date many striatal and extrastriatal D2 receptor studies have been investigated in rodents, nonhuman primates and humans using [18F]-fallypride [41-43]. Finally, [18F]-fallypride offers a maximum binding potential in D2/D3 receptor rich regions during reasonable task timing. Time activity curves (TACs) of [18F]-fallypride in striatum have been plotted in various species of animals [44-47]. Also, [18F]-fallypride can easily cross the blood brain barrier and reach maximal uptake activity within 30 min after a single bolus IV injection. An equilibrium state may last at least two hours before gradual clearance. Therefore, the relatively slow washout and long half-life (110 minutes) of [18F]-fallypride are two key factors which support our study designs [48]. Thus, [18F]-fallypride was selected in this study to evaluate the potential effect of prolonged sevoflurane exposure on D2 and D3 dopamine receptor expression levels. In present the study both control and sevoflurane-exposed animals were initially scanned 30 min after intravenous injection of [18F]-fallypride and the data recorded for 90 min in order to collect as many signals as possible. Our microPET data demonstrated that the accumulation of the [18F]-fallypride signal was heavily expressed in the striatum as evidenced by horizontal, coronal and sagittal examination (Figure 1), in both control and sevoflurane-exposed animals.
It should be mentioned that currently only a few studies have investigated the roles of monoaminergic neurotransmission in sevoflurane anesthesia [10]. In general, dopamine and dopamine receptors help control brain reward and pleasure, and their dysregulation and/or deficiency can result in neuronal degeneration and cognitive-disorders including Parkinson’s disease [49]. Also, numerous behavioral studies have disclosed that long-term sevoflurane exposure in developing brains may impair cognition, memory and learning ability later in life [15-17,50,51]. It has been reported that altered dopamine neurotransmission, especially dopamine receptor expression levels, may mediate the capacity of inhaled anesthetics to provide immobility and decrease or increase MAC (the minimum alveolar concentration of an inhaled anesthetic required to suppress movement in response to a noxious stimulus in 50% of test subjects) [52,53]. Therefore, it is originally proposed that any kind of D2/D3 receptor dysregulation or dysfunction, after prolonged anesthetic exposure, might subsequently result in increased neuronal damage in various brain areas including striatum. Since imaging tools provide a means to analyze the mechanism underlying behavior dynamically, the subjects (experimental animals) could receive “follow up” scans repeatedly. Thus, minimally invasive microPET/CT imaging was applied in the present study to detect subtle variations in vivo and to measure the molecular level variation of the dopaminergic system following 1, 2, 3, and 4 weeks of sevoflurane exposure. Particularly, striatal dopamine receptor levels were monitored to determine whether the dynamic alteration of D2 and D3 receptors could be associated with the sevoflurane-induced neuro-apoptosis, which was previously demonstrated [14,54]. Our imaging data demonstrate that no statistically significant changes in D2/D3 receptors were detected in striatum on PND 14 or older ages after an 8-hour sevoflurane exposure in the developing rodent brain. Our previous publication demonstrated that the frontal cortex was one of the most vulnerable areas of anesthetic-drug induced neural damage [55], so we also evaluated the D2/D3 receptor expression levels in this area. No obvious changes were found in frontal cortex at multiple time points (data not shown). These data, plus data from studies by others [56], indicate that dopamine receptors do not mediate the immobility or altered MAC produced by inhaled anesthetics, including sevoflurane, and that D2/D3 receptors in striatum have no contribution to sevoflurane-induced behavioral disturbance and sevoflurane-induced neurotoxicity [14,54].
Commonly used anesthetics may induce neuro-apoptosis in immature animal brains. Such mechanisms have been widely categorized and associated deficits of cognitive behavior are usually caused by brain and/or neuronal damage in areas that affect learning and memory. According to our PET and immunochemical data, D2/D3 neurons in the striatum may be less sensitive or completely not related to sevoflurane-induced neuronal damage and behavioral deficits. Accumulating evidence indicates that anesthetic-induced neuronal damage could be closely associated with receptor subtype activation and the maturity of animals at the time of exposure [57,58]. Therefore, neuro-apoptosis induced by prolonged sevoflurane could be mediated via all mechanisms whereby sevoflurane acts; including sevoflurane-induced depression of glutamate release [59], GABA receptor excitation [60], increased intracellular calcium influx [61], anesthetics-induced decrease in neurotrophic factors [62] and anesthetic-induced activation of inositol 1,4,5,-trisphosphate (IP3) receptors [63]. Thus, our immediate priority for future projects is to search and identify specific radiotracers to target potential alteration of glutamate neurotransmission and GABA excitation after gaseous anesthetic exposure. Also, the effects of anesthetics on mitochondria could be another factor as why immature neurons are subject to anesthetic-induced apoptosis.
The limitation of molecular imaging studies is that a variety of different brain cell types cannot be characterized using microPET/CT scanning. There are many different kinds of cells in the central nervous system, including neurons, astrocytes, oligodendrocytes and microglia. An obvious question for further studies is to categorize, quantify and determine which types of brain cells can be affected under the situation of sevoflurane exposure. Neural cell and apoptosis-related biomarkers may be useful to solve the limitation [64].
In summary, microPET/CT provides a minimally invasive way to interrogate brain status affected by general anesthesia during development. The current results demonstrate that dopamine neurotransmission, especially in the D2/D3 receptor subtypes, is not affected by prolonged sevoflurane-induced general anesthesia. D2/D3 receptors in striatum do not contribute to sevoflurane-induced neurotoxicity.
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval dose not signify that the contents necessarily reflect the position or opinions of the FDA. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
- Delgado-Herrera L, Ostroff RD, Rogers SA (2001) Sevoflurane: Approaching the ideal inhalational anesthetic a pharmacologic, pharmacoeconomic, and clinical review. CNS Drug Rev 7: 48-120. Link: https://goo.gl/SbQa8C
- De Hert S, Moerman A (2015) Sevoflurane. F1000Res 4: 626. Link: https://goo.gl/E5QK92
- Eckle VS, Hauser S, Drexler B, Antkowiak B, Grasschoff C (2013) Opposing actions of sevoflurane on GABAergic and glycinergic synaptic inhibition in the spinal ventral horn. PLoS One 8: e60286. Link: https://goo.gl/aAcQ83
- Hapfelmeier G, Schneck H, Kochs E (2001) Sevoflurane potentiates and blocks GABA-induced currents through recombinant alpha1beta2gamma2 GABAA receptors: implications for an enhanced GABAergic transmission. Eur J Anaesthesiol 18: 377-383. Link: https://goo.gl/QfEQAz
- Kria T, Harata N, Sakata T, Akaike N (1998) Kinetics of sevoflurane action on GABA- and glycine-induced currents in acutely dissociated rat hippocampal neurons. Neuroscience 85: 383-394. Link: https://goo.gl/82CaEV
- Paul S Garcia, Scott E Kolesky, Andrew Jenkins (2010) General anesthetic actions on GABAA receptors. Curr Neuropharmacol 8: 2-9. Link: https://goo.gl/dwZkxy
- Robert J Brosnan, Roberto Thiesen (2012) Increased NMDA receptor inhibition at an increased sevoflurane MAC. BMC Anesthesiol 12: 9. Link: https://goo.gl/zNDMEi
- Scheller M, Bufler J, Schneck H, Kochs E, Franke C (1997) Isoflurane and sevoflurane interact with the nicotinic acetylcholine receptor channels in micromolar concentrations. Anesthesiology 86: 118-127. Link: https://goo.gl/RCz5aQ
- Suzuki T, Koyama H, Sugimoto M, Uchida I, Mashimo T (2002) The diverse actions of volatile and gaseous anesthetics on human-cloned-5-hydroxytryptamine 3 receptor expressed in Xenopus oocytes. Anesthesiology 96: 699-704. Link: https://goo.gl/atCo2A
- Silva JH, Gomez RS, Diniz PH, Gomez MV, Guatimosim C (2007) The effect of sevoflurane on the release of [3H] dopamine from rat brain cortical slices. Brain Res Bull 72: 309-314. Link: https://goo.gl/vjVaER
- Tanifuji Y, Zhang Y, Liao M, Eger EI 2, Laster MJ, et al. (2006) Do dopamine receptors mediate part of MAC? Anesth Analg 103: 1177-1181. Link: https://goo.gl/21Jc3R
- Kimura-Kuroiwa K, Adachi YU, Mimuro S, Obata Y, Kawamata M, et al. (2012) The effect of aging on dopamine release and metabolism during sevoflurane anesthesia in rat striatum: an in vivo microdialysis study. Brain Res Bull 89: 223-230. Link: https://goo.gl/qS5NeR
- Liu SL, Paule MG, Zhang X, Newport GD, Patterson TA, et al. (2014) Positron emission tomography with [18F]FLT revealed sevoflurane-induced inhibition of neural progenitor cell expansion in vivo. Frontiers in Neurology 5: 234. Link: https://goo.gl/VrvbCP
- Zhang X, Liu SL, Newport GD, Paule MG, Callicott R, et al. (2016) In Vivo monitoring of sevoflurane-induced adverse effects in neonatal nonhuman primates using small-animal positron emission tomography. Anesthesiology 125: 133-146. Link: https://goo.gl/dF6v2i
- Xiao H, Liu B, Chen Y, Zhang J (2016) Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int J Dev Neurosci 48: 38-49. Link: https://goo.gl/XHCK38
- Satomoto M, Satoh Y, Terui K, Miya H,Takishima K, et al. (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110: 628-637. Link: https://goo.gl/GnbkiF
- Stratmann G, Lee J, Sall JW, Lee BH, Alvi RS, et al. (2014) Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology 39: 2275-2287. Link: https://goo.gl/ffsVsb
- Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling and pharmacology of dopamine receptors. Pharmacol Rev 63:182-217. Link: https://goo.gl/TKHbJd
- Jaber M, Robinson SW, Missale C, Caron MG (1996) Dopamine receptors and brain function. Neuropharmacology 35: 1503-1519. Link: https://goo.gl/2C9VHz
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, et al. (1991) Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology 5: 231-242. Link: https://goo.gl/ELdL7L
- Parsons B, Stanley M, Javitch J (1993) Differential visualization of dopamine D2 and D3 receptors in rat brain. Eur J Pharmacol 234: 269-272. Link: https://goo.gl/5iKQoo
- Raymundo Baez-Mendoza, Wolfram Schultz (2013) The role of the striatum in social behavior. Front Neurosci 7: 233. Link: https://goo.gl/ShTRPq
- Constantinescu CC,Coleman RA, Pan ML, Mukherjee J (2011) Striatal and extrastriatal microPET imaging of D2/D3 dopamine receptors in rat brain with [18F]-fallypride and [18F]Desmethoxyfallypride. Synapse 65:778-787. Link: https://goo.gl/LWkeVf
- Mukherjee J, Christian BT, Narayanan TK, Shi B, Collins D (2005) Measurement of d-amphetamine-induced effects on the binding of dopamine D-2/D-3 receptor radioligand, 18F-fallypride in extrastriatal brain regions in non-human primates using PET. Brain Res1032: 77-84. Link: https://goo.gl/WSJUV8
- Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, et al. (2010) Striatal and extrastriatal dopamine release measured with PET and [18F]fallypride. Synapse 64:350-362. Link: https://goo.gl/2aFPgQ
- Wang C (2016) Advanced techniques to study anesthetic effect on the nervous system. Glob J Anesthesiol 3: 007-010. Link: https://goo.gl/MUrAxN
- Zhang X, Paule MG, Newport GD, Zou XJ, Sadovova N, et al. (2009) A minimally invasive, translational biomarker of ketamine-induced Neuronal death in rats: micro PET imaging using 18F-annexin V. Toxicol Sci 111: 355-361. Link: https://goo.gl/LppWNn
- Mukherjee J, Yang ZY, Das MK, Brown T (1995) Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nuclear Medicine and Biology 22: 283-296. Link: https://goo.gl/bcVxJq
- Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, et al. (1999) Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med and Biol 26: 519-527. Link: https://goo.gl/v7vcKt
- Liu F, Rainosek SW, Sadovova N, Fogle CM, Patterson TA, et al. (2014) Protective effect of acetyl-L-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 42: 49-57. Link: https://goo.gl/RGxbQj
- Rastogi A, Bhattacharya A, Prakash M, Sharma S, Mittal BR, et al. (2016) Utility of PET/CT with fluorine-18-fluorodeoxyglucose-labeled autologous leukocytes for diagnosing diabetic foot osteomyelitis in patients with Charcot’s neuroarthropathy. Nucl Med Commun 37: 1253-1259. Link: https://goo.gl/xfMteh
- Kinahan PE, Fletcher JW (2010) Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR 31: 496-505. Link: https://goo.gl/tAvbGS
- Yoder KK, Mock BH, Zheng QH, McCarthy BP, Riley AA, et al. (2011) Assessment of i.p. injection of [18F]-fallypride for behavioral neuroimaging in rats. J Neurosci Methods 196: 70-75. Link: https://goo.gl/oq2dsg
- Zhang X, Liu F, Slikker W Jr, Wang C, Paule MG. (2017)Minimally invasive biomarkers of general anesthetic-induced developmental neurotoxicity. Neurotoxicol Teratol 60: 95-101. Link: https://goo.gl/vn659i
- Ehrin E, Farde L, de Paulis T, Eriksson L, Greitz T, et al. (1985) Preparation of 11C-labelled Raclopride, a new potent dopamine receptor antagonist: preliminary PET studies of cerebral dopamine receptors in the monkey. Int J Appl Radiat Isot 36: 269-273. Link: https://goo.gl/Z3qASm
- Kung HF, Kasliwal R, Pan SG, Kung MP, Mach RH, et al. (1988) Dopamine D-2 receptor imaging radiopharmaceuticals: synthesis, radiolabeling, and in vitro binding of (R)-(+)-and (S)-(-)-3-iodo-2-hydroxy-6-methoxy-N-[(1-ethyl-2-pyrrolidinyl)methy]benzamide. J Med Chem 31: 1039-1043.
- Halldin C, Farde L, Hogberg T, Mohell N, Hall H, et al. (1995) Carbon-11-FLB457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 36: 1275-1281. Link: https://goo.gl/YVZE6m
- Kessler RM, Mason NS, Votaw JR, De Paulis T, Clanton JA, et al. (1992) Visualization of extrastriatal dopamine D2 receptors in the human brain. Eur J Pharmacol 223: 105-107. Link: https://goo.gl/ykzCKM
- Slifstein M, Hwang DR, Huang Y, Guo N, Sudo Y, et al. (2004) In vivo affinity of [18F]-fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman primates. Psychopharmacology 175: 274-286. Link: https://goo.gl/aWCq2s
- Lawrence S. Kegeles, Mark Slifstein, Xiaoyan Xu, Nina Urban, Judy L. Thompson, et al. (2010) Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride PET. Biol Psychiatry 68: 634-641. Link: https://goo.gl/4hq6qb
- Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, et al. (2010) D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. Neuroimage 50: 1402-1407. Link: https://goo.gl/orz2xe
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, et al. (2010) High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab 30:994-1007. Link: https://goo.gl/1ww5je
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, et al. (2002) Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 46: 170-188. Link: https://goo.gl/v9JXr8
- Tantawy MN, Jones CK, Baldwin RM, Ansari MS, Conn PJ, et al. (2009) [(18)F] Fallypride dopamine D2 receptor studies using delayed microPET scans and a modified Logan plot. Nucl Med Biol 36:931-940. Link: https://goo.gl/1tM97e
- Christian BT, Vandehey NT, Fox AS, Murali D, Oakes TR, et al. (2009) The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage 44: 1334-1344. Link: https://goo.gl/nSizcc
- Slifstein M, Narendran R, Hwang DR, Sudo Y, Talbot PS, et al. (2004) Effect of amphetamine on [(18)F]Fallypride in vivo binding to D(2) receptors in striatal and extrastriatal regions of the primate brain: Single bolus and bolus plus constant infusion studies. Synapse 54: 46-63. Link: https://goo.gl/y3r2kZ
- Ceccarini J, Vrieze E, Koole M, Muylle T, Bormans G, et al. (2012) Optimized in vivo detection of dopamine release using 18F-fallypride PET. J Nucl Med 53: 1565-1572. Link: https://goo.gl/hZH3aE
- Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, et al. (2011) The applicability of SRTM in [(18)F]Fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab 31:1958-1966. Link: https://goo.gl/DPZ3Qg
- Seeman P, Niznik HB (1990) Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J 4: 2737-2744. Link: https://goo.gl/yxLwKq
- Zhang X, Shen F, Xu D, Zhao X (2016) A lasting effect of postnatal sevoflurane anesthesia on the composition of NMDA receptor subunits in rat prefrontal cortex. Int J Dev Neurosci 54: 62-69. Link: https://goo.gl/QdCXxP
- Tian Y, Guo S, Wu X, Ma L, Zhao X (2015) Minocycline alleviates sevoflurane-induced cognitive impairment in aged rats. Cell Mol Neurobiol 35:585-594. Link: https://goo.gl/ghMxEA
- Segal IS, Walton JK, Irwin I, DeLanney LE, Ricaurte GA, et al. (1990) Modulating role of dopamine on anesthetic requirements. Eur J Pharmacol 186: 9-15. Link: https://goo.gl/EBkHjw
- Onozawa H, Miyano K, Tanifuji Y (1999) Effect of dopamine content in rat brain striatum on anesthetic requirement: an in vivo microdialysis study. Brain Res 817:192-195. Link: https://goo.gl/naJmmM
- Liu F, Rainosek SW, Frisch-Daiello JL, Patterson TA, Paule MG, et al. (2015) Potential adverse effects of prolonged sevoflurane exposure on developing monkey brain: from abnormal lipid metabolism to neuronal damage. Toxicol Sci 147:562-572. Link: https://goo.gl/qhRv6R
- Wang C, Slikker W Jr (2008) Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg 106:1643-1658. Link: https://goo.gl/RdNCnj
- Tanifuji Y, Zhang Y, Liao M, Eger EI 2nd, Laster MJ, et al. (2006) Do dopamine receptors mediate part of MAC? Anesth Analg 103: 1177-1181 Link: https://goo.gl/RPhcQE
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, et al. (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961-973. Link: https://goo.gl/4D5F7r
- Wang C, Kaufmann JA, Sanchez-Ross MG, Johnson KM (2000) Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J Pharmacol Exp Ther 294: 287-295. Link: https://goo.gl/BRcZsN
- Ishizeki J, Nishikawa K, Kubo K, Saito S, Goto F (2008) Amnestic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology 108:447-456. Link: https://goo.gl/HrXZQX
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, et al. (2001) Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol 62:401-405. Link: https://goo.gl/BPt8n5
- Uvarov P, Ludwig A, Markkanen M, Pruunsild P, Kaila K, et al. (2007) A novel N-terminal isoform of the neuron-specific K-Cl cotransporter KCC2. J Biol Chem 282: 30570-30576. Link: https://goo.gl/qFecg9
- Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11: 1603-1615. Link: https://goo.gl/wzD3g1
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, et al (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1, 4, 5-trisphosphate receptors. Anesthesiology 108: 251-260. Link: https://goo.gl/yjHsLn
- Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, et al. (2012) Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol 72: 525-535 . Link: https://goo.gl/FiapWA

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
