Global Journal of Anesthesiology
MicroPET/CT Assessment of Minocycline Effects on Anesthetic-Induced Neuronal Injury in Developing Rats
Xuan Zhang1*, Merle G Paule1, Mackean Maisha1, Glenn D Newport1, Marc S Berridge2, Scott M Apana2, William Slikker Jr1 and Cheng Wang1
23D Imaging, LLC, Little Rock, AR 72113 and University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA
Cite this as
Zhang X, Paule MG, Maisha M, Newport GD, Berridge MS, et al. (2017) MicroPET/CT Assessment of Minocycline Effects on Anesthetic-Induced Neuronal Injury in Developing Rats. Glob J Anesth 4(3): 041-047. DOI: 10.17352/2455-3476.000039Ketamine is a dissociative anesthetic that is frequently used for the induction and maintenance of general anesthesia in children. It has been reported that blockade of NMDA receptors by ketamine may cause neurotoxicity in neonatal rats when given over a 12 hour period during the brain growth spurt. Noninvasive, quantitative imaging of rodent brains may allow for the detection of functional, morphological and metabolic alterations induced by ketamine. Since it is known that level of the mitochondrial translocator protein (TSPO), formerly known as the peripheral benzodiazepine receptor (PBR) increase in areas of neuronal injury following exposure to neurotoxicants, TSPOs are widely recognized as important targets for imaging using positron emission tomography (PET). In this study, the effect of ketamine on the uptake and retention of [18F]-FEPPA (a TSPO ligand) in the brains of rats and the potential protective effect of minocycline, an anti-inflammatory agent, on anesthetic-induced neuronal cell death were investigated using microPET/CT imaging. On postnatal day 7 (PND 7), rat pups in the experimental group were exposed to 6 injections of ketamine (20 mg/kg at 2 h intervals) with or without minocycline (45mg/kg i.p. 30 minutes prior to, and 4 hours after exposure); control pups received 6 injections of saline. On PNDs 14, 21, 28 and 35, [18F]-FEPPA (18.5 MBq) was injected into the tail vein of treated and control rats and microPET/CT images were obtained over the next 90 minutes. Radiolabeled tracer accumulation in regions of interest (ROIs) in the frontal cortex was converted into Standard Uptake Values (SUVs). In PND 14 and 21 rats the uptake of [18F]-FEPPA was significantly increased and the duration of tracer wash-out was prolonged in ketamine-treated rats. The increased uptake of the tracer was attenuated by the co-administration of minocycline. As expected, no significant difference in radiotracer uptake in the frontal cortex was observed at 28 or 35 days after anesthesia. This preliminary study demonstrates that microPET imaging is capable of distinguishing differences in retention of [18F] -FEPPA in the brains of rodents and suggests that this approach may provide a minimally-invasive biomarker of pathogenic process associated with neurotoxicity induced by ketamine. Minocycline effectively blocks the neuronal injury caused by ketamine anesthesia in the developing rat brain.
Introduction
As a noncompetitive NMDA receptor antagonist, ketamine is a commonly used anesthetic in pediatric patients for the induction and maintenance of general anesthesia, usually in combination with other sedative and anesthetic drugs [1,2]. However, numerous studies of both the developing animal brain and primary cultured neurons have reported that prolonged exposure to ketamine during the brain growth spurt period induces widespread neurotoxicity as shown by increased neuroapoptosis, enhanced neuroinflammation and impaired neurogenesis [3-11]. General anesthesia maintained by ketamine during brain development can result in long-lasting deficits in brain function in nonhuman primates [12]. These observations have raised concerns regarding the use of ketamine-induced anesthesia for surgery or other clinical procedures in early childhood. Recently, retrospective reports have indicated that the development of cognitive abnormalities and learning disabilities in children correlate with anesthetic exposure during surgery before 4 years of age [2,10,13-18]. Thus, practical neuroprotective strategies that can serve to protect the developing brain from ketamine or other general anesthetic-induced neuronal injury and long-lasting cognitive impairments are urgently needed.
Minocycline is a semi-synthetic, tetracycline derivative that has antibiotic activity against a broad spectrum of bacterial types [19-21]. In addition to its broad antimicrobial activity, minocycline effectively crosses the blood-brain barrier and has exhibited neuroprotective effects in various types of experiment models [18-24]. Minocycline is approved by the FDA and indicated up to 200 mg in 24 hours for a variety of infections. With high bioavailability in humans, minocycline is efficiently absorbed by the gastrointestinal tract the average half-life is around 15 h [25,26]. The mechanisms underlying the neuroprotective effect of minocycline are not clear: minocycline may exert its neuroprotection through inhibition of microglial activation, anti-inflammatory effects, reduction of nitric oxide synthesis and/or prevention of apoptotic cell death [19-22,24,27].
In our previous studies, the administration of multiple doses of ketamine (20 mg/kg every 2 hours, 6 times, s.c.) to PND 7 rat pups, induced widespread neuronal damage in the developing brain as indicated by increased caspase-3-, silver stain-, and Fluoro-Jade C-positive cells in neocortical areas, especially in layers II and III of the frontal cortex [11]. MicroPET scans using [18F]-radiolabelled]-N-(2-(2-fluoroethoxy) benzyl)-N-(4-phenoxypyridin-3-yl) acetamide) ([18F]-FEPPA) [28], indicated that anesthetic exposure significantly increased the uptake of [18F]-FEPPA in the temporal and frontal lobes of exposed nonhuman primates in a time-dependent manner. In the current study, we initiated a microPET protocol to measure [18F]-FEPPA uptake as a quantitative marker of neuroinflammation and, presumably, neuronal injury in the rat brain in vivo.
Materials and Methods
Drugs
Ketamine hydrochloride (Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA, USA) for injection was diluted in saline. The purity of ketamine (> 99%) was identified and confirmed via HPLC (High-performance liquid chromatography) and mass spectrometry. Minocycline hydrochloride was purchased from Sigma-Aldrich, USA) and dissolved in sterile saline.
Animals
All animal procedures were approved by the National Center for Toxicological Research (NCTR) Institutional Animal Care and Use committee and conducted in full accordance with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals.
Animals included in current study were obtained from and maintained in the animal facility at NCTR with dam (PND 7-PND 21) or 3 rats/cage after PND 21. The room temperature in the animal facility was kept at 22±2°C. The animals were maintained ad lib on regular rat chow and water under a light/dark cycle of 12hr/12hr. The light cycle for these animals began at 7:00 am. Seven-day-old (PND-7) Sprague Dawley (male and female) rat pups (average body weight 11–18 g) were randomly assigned to control, control plus minocycline, ketamine plus saline, and ketamine plus minocycline groups (n=3-5/group, with the same rats used on PNDs 14, 21,28 and 35). Ketamine hydrochloride or saline (10µl/g) was applied subcutaneously using a 29-gauge needle. Similar to our previous studies [11], doses of ketamine (20 mg/kg/injection) or saline were given in six injections within 12 hours (2-h/interval). In order to maintain body temperature and lessen potential stressors, pups were accommodated with their dam during the 2-h injection interval [11]. Rat pups in the groups with minocycline were given i.p. injections of 45 mg/kg minocycline (100-200µl) in saline 1/2 hr before and 4 hr after the start of ketamine administration. The minocycline solution was prepared within 24h of the experiment and kept on ice between the two injections.
After exposure on PND 7, rat pups in all groups were returned to the rodent facility until microPET scanning on PNDs 14, PND 21, PND 28 and PND 35.
Radiotracer preparation
[18F]-FEPPA was prepared by 3D-Imaging LLC (Little Rock, AR) according to Wilson et al. [29], from the corresponding tosylate as described therein with minor modifications to the procedure. [18F]-FEPPA was produced in > 98% purity at a specific activity EOS of 1.1-3.7 TBq (30-100 Ci)/μmole, as compared to the previously reported value of 11-37 MBq (0.3-1 Ci)/μmole [30]. Specific activity at the time of use was one tenth to two-thirds of the end of synthesis (EOS) value.
MicroPET
MicroPET images of the rat brains were acquired quantitatively using a commercial high resolution small animal PET scanner, Focus 220 (Siemens Preclinical Solution, Knoxville, USA). With 96 lutetium oxyortho-silicate detectors, Focus 220 provides microPET images with a transaxial resolution of 1.35 mm full-width at half-maximum. List mode data were collected in a 128x128x95 matrix with a pixel width of 0.475 mm and a slice thickness of 0.815 mm.
MicroPET scan
MicroPET images were recorded on PNDs 14, 21, 28 and 35. General anesthesia was initially induced in rat pups using 1.5-2% isoflurane gas delivered through a custom face mask: anesthesia was maintained using 1-1.5% isoflurane throughout the PET imaging procedure. For each imaging procedure, [18F]-FEPPA (18.5 MBq) was injected into the tail vein of each animal. Following the injection, a set of serial microPET images was collected over 90 minutes (18 frames, 5 min each) to assess the uptake of the radiotracer.
MicroPET data analysis
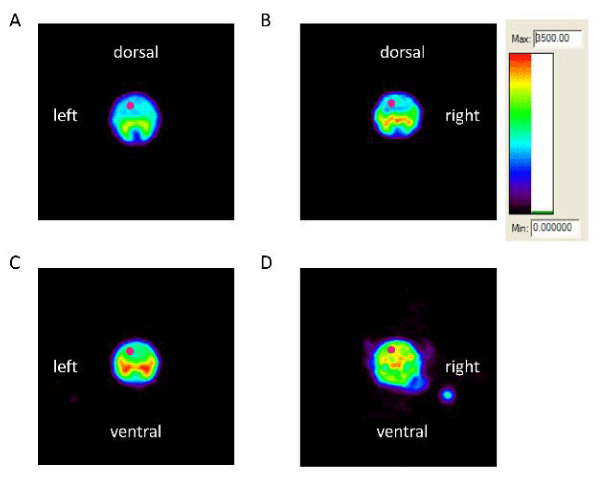
Medical image analysis software, ASIProTM (Concorde Microsystems, Inc, Knoxville, TN) was used in the analyses for each Region of Interest (ROI). ROIs were defined and quantified using ROI tools provided by ASIProTM. As shown in Figure 1, all images were displayed using the same color scale. Based on our histological data, the frontal cortex was the most vulnerable region to ketamine-induced neuronal cell death [11], and thus, was selected as our main ROI. Tracer accumulation in the ROI in the left frontal cortex was converted to Standard Uptake Values (SUVs) which were computed as = average concentration of radioactivity in the ROI (mCi/mL) x body weight (gram)/injected dose (mCi).
The SUVs calculated for each ROI at different time points are displayed as means ± standard errors (SE). A repeated measures ANOVA profile model was used to determine the effect of ketamine on the uptake and the potential protective effect of minocycline on anesthetic-induced neuronal injury on the basis of the three ketamine treatment groups (minocycline; ketamine; minocycline plus ketamine) and a saline control group. The SUVs were modeled as a linear function of treatment and time profile within each PND time point. A Heterogeneous Autoregressive covariance structure was used to model the within-animal correlations for measurements on the same rat. The SUVs at different time points were compared between ketamine groups and the saline control using Student’s t-tests. All statistical tests were two sided and adjusted for multiplicity testing in order to control the nominal Type I Error rate. Dunnett’s adjustment method was used for pairwise treatment comparisons while Bonferroni correction was used for comparisons between observation times. Statistical significance was assessed at the 5% level. The SAS System for Windows (v9.3) was used for statistical analysis while Sigma Plot for windows (v11.0) was used to generate graphs.
Results
On PNDs 14, 21, 28 and 35, dynamic microPET scans were performed on each rat for 90 minutes. Acquisition of microPET data was started following i.v. injection of [18F]-FEPPA. PET image for each scan was constructed in a set of eighteen frames, in which every frame including the total radioactivity of the tissues collected over a 5 min period. Time Activity Curves (TACs) for the ROI in the frontal cortex [31,32], were obtained and tracer accumulation converted to SUVs. Figure 1 show 4 representative microPET images, recorded on PND 14, of the brain from a control rat (saline only), a control rat given minocycline, a ketamine-exposed rat given minocycline and a ketamine-exposed rat given saline. Images in figure 1 illustrate the distribution of radiotracer 0-5 minutes following the [18F]-FEPPA injection. The radioactivity accumulated in the ROI in frontal cortex of ketamine+saline treated rat was significantly higher 0-5 min after injection of radiotracer compared to the radioactivity in the same ROI of brains of saline, saline + minocycline, and ketamine+minocycline treated rats.
Dynamic [18F]-FEPPA uptake in the brain at different time points
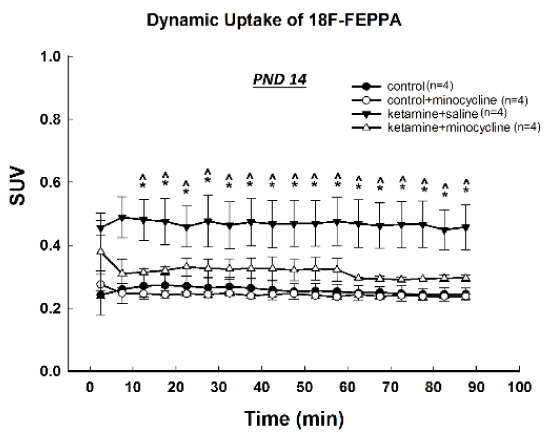
On PND14, about one week after the anesthetic exposure, SUVs in the ROI of frontal cortical area were higher in the ketamine+saline treated animals than in the saline controls and the minocycline +saline and ketamine+minocycline animals at almost every time points, indicating a significantly enhanced uptake and retention of [18F]-FEPPA in subjects that exposed to ketamine (Figure 2). Co-administration of minocycline effectively blocked this increase and there was no significant effect of minocycline on SUVs when given alone.
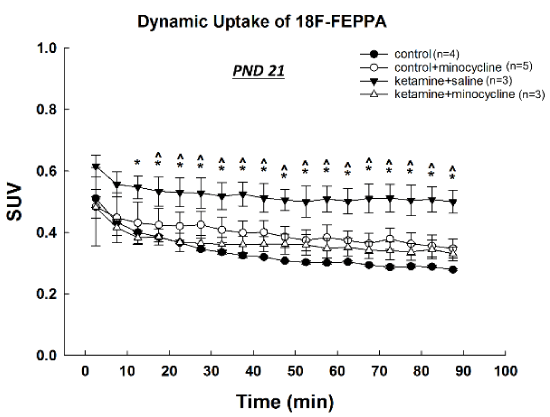
On PND 21, 2 weeks after anesthetic exposure, SUVs of [18F]-FEPPA in the same ROI were still significantly higher in the ketamine tested animals than in all other groups at most of the time points, showing a prolonged increase of [18F]-FEPPA uptake and retention in treated subjects (Figure 3). Compared with controls, there was no significant difference in SUVs detected in animals treated with ketamine plus minocycline.
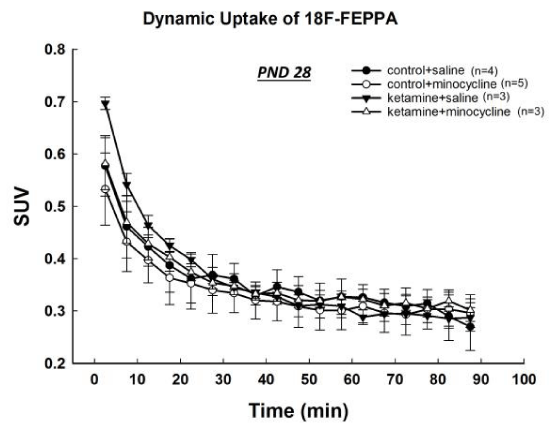
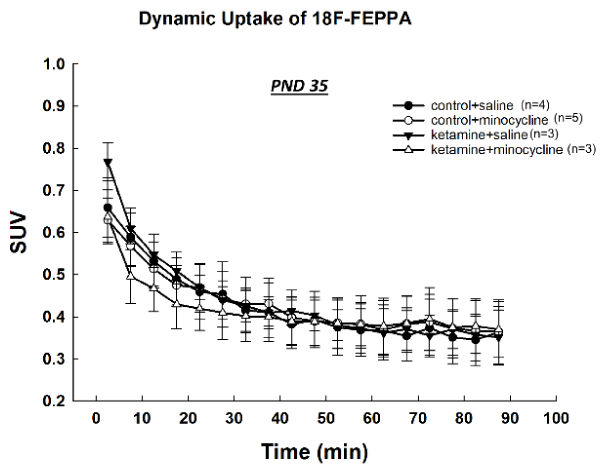
At PNDs 28 and 35, the uptake of [18F]-FEPPA in the ketamine-exposed rats was similar to that of control animals and no significant differences were observed at any time points (Figures 4,5).
Discussion
Ketamine is commonly used to induce and maintain general anesthesia in pediatric patients [33]. Ketamine is thought to exert its anesthetic effect through blockade of NMDA receptors, resulting in inhibition of neuronal activity in CNS. Prolonged exposure to ketamine causes widespread apoptotic neurodegeneration in the developing animal brain [3,7,9,34-37]. Previous studies in our lab demonstrated that neuronal apoptosis in several major area of developing brain can be induced by multiple ketamine injections (20 mg/kg given every 2 hours for 6 times) to PND 7 rats, especially in the frontal cortex [11,34]. Ketamine-induced neuronal apoptosis has also been shown to occur in newborn rhesus monkeys [38], in fetal NHP brains [34], and primary cultured rat neurons [6,39]. Early exposure to ketamine leads to persistent cognitive deficits, including impaired learning and memory in rodent and nonhuman primate models [12,40].
In the present study, microPET imaging using [18F]-FEPPA was used to repeatedly detect and quantify, in the same animal, aspects of neuronal activity thought to be associated with brain cell damage caused by ketamine-induced general anesthesia during early development. As a specific ligand for the Translocator protein (TSPO), a biomarker of activated microglia [41-43], the synthesis of [18F]-FEPPA has proven efficiently with high radiochemical yields. [18F]-FEPPA was applied successfully in previous nonhuman primate and rodent studies [29,44-50]. In response to a variety of neuronal insults, glial cells are activated and the expression of TSPO in those cells, especially microglia and astrocytes, is significantly upregulated. The upregulation of TSPOs is associated with several neurological and psychological disorders including multiple sclerosis, cerebral ischemia and stroke, epilepsy, brain injury, brain infection, neurodegenerative diseases and schizophrenia [43,51-56]. By monitoring the increased expression of TSPO in the same animal at different time points, anesthetic-induced neurotoxicity can be investigated dynamically via microPET imaging using [18F]-FEPPA [47-49].
According to our PET imaging data obtained on PNDs 14 and 21, treatment of rat pups with multiple doses of ketamine on PND 7 increased microglial activation in the frontal cortex for at least two weeks. This effect had abated completely 3 weeks after exposure. These observations demonstrate that seemingly adverse effects of ketamine-induced anesthesia persist long after animals have recovered from anesthesia when levels of ketamine are no longer detectable.
Although previous studies and the current data provide evidence of general anesthesia-induced developmental neurotoxicity, it is simply not possible to eliminate the need for general anesthesia in pediatric and neonatal patients. Thus, practical strategies that can protect the developing brain from general anesthesia-induced neuronal injury and possibly the long-lasting cognitive impairments that may ensue are urgently needed. In addition to other agents that have been shown to be effective in ameliorating general anesthetic-induced neurotoxicity such as L-carnitine, acetyl-L-carnitine, and 7-nitroindazole [32,57,58], the neuroprotective effect of minocycline was investigated in the present study.
Minocycline is an antibiotic effective against a broad spectrum of bacteria and can easily traverse the blood-brain barrier, probably because of its high lipophilicity [19-21]. Recently, minocycline has been reported to have other properties in addition to its antibiotic activity including neuroprotection [19-22,24,27,59]. One of the possible mechanisms underlying the neuroprotective effect of minocycline is its ability to inhibit microglial activation [21,59-61]. Microglial activation in response to neuronal insults enhances the clearance of necrotic and apoptotic neurons and the survival of surrounding healthy neurons. However, microglia activation can also be toxic to the CNS due to the associated release of free radicals and inflammatory cytokines [59,62,63]. Based on our previous microPET data showing general anesthesia related retention of FEPPA indicating microglial activation, we sought in the present study to assess the protective effects of minocycline using microglial activation as the metric.
The results presented here indicate that microPET imaging is capable of distinguishing differences in the retention of [18F]-FEPPA in the brains of rodents with a history of general anesthesia. This approach seemingly provides a minimally invasive way in which to monitor pathogenic processes associated with the developmental neurotoxicity caused by general anesthetics such as ketamine. The data presented here also suggest that minocycline can protect the neonatal brain from ketamine-induced neuronal damage, presumably--at least in part-- by inhibition of microglial activation. That minocycline might be employed as a neuroprotective agent in pediatric patients and infants in need of general anesthesia should be seriously considered. However, much yet need to be described concerning the precise timing and dosing that would be appropriate for minocycline in the pediatric setting. Toward this end, further animal studies that focus on examining other neuroprotective mechanisms underlying the effects of minocycline would be helpful as would a thorough assessment of its potential adverse effects.
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The views expressed in this report are those of the authors and do not necessarily reflect the views of the FDA.
This work was supported by the National Center for Toxicological Research (NCTR)/U.S. Food and Drug Administration (FDA). The authors are grateful for the technical expertise provided by the animal care staff of Priority One Services.
- Kohrs R, Durieux ME (1998) Ketamine: teaching an old drug new tricks. Anesth Analg 87: 1186-1193. Link: https://goo.gl/bmhyE8
- Mellon RD, Simone AF, Rappaport BA (2007) Use of anesthetic agents in neonates and young children. Anesth Analg 104: 509-520. Link: https://goo.gl/dZve1S
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, et al. (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283: 70-74. Link: https://goo.gl/hRVZpz
- Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, et al. (2013) Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth 111: 143-151. Link: https://goo.gl/FBztjo
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, et al. (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23: 876-882. Link: https://goo.gl/Zjceg3
- Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, et al. (2013) Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci 131: 548-557. Link: https://goo.gl/oGWH4E
- Scallet AC, Schmued LC, Slikker W Jr, Grunberg N, Faustino PJ, et al. (2004) Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci 81: 364-370. Link: https://goo.gl/GnnUZa
- Slikker W Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, et al. (2007) Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 98: 145-158. Link: https://goo.gl/YWk6CJ
- Wang C, Sadovova N, Fu X, Schmued L, Scallet A, et al. (2005) The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience 132: 967-977. Link: https://goo.gl/Ejk5AA
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, et al. (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110: 796-804. Link: https://goo.gl/U4MTPJ
- Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, et al. (2009) Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci 108: 149-158. Link: https://goo.gl/vYsArw
- Paule MG, Li M, Allen RR, Liu F, Zou X, et al. (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33: 220-230. Link: https://goo.gl/iqM9Zm
- DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G (2009) A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 21: 286-291. Link: https://goo.gl/csW9xU
- Istaphanous GK, Loepke AW (2009) General anesthetics and the developing brain. Curr Opin Anaesthesiol 22: 368-373. Link: https://goo.gl/wtXdny
- Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, et al. (2012) Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology 117: 494-503. Link: https://goo.gl/MLf37A
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, et al. (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128: e1053-1061. Link: https://goo.gl/dRx34j
- Ma W, Cao YY, Qu S, Ma SS, Wang JZ, et al. (2016) Remote ischemic preconditioning provides neuroprotection: impact on ketamine-induced neuroapoptosis in the developing rat brain. Eur Rev Med Pharmacol Sci 20: 4972-4979. Link: https://goo.gl/xzTvrE
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, et al. (2012) Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87: 120-129. Link: https://goo.gl/dzMxuU
- Monte AS, de Souza GC, McIntyre RS, Soczynska JK, dos Santos JV, et al. (2013) Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: Possible involvement of antioxidant and nitrergic pathways. J Psychopharmacol 27: 1032-1043. Link: https://goo.gl/ZuqyZc
- Song Y, Wei EQ, Zhang WP, Ge QF, Liu JR, et al. (2006) Minocycline protects PC12 cells against NMDA-induced injury via inhibiting 5-lipoxygenase activation. Brain Res 1085: 57-67. Link: https://goo.gl/LbK1oK
- Zemke D, Majid A (2004) The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol 27: 293-298. Link: https://goo.gl/wp7FPt
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, et al. (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology 32: 2393-2404. Link: https://goo.gl/pXv168
- Tian Y, Guo S, Wu X, Ma L, Zhao X (2015) Minocycline alleviates sevoflurane-induced cognitive impairment in aged rats. Cell Mol Neurobiol 35: 585-594. Link: https://goo.gl/ZQe1jv
- Wang HL, Liu H, Xue ZG, Liao QW, Fang H (2016) Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med 20: 1632-1639. Link: https://goo.gl/mma8VQ
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M (2004) Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis 17: 359-366. Link: https://goo.gl/5EdfyS
- FDA: Drug Approval Package: Solodyn (minocycline Hydrochloride) Extended-Release Tablets. In.; 2015.
- Apoorv TS, Babu PP (2017) Minocycline prevents cerebral malaria, confers neuroprotection and increases survivability of mice during Plasmodium berghei ANKA infection. Cytokine 90: 113-123. Link: https://goo.gl/TgQAGA
- Zhang X, Paule MG, Newport GD, Liu F, Callicott R, et al. (2012) MicroPET/CT Imaging of [18F]-FEPPA in the Nonhuman Primate: A Potential Biomarker of Pathogenic Processes Associated with Anesthetic-Induced Neurotoxicity. ISRN Anesthesiology 2012: 11. Link: https://goo.gl/w7H9Np
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, et al. (2008) Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol 35: 305-314. Link: https://goo.gl/8ccsKM
- Berridge MS, Apana SM, Hersh J (2009) Teflon radiolysis as the major source of carrier in fluorine-18. J Label Compd Radiopharm 52: 543-548. Link: https://goo.gl/2MCMQE
- Zou X, Liu F, Zhang X, Patterson T, Callicott R, et al. (2011) Inhalation anesthetic-induced neuronal damage in the developing Rhesus monkey. Neurotoxicology and Teratology 33: 592-597. Link: https://goo.gl/Kb5HXk
- Zou X, Sadovova N, Patterson TA, Divine RL, Hotchkiss CE, et al. (2008) The effects of L-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience 151: 1053-1065. Link: https://goo.gl/TqKLLv
- Kurdi MS, Theerth KA, Deva RS (2014) Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res 8: 283-290. Link: https://goo.gl/bgxWhh
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, et al. (2012) Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 116: 372-384. Link: https://goo.gl/ydRtpY
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, et al. (2002) Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol 12: 488-498. Link: https://goo.gl/EVTexx
- Slikker W Jr, Paule MG, Wright LK, Patterson TA, Wang C (2007) Systems biology approaches for toxicology. J Appl Toxicol 27: 201-217. Link: https://goo.gl/TdsNRs
- Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, et al. (2006) Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci 91: 192-201. Link: https://goo.gl/81zibb
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, et al. (2009) Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci 27: 727-731. Link: https://goo.gl/Z8352M
- Li J, Wang B, Wu H, Yu Y, Xue G, et al. (2014) 17beta-estradiol attenuates ketamine-induced neuroapoptosis and persistent cognitive deficits in the developing brain. Brain Res 1593: 30-39. Link: https://goo.gl/zzgZgn
- Huang L, Liu Y, Jin W, Ji X, Dong Z (2012) Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res 1476: 164-171. Link: https://goo.gl/R3Levt
- Braestrup C, Albrechtsen R, Squires RF (1977) High densities of benzodiazepine receptors in human cortical areas. Nature 269: 702-704. Link: https://goo.gl/oG8MUv
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, et al. (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27: 402-409. Link: https://goo.gl/R9Z8Bo
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX (2006) Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 138: 749-756. Link: https://goo.gl/1QrH9i
- Bennacef I, Salinas C, Horvath G, Gunn R, Bonasera T, et al. (2008) Comparison of [11C]PBR28 and [18F]FEPPA as CNS peripheral benzodiazepine receptor PET ligands in the pig. J Nucl Med 49: 81. Link: https://goo.gl/Dm9Vnq
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, et al. (2011) Quantitation of translocator protein binding in human brain with the novel radioligand [(18)F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 31: 1807-1816. Link: https://goo.gl/P4FJPF
- Schweitzer PJ, Fallon BA, Mann JJ, Kumar JS (2010) PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discov Today 15: 933-942. Link: https://goo.gl/oBHSaM
- Zhang X, Newport GD, Paule MG, Liu S, Berridge MS, et al. (2013) Quantitative Assessment of Acetyl-carnitine Effects on Anesthetic-Induced Neuronal Death using MicroPET/CT Imaging. Journal of Drug and Alcohol Research 2. Link: https://goo.gl/mQJwzR
- Zhang X, Paule MG, Newport GD, Liu F, Callicott R, et al. (2012) MicroPET/CT imaging of [18F]-FEPPA in the nonhuman primate: a potential biomarker of pathogenic processes associated with anesthetic-induced neurotoxicity. ISRN Anesthesiology 11. Link: https://goo.gl/dZ9nVf
- Zhang X, Paule MG, Wang C, Slikker W Jr (2013) Application of microPET imaging approaches in the study of pediatric anesthetic-induced neuronal toxicity. J Appl Toxicol 33: 861-868. Link: https://goo.gl/R2fXXq
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, et al. (2011) Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. Journal of Cerebral Blood Flow & Metabolism 31: 1807-1816. Link: https://goo.gl/2xuXX2
- Benavides J, Fage D, Carter C, Scatton B (1987) Peripheral type benzodiazepine binding sites are a sensitive indirect index of neuronal damage. Brain Res 421: 167-172. Link: https://goo.gl/uathua
- Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, et al. (2008) Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem 51: 17-30. Link: https://goo.gl/fvQz2g
- Lang S (2002) The role of peripheral benzodiazepine receptors (PBRs) in CNS pathophysiology. Curr Med Chem 9: 1411-1415. Link: https://goo.gl/z566Kt
- Oku N, Kashiwagi T, Hatazawa J (2010) Nuclear neuroimaging in acute and subacute ischemic stroke. Ann Nucl Med 24: 629-638. Link: https://goo.gl/T7HYrG
- Kuszpit K, Hollidge BS, Zeng X, Stafford RG, Daye S, et al. (2017) [18F]DPA-714 PET Imaging Reveals Global Neuroinflammation in Zika Virus-Infected Mice. Mol Imaging Biol. Link: https://goo.gl/TP9drq
- Selvaraj S, Bloomfield PS, Cao B, Veronese M, Turkheimer F, et al. (2017) Brain TSPO imaging and gray matter volume in schizophrenia patients and in people at ultra high risk of psychosis: An [11C]PBR28 study. Schizophr Res. Link: https://goo.gl/rP4wtp
- Wang C, Sadovova N, Patterson TA, Zou X, Fu X, et al. (2008) Protective effects of 7-nitroindazole on ketamine-induced neurotoxicity in rat forebrain culture. Neurotoxicology 29: 613-620. Link: https://goo.gl/VewcvV
- Zhang X, Liu S, Newport GD, Paule MG, Callicott R, et al. (2016) In Vivo Monitoring of Sevoflurane-induced Adverse Effects in Neonatal Nonhuman Primates Using Small-animal Positron Emission Tomography. Anesthesiology 125: 133-146. Link: https://goo.gl/QEe3X7
- Chen Y, Cai Z, Ke Z (2017) Antineuroinflammation of Minocycline in Stroke. Neurologist 22: 120-126. Link: https://goo.gl/H1pndD
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95: 15769-15774. Link: https://goo.gl/ySi2ju
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, et al. (1999) A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96: 13496-13500. Link: https://goo.gl/aKZAgp
- Boridy S, Soliman GM, Maysinger D (2012) Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine (Lond) 7: 1149-1165. Link: https://goo.gl/N5Koae
- Chen G, Mao J, Zhao J, Zhang Y, Li T, et al. (2016) Arsenic trioxide mediates HAPI microglia inflammatory response and the secretion of inflammatory cytokine IL-6 via Akt/NF-kappaB signaling pathway. Regul Toxicol Pharmacol 81: 480-488. Link: https://goo.gl/Rv36D2

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
