Global Journal of Anesthesiology
Lidocaine Infusion Decreases Pain Scores in a Fibromyalgia Pain Population with Significant Differential Pain Relief Secondary to Smoking Status
Yang Hyung Kim1, Dan Moyse2, Christian Horazeck2, Hung-Lun Hsia3, Carlos J Roldan4,5, Billy Huh4 and Lance Roy2*
2Department of Anesthesiology, Duke University, Durham, NC 27705, USA
3Department of Anesthesiology, Durham Veterans Affairs Hospital, Durham, NC 27705, USA
4Department of Pain Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, TX 77030, USA
5Department of Emergency Medicine, The University of Texas, Houston, TX 77030, USA
Cite this as
Kim YH, Moyse D, Horazeck C, Hsia HL, Roldan CJ, et al. (2017) Lidocaine Infusion Decreases Pain Scores in a Fibromyalgia Pain Population with Significant Differential Pain Relief Secondary to Smoking Status. Glob J Anesth 4(2): 016-022. DOI: 10.17352/2455-3476.000032Study Objective: Our hypothesis is that systemic lidocaine can significantly reduce chronic pain associated with fibromyalgia. A secondary goal of the study is to determine if other patient demographics can affect the response to IV lidocaine.
Design: Retrospective chart review.
Setting: Outpatient pain clinic.
Patients: 55 patients being treated for fibromyalgia by lidocaine infusion
Interventions: Intravenous infusion of lidocaine 5mg/kg over 1 hour.
Measurements: Two-tailed paired t-tests (α=0.05) were performed to test for significant change from pre- to post-infusion in BPI, PI, and VAS scores. Smoking status and race classification were also identified.
Main Results: Mean BPI score decreased from 83.2±16.5 to 73.7±21.6 after infusion of 4 to 5 mg/kg of lidocaine in 500 ml 5% dextrose (p-value<0.0001). Mean VAS decreased from 7.6±1.6 to 5.8±2.2 (p<0.0001). The post-infusion BPI was significantly lower in non-smokers 68.5 ±19.6 vs. smokers 80.3 ±17.2 (p=0.028) and Caucasians 69.3 ±20.3 vs. African-Americans 83.6±21.4 (p=0.027).
Conclusions: A retrospective analysis of 55 fibromyalgia patients who received IV lidocaine shows a statistically significant decrease in pain scores. A subgroup analysis shows that baseline pain scores are higher in smokers, and response to IV lidocaine is attenuated in smokers compared to non-smokers.
Introduction
Fibromyalgia is a complex chronic pain syndrome that represents the extreme spectrum of musculoskeletal pain in the general population. It is defined as widespread pain lasting for more than three months, along with the presence of tenderness, discomfort, or pain in 11 or more of 18 tender points in the body [1]. In addition, fibromyalgia is often associated with other constitutional symptoms such as disturbed sleep, emotional distress, and pronounced fatigue. Like many other clinical syndromes, fibromyalgia has no single specific feature but represents a symptom complex of self-reported or elicited findings [2].
Fibromyalgia is a relatively common disorder that is present in about 2% of the population with higher rates among women when compared to men, 3.4% and 0.5%, respectively. The prevalence of fibromyalgia increases with age. The highest frequency is between 60 and 79 years (greater than 7.0% in women). Characteristic features of fibromyalgia including altered pain threshold levels and symptoms complexes are similar in community and pain clinic populations, but the overall severity and functional disability are more severe in the pain clinic population [3]. In fact, the chronic pain associated with fibromyalgia is significantly associated with a decrease in health-related quality of life. Effective management of fibromyalgia symptoms, especially the chronic pain component, is associated with an increase in quality of life [4].
Despite new insights into the pathogenesis and treatment of fibromyalgia, it remains a condition that is difficult to treat. Many patients have suboptimal response to conventional pharmacologic treatment, and many often struggle with comorbid medical conditions, including depression and anxiety. Intravenous (IV) lidocaine infusions have shown efficacy in treating a wide variety of chronic pain conditions, including fibromyalgia. Prior studies have demonstrated the therapeutic effect of intravenous lidocaine infusions and improvement in pain scores in patients with fibromyalgia [5]. Evidence that suggests systemic lidocaine can induce a significant and selective reduction of several components of pain. The observed preferential anti-hyperalgesic and anti-allodynic effects of this drug suggest a selective central action on the mechanisms underlying these [6].
As such, the goal of this study is to corroborate and strengthen earlier findings in the literature regarding reductions in pain, as well as identify patient characteristics that may affect pain levels or response to treatment. Our hypothesis is that systemic lidocaine can significantly reduce the chronic pain associated with fibromyalgia. A secondary goal of the study is to determine if other patient demographics can affect the response to IV lidocaine.
The pharmacologic treatment of fibromyalgia often is comprised of a multi-faceted approach. Classes of drugs commonly prescribed include antidepressants —primarily duloxetine, milnacipran, tricyclic antidepressants, and antiepileptics, mainly pregabalin. Other drug classes commonly prescribed include opioid analgesics, muscle relaxants and sleep agents [7]. Often, complementary treatments such as psychological therapy, cognitive behavioral therapy [5], and mind-body techniques including hypnosis and biofeedback [8], are used to help with symptom management. However, regardless of these therapeutic options, many patients still have significant chronic pain complaints associated with fibromyalgia.
Certain behavioral factors can affect how people perceive and experience pain. For example, smoking status is independently associated with chronic pain, with smoking associated with musculoskeletal pain despite adjustment for confounding factors [9]. Though there are no clear associations between pain threshold levels and smoking status [10], habitual cigarette smoking correlates with lower physical and psychological indices of chronic pain. Many chronic pain patients frequently mitigate their pain by smoking. 57% of patients who smoke report having a need to smoke when they are in pain [11]. However, when smoking and non-smoking back pain patients were compared, smokers showed significantly higher levels of emotional distress, tended to remain inactive, and relied on medication more often than the nonsmoking patients [11].
Additionally, several studies have found an association between race and pain tolerance levels. Studies have shown that Caucasians typically have higher pain thresholds, African Americans have an intermediate level, and Asian Americans have the lowest pain threshold level [12,13]. The genetic and cultural differences of pain perception among various races presents a challenge, as a patient’s genetic background, cultural views, and support structure must be accounted for in the development and treatment of evolving chronic pain. As such, there remains a need for effective treatments for patients with fibromyalgia that also account for the potential impact of factors such as race.
Local anesthetics have been used in medical treatments for many years. Traditionally local anesthetics, such as lidocaine, have been used as an anesthetic for local and regional anesthesia as well as an antiarrhythmic agent. The variability in the effects of local anesthetics is attributable to differential blockade of various cellular systems at different concentrations. Lidocaine blocks sodium channels to inhibit neuronal transmission of pain signals but also has systemic effects in addition to inhibiting neuronal conduction [14]. Intravenous (IV) lidocaine has been utilized in various areas of medicine owing to its analgesic, anti-hyperalgesic, and anti-inflammatory properties [15]. In the perioperative setting, there is data supporting the use of IV lidocaine to reduce post-operative opioid consumption, early return of bowel function, and decreased the length of hospital stay [16-18]. In response to emerging literature, there have been increased efforts to elucidate fully the role of intravenous lidocaine, especially in treating chronic pain states that have failed conservative therapy.
Recently, there has been renewed interest in IV lidocaine infusions as a therapy in the treatment of chronic pain states. It has been used as a therapy for a wide range of chronic pain syndromes, such as fibromyalgia and complex regional pain syndrome (CRPS) [19,20]. IV lidocaine has also proved efficacious in the treatment of a variety of neuropathic pain conditions, which include post-stroke pain, peripheral neuropathy, diabetic neuropathy, and CRPS type 1 and 2 [21]. In a study on CRPS patients, significant reductions in multiple pain parameters were demonstrated, including dynamic and static mechano-allodynia, deep muscle pain, joint pain, and thermal allodynia [21]. Also, in a fibromyalgia patient population, subsequent IV lidocaine infusions over a five day period, demonstrated significant improvements in quality of life and visual analog scale (VAS) scores [19]. In addition, IV lidocaine (5 mg/kg body weight) has been shown to be effective in relieving the signs and symptoms of painful diabetic neuropathy in the short term [22,23]. Patients with peripheral nervous system (PNS) injury reported substantially more pain relief than those with central nervous system (CNS) injury or with the pain of unknown etiology [24]. This suggests that the pathophysiology of chronic pain due to PNS injury is different from that due to CNS injury and idiopathic pain and that pain due to PNS damage may be blocked by local anesthetic agents [24].
Unfortunately, the exact mechanisms involved in the improvement of fibromyalgia pain through lidocaine infusion have not been clearly defined. Intravenous lidocaine affects both peripheral and central voltage-gated sodium channels with more affinity for open channels. Studies in rats suggest the effect on peripheral voltage gated sodium channels is the major contributor to reducing the pain of skin incision. In contrast, it appears that intravenous lidocaine reduces neuropathic pain via reduction of central hyperexcitability [14]. Lidocaine also has actions separate from effects on central and peripheral voltage gated Na+ channels. Additional lidocaine mechanisms of action include increase in spinal acetylcholine, inhibition of spinal glycine receptors, release of endogenous opioids, activation of NMDA receptors, and decrease of inflammation through the release of ATP and activation of potassium channels [15].
Methods
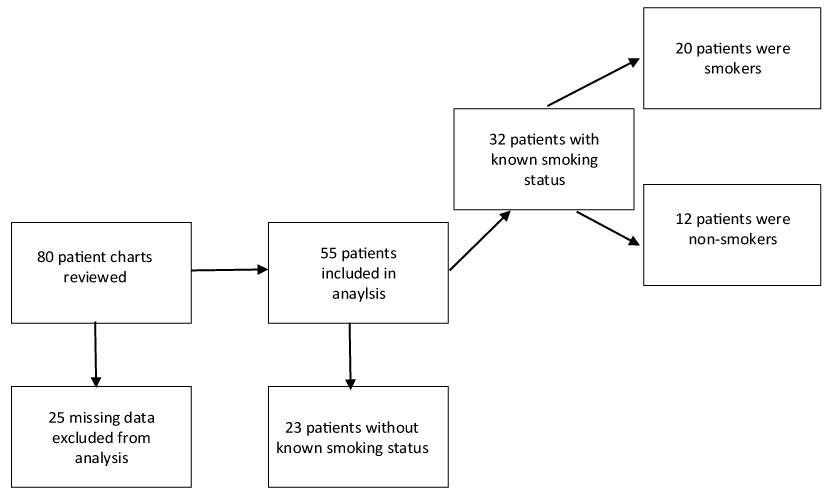
After Duke University Institutional Review Board (IRB) approval, a retrospective chart review was performed on 55 patients being actively treated for fibromyalgia under a waiver of consent conditions. The demographic information for these subjects is included in table 1. In this group of fibromyalgia patients, most of the individuals had significant pain that was refractory to other more conservative treatments. All patients underwent lidocaine infusion using 5mg/kg of lidocaine in D5W infused intravenously over 1 hour. The maximum dose used regardless of weight was 500mg. Patient information retrieved from the chart review included: sex, race, weight, pain duration, duration of relief after lidocaine infusion, smoking status, and individual pain scores as measured by brief pain inventory (BPI), visual analog scale (VAS), and pain interference scores (PIS).
As seen in figure 1, smoking status is known in only 32 of our 55 patients. Because of the importance of smoking as a potential effect, we proceeded with the analysis in that subset. Although results involving smoking status are based only on that subset, we have no reason to think it is not representative of the sample, nor to suspect any bias in data collection.
As a normal distribution of the population could not be assured, Wilcoxon signed rank tests were also performed on each paired measure with α=0.05. Then, separate repeated-measures ANOVA’s were performed to test the effects of smoking status, sex, or race on overall BPI, PI, and VAS scores, as well as on pre-post change in these scores, with α=0.05.
Finally, a two-tailed t-tests (α=0.05) were performed to compare post infusion BPI, PI, and VAS scores based on smoking status and race groupings, as both were found to have significant differences on the F-test among groups.
Results
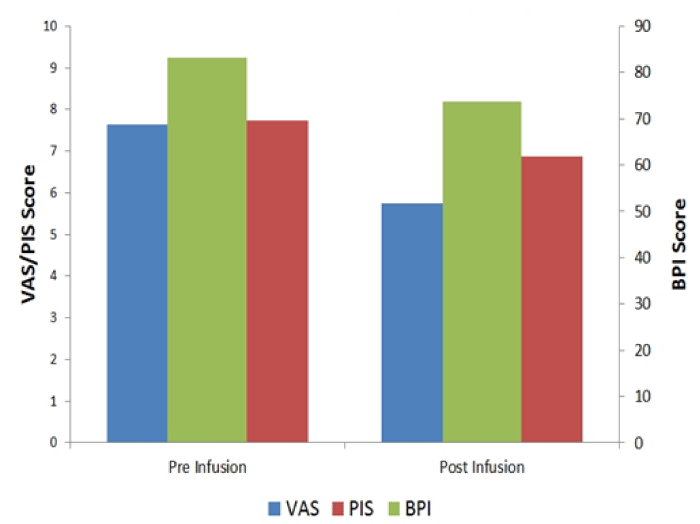
As seen in figure 2, mean BPI score was found to be 83.2±16.5 before and 73.7±21.6 after infusion of 4 to 5 mg/kg of lidocaine in 500 ml 5% dextrose. The mean improvement in BPI score was significant both in the t-test and Wilcoxon test (both p<0.0001).< /p>
Pain intensity score (PIS) was converted to 0-10 scale. Mean of PIS was 7.7±1.9 before lidocaine infusion, and 6.9±2.1 after lidocaine infusion, showing significant decrease both by the t-test (p <0.0001) and the Wilcoxon test (p<0.0001).
The mean scores of the VAS before and after infusion were 7.6±1.6 and 5.8±2.2 respectively, again showing a significant post-treatment decrease (p<0.0001 by both tests).
The post-infusion BPI was significantly lower in non-smokers 80.3 ±17.2 vs. smokers 68.5 ±19.6 (p=0.028) and Caucasians 69.3 ±20.3 vs. African-Americans 83.6 ±21.4 (p=0.027), with no statistically significant changes seen of pre-infusion BPI scores compared between these groups.
The post-infusion PIS was significantly lower in non-smokers 4.5 ±1.1 vs. smokers 5.2 ±1.2 (p=0.0037) and Caucasians 4.5 ±1.4 vs. African-Americans 5.6 ±1.2 (p=0.028), with no statistically significant changes seen of pre-infusion BPI scores compared between these groups.
Finally, a similar, statistically significant difference was observed between the post-infusion VAS in non-smokers 5.4 ±1.9 vs. smokers 6.3 ±2.1 (p=0.039) and Caucasians 5.5 ±2.0 vs. African-Americans 6.3 ±2.7 (p=0.018), with no statistically significant changes seen of pre-infusion BPI scores compared between these groups.
A difference between sexes could not be analyzed for either variable, as too few male participants were available for a reliable test.
Analysis
Statistical analysis was able to reject the null hypothesis of equal means between pre-infusion and post-infusion measurements of all three pain scales used. When paired with the significance of the one-tailed analysis, the results show a statistically significant reduction in pain after an infusion of 4 to 5 mg/kg of lidocaine in 500 ml 5% dextrose. These results were confirmed by a Wilcoxon signed rank test. In all cases, the null hypothesis could be rejected with p<0.0001. All three pain scales showed an equal and comparable result, suggesting that all three are reliable measurements of pain levels in patients both before and after interventional procedures.
The analysis of repeated measures matched pairs, taking into consideration a second variable, provided important information about the efficacy of treatment based on different patient characteristics. When using smoking status as a second variable, a significant difference was found between treatment results in non-smoking vs. smoking groups. For every pain scale used in our analysis, the patients that identified as smokers had a higher baseline pain score and a less robust decrease in pain score following intravenous lidocaine infusion when compared to non-smokers. The overall mean of VAS for smokers was found to be 7.4, while for non-smokers it was calculated as 6.8. When only post-infusion VAS scores were compared between smokers and non-smokers, statistically significant differences were found between both groups using all three scales. In all cases, smokers had a higher level of post-infusion pain and less improvement in pain scores after lidocaine infusion than non-smokers receiving a comparable treatment.
Our conclusions about smoking effects are limited by the fact that 23 of 55 patients were unable to be assigned a smoking status, as a retrospective chart review was unrevealing. However, we have no reason to suspect any bias in data collection, or to think this subset was not representative. Even in our subset of 32 patients whose smoking status was known, the effect of smoking was large enough to achieve significance.
A similar difference was found between patients grouped by race. Caucasians had a greater amount of pain relief than African-Americans in both post-infusion BPI and PIS scores. No such difference was found on post-infusion VAS. At the same time, grouped matched pair analysis showed statistically significant differences between racial groups for all three scales. There was not sufficient diversity in the study population to analyze race groups other than Caucasians and African Americans. There were too few males in the study to support reliable conclusions about gender differences in treatment.
Discussion
The analysis of a cohort of 55 fibromyalgia patients treated with IV Lidocaine shows a statistically significant decrease in pain as measured by mean Brief Pain Inventory (BPI) score, Pain Intensity Score (PIS), and Visual Analogue Score (VAS). Our results confirm what other studies have demonstrated in the past: intravenous lidocaine infusions are efficacious in treating patients with fibromyalgia [5].
However, further analyzing our data for patient’s smoking status in the subanalysis of matched pairs, we found that tobacco smokers had higher levels of baseline pain as measured by BPI, VAS, and PIS, along with a lower absolute reduction in pain scores after IV lidocaine infusions, as compared to non-smokers. This higher baseline pain score in smokers, as well as a lower absolute reduction in pain scores after lidocaine infusion compared to non-smokers, were noteworthy.
Tobacco use and chronic pain states, such as fibromyalgia, are a well-known, often comorbid health problem. Tobacco abuse remains the single most preventable cause of death in the United States, with an estimated 443,000 deaths annually attributed to tobacco abuse, costing the United States 96 billion dollars in direct medical expenses, and 97 billion in lost productivity every year [25].
However, the association between smoking and pain is not straightforward. Tobacco abuse and its association with pain have been documented by numerous studies in the past. Tobacco use has been shown to be proportionately greater in populations with chronic pain than in populations without chronic pain [26]. Direct comparison of any chronic pain in smokers as compared to the chronic pain in non-smokers is fraught with confounders, including comorbid medical, psychological, and behavioral conditions that are prevalent in this population. In essence, when focusing on comorbid pain and smoking, the question is whether a patient’s pain affects tobacco use, or that tobacco use affects and modulates a patient’s response to pain.
Nicotine has been shown to have direct pain-inhibitory effects in numerous animal studies, when administered subcutaneously, as measured by tail flick and hot plate models [27-29]. Also, nicotine has been demonstrated to provide the same analgesic effects via indirect administration in animals, as in tobacco smoke [30]. Nicotine exerts its complex physiologic effects by interacting with the nicotinic acetylcholine receptor (nAChR), which is a transmembrane complex composed of five proteins that mediate transmembrane passage of Na+, Ca2+, and K+ ions [31].
These ubiquitous receptors are located in both the peripheral and central nervous system. Nicotinic acetylcholine receptors composed of alpha 4, beta two subunits have been shown to be present in the dorsal horn of the spinal cord, and the thalamus, likely modulating the transmission of pain in the central nervous system [32,33]. A supraspinal component of nicotine’s pain modulating effect is likely due to a complex interplay with the descending endogenous opioid system. In rats, as measured by the tail-flick test, the opioid antagonist, naltrexone, and a centrally acting nicotinic receptor antagonist, mecamylamine, has been shown to block the analgesic effects of nicotine [34].
In human studies, nicotine has shown to have an acute analgesic effect in experimental models [35]. Much like the well-studied animal models, nicotine likely modulates the perception of pain at the dorsal horn in humans and modulates pain processing via central mechanisms such as descending inhibitory opioid pathways.
However, epidemiologic and prospective cohort studies have shown a positive association with smoking and chronic pain conditions [36,37]. This change is likely due to receptor desensitization and tolerance due to chronic exposure of nicotine, blunting the acute effects of nicotine on the body. Smokers who have tobacco withheld from them have shorter pain latency to heat pain and reduced tolerance to electrical pain stimulation [38,39]. Smokers have been demonstrated to have higher levels of Substance P in the CSF, than non-smokers [40]. Smokers have lower beta-endorphin levels as compared to non-smokers [41], while patients with fibromyalgia have lower beta-endorphin levels than controls [42].
Studies have assessed the relationship between smoking and fibromyalgia, as in our study patient population. Several studies have implicated smoking with an increase in widespread musculoskeletal pain compared to non-smokers [36,43]. In a cohort of American women, active smokers have been shown to have more chronic musculoskeletal pain than former smokers or non-smokers [44]. Data from Yunus et al. of 233 female patients with fibromyalgia showed that the smoking group reported more pain, numbness, global patient severity, and functional difficulties as compared to non-smokers [45]. These variables were noted to have a dose-effect relationship, with greater smoking causing greater functional impairment.
The association between tobacco abuse and its effects on disease modification of fibromyalgia is far from certain. Many studies have failed to show a definitive association between fibromyalgia and tobacco abuse. Costallat et al. could not show any difference in health status when comparing smoking status in 108 Brazilian women with fibromyalgia [46]. Another study has showed that chronic pain patients who smoke have greater pain intensity and increase in some painful areas [47]. However, Yunus et al. found no difference in tender points when comparing smoking status amongst patients with fibromyalgia [45].
Many of the population studies conducted on fibromyalgia patients of various ethnic backgrounds are small and cross-sectional. Smokers often have higher levels of depression compared to non-smokers. Hence, smoking can have an impact on the severity of fibromyalgia symptoms [48]. Our results also show that response to IV lidocaine infusions is different between races. African American patients reported less pain relief than Caucasian patients from an IV lidocaine infusion. The racial differences seen are multifactorial; likely a summation of differing social and psychological racial constructs, known genetic differences, and new, evolving research into the contribution of epigenetics, all contributing to differences in pain perception.
The analysis of race and its effect on pain is relatively new in the scientific literature. Early medical research in the United States excluded African-Americans [49]. The race was merely associated with stereotyped behavior and emotional characteristics [49]. However, as we progressed towards the twenty-first century, our inquest into elucidating racial differences in pain has expanded. Nevertheless, our examination of racial differences in pain is tempered by various contemporary social factors including prevalent socioeconomic disparities. Underserved populations have less access to health care, and persistent medical problems such as pain may be undertreated and cause greater psychological and physical distress [50]. An analysis of a cohort of African American and Caucasian women with fibromyalgia showed that African American women had more widespread pain as compared to Caucasian women, who were noted to have more tender points and pain intensity at these points. They also noted greater depressive symptoms in African American cohort compared to the Caucasian cohort [51].
Complex genetic differences in pain modulation in African Americans versus Caucasians may also contribute to clinical disparities. African Americans have been shown to have more pain associated with chronic medical conditions than Caucasians [49]. Experimental laboratory studies have shown that African Americans have reduced pain tolerance for various modalities of pain, including extremes of temperature and pressure pain [52,53]. Altered pain regulatory mechanisms could account for clinical differences in subjective pain relief. African-Americans showed less blood pressure response and change in norepinephrine levels after both rest and stress conditions compared to Caucasians [53].
The limitations of our study should be noted. Our study was retrospective in nature, relying on documentation of multiple providers. Our sample size was only 55 patients who received treatment at one tertiary care referral site. Although the sample size was sufficient to reject the null hypothesis, it is inadequate to make generalizations to entire populations. Our study only contained three male patients, consistent with the known gender ratio of fibromyalgia, preventing any meaningful analysis of gender effect.
Although we were not able to obtain the smoking status of 23 of the 55 patients, the impact of tobacco on outcome still had adequate power, and the results are statistically significant.
Conclusions
A retrospective analysis of a cohort of 55 fibromyalgia patients who received IV lidocaine shows a statistically significant decrease in pain scores. A subgroup analysis shows that baseline pain scores are higher in smokers, and response to IV lidocaine is attenuated in smokers compared to non-smokers. Moreover, race also seems to influence the response to IV lidocaine. Therefore, it is reasonable to stress smoking cessation in this population, and further research is needed to understand better the effect of tobacco use and race in the management of fibromyalgia pain.
- Wolfe F, Smyth HA, Yunus MB, Bennett RM, Bombardier C, et al (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160-172. Link: https://goo.gl/tlB3L2
- Staud R (2006) Biology and therapy of fibromyalgia: pain in fibromyalgia syndrome. Arthritis Res Ther 8: 208. Link: https://goo.gl/TCGa1X
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38: 19-28. Link: https://goo.gl/EimxRG
- Bennett RM, Schein J, Kosinski MR, Hewitt DJ, Jordan DM, et al. (2005) Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum 53: 519-527. Link: https://goo.gl/7W5Gsk
- Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, et al. (2010) Psychological treatments for fibromyalgia: a meta-analysis. Pain 151: 280-295. Link: https://goo.gl/UICA8e
- Attal N, Gaude V, Brasseur L, Dupuy M, Guirimand F, et al. (2000) Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study. Neurology 54: 564-574. Link: https://goo.gl/MRYxcX
- Dussias P, Kalali AH, Staud RM (2010) Treatment of fibromyalgia. Psychiatry (Edgmont) 7: 15-18. Link: https://goo.gl/RpGhEA
- Berman BM, Swyers JP (1999) Complementary medicine treatments for fibromyalgia syndrome. Baillieres Best Pract Res Clin Rheumatol, 13: 487-492. Link: https://goo.gl/YcT1Il
- Brage S, Bjerkedal T (1996) Musculoskeletal pain and smoking in Norway. J Epidemiol Community Health 50: 166-169. Link: https://goo.gl/2dF7K6
- Pauli P, Rau H, Zhuang P, Brody S, Birbaumer N (1993) Effects of smoking on thermal pain threshold in deprived and minimally-deprived habitual smokers. Psychopharmacology (Berl) 111: 472-476. Link: https://goo.gl/d3X2xP
- Jamison RN, Stetson BA, Parris WC (1991) The relationship between cigarette smoking and chronic low back pain. Addict Behav 16: 103-110. Link: https://goo.gl/M6ilJT
- Woodrow KM, Friedman GD, Siegelaub AB, Collen MF (1972) Pain tolerance: differences according to age, sex and race. Psychosom Med 34: 548-556. Link: https://goo.gl/bPx1Th
- Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS (2000) Race and sex differences in cutaneous pain perception. Psychosom Med 62: 517-523. Link: https://goo.gl/Ezx1hs
- Hollmann MW, Durieux ME (2000) Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93: 858-875. Link: https://goo.gl/2N3h6Y
- Koppert W, Ostermeier N, Sittl R, Weidner C, Schmelz M (2000) Low-dose lidocaine reduces secondary hyperalgesia by a central mode of action. Pain 85: 217-224. Link: https://goo.gl/TCmNl5
- Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME (2007) Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 106: 11-18. Link: https://goo.gl/KCzlJZ
- Groudine SB, Fisher HA, Kaufman RP Jr, Patel MK, Wilkins LJ, et al (1998) Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg 86: 235-239. Link: https://goo.gl/NQTjJo
- Cassuto J, Wallin G, Hogstrom S, Faxen A, Rimback G (1985) Inhibition of postoperative pain by continuous low-dose intravenous infusion of lidocaine. Anesth Analg 64: 971-974. Link: https://goo.gl/8AOtKV
- Schafranski MD, Malucelli T, Machado F, Takeshi H, Kaiber F, et al. (2009) Intravenous lidocaine for fibromyalgia syndrome: an open trial. Clin Rheumatol 28: 853-855. Link: https://goo.gl/TBNtoa
- Schwartzman RJ, Patel M, Grothusen JR, Alexander GM (2009) Efficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med 10: 401-412. Link: https://goo.gl/dqmdxo
- Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB (2005) Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg 101: 1738-1749. Link: https://goo.gl/W0yNTd
- Kastrup J, Petersen P, Dejgard A, Angelo HR, Hilsted J (1987) Intravenous lidocaine infusion--a new treatment of chronic painful diabetic neuropathy? Pain 28: 69-75. Link: https://goo.gl/f77XpB
- Kastrup J, Angelo H, Petersen P, Dejgard A, Hilsted J (1986) Treatment of chronic painful diabetic neuropathy with intravenous lidocaine infusion. Br Med J 292: 173. Link: https://goo.gl/EqqTD4
- Galer BS, Miller KV, Rowbotham MC (1993) Response to intravenous lidocaine infusion differs based on clinical diagnosis and site of nervous system injury. Neurology 43: 1233-1235. Link: https://goo.gl/p8Mr2K
- Centers for Disease, C. and Prevention (2012) Current cigarette smoking among adults - United States, 2011. MMWR Morb Mortal Wkly Rep 61: 889-894. Link: https://goo.gl/m7izlc
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ (2009) Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res 11: 1407-1414. Link: https://goo.gl/h7kCYv
- Sahley TL, Berntson GG (1979) Antinociceptive effects of central and systemic administrations of nicotine in the rat. Psychopharmacology 65: 279-283. Link: https://goo.gl/2z8Kw3
- Rowley TJ, Payappilly J, Lu J, Flood P (2008) The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth Analg 107: 1052-1057. Link: https://goo.gl/1WFoF4
- Aceto MD, Awaya H, Martin BR, May EL (1983) Antinociceptive action of nicotine and its methiodide derivatives in mice and rats. Br J Pharmacol 79: 869-876. Link: https://goo.gl/QxdAeV
- Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI (2004) Antinociception induced by chronic exposure of rats to cigarette smoke. Neurosci Lett 366: 86-91. Link: https://goo.gl/hLHmZR
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8: 733-750. Link: https://goo.gl/Z5KD5P
- Cucchiaro G, Chaijale N, Commons KG (2005) The dorsal raphe nucleus as a site of action of the antinociceptive and behavioral effects of the alpha4 nicotinic receptor agonist epibatidine. J Pharmacol Exp Ther 313: 389-394. Link: https://goo.gl/cztCwe
- Rashid MH, Furue H, Yoshimura M, Ueda H (2006) Tonic inhibitory role of alpha4beta2 subtype of nicotinic acetylcholine receptors on nociceptive transmission in the spinal cord in mice. Pain 125: 125-135. Link: https://goo.gl/O6h8R7
- Simons CT, Cuellar JM, Moore JA, Pinkerton KE, Uyeminami D, et al. (2005) Nicotinic receptor involvement in antinociception induced by exposure to cigarette smoke. Neurosci Lett 389: 71-76. Link: https://goo.gl/OVqZKM
- Jamner LD, Girdler SS, Shapiro D, Jarvik ME (1998) Pain inhibition, nicotine, and gender. Exp Clin Psychopharmacol 6: 96-106. Link: https://goo.gl/N5h7Rp
- Eriksen WB, Brage S, Bruusgaard D (1997) Does smoking aggravate musculoskeletal pain? Scand J Rheumatol 26: 49-54. Link: https://goo.gl/nqdM9d
- Lanier DC, Stockton P (1998) Clinical predictors of outcome of acute episodes of low back pain. J Fam Pract 27: 483-489. Link: https://goo.gl/dN80dL
- Nesbitt PD (1973) Smoking, physiological arousal, and emotional response. J Pers Soc Psychol 25: 137-144. Link: https://goo.gl/pDQQbu
- Silverstein B (1982) Cigarette smoking, nicotine addiction, and relaxation. J Pers Soc Psychol 42: 946-950. Link: https://goo.gl/5wM0o0
- Vaeroy H, Helle R, Forre O, Kass E, Terenius L (1988) Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain 32: 21-26. Link: https://goo.gl/v53exh
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, et al. (2005) Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain 114: 372-385. Link: https://goo.gl/tQM7K9
- Panerai AE, Vecchiet J, Panzeri P, Meroni P, Scarone S, et al. (2002) Peripheral blood mononuclear cell beta-endorphin concentration is decreased in chronic fatigue syndrome and fibromyalgia but not in depression: preliminary report. Clin J Pain 18: 270-273. Link: https://goo.gl/APKZl6
- Andersson H, Ejlertsson G, Leden I (1998) Widespread musculoskeletal chronic pain associated with smoking. An epidemiological study in a general rural population. Scand J Rehabil Med 30: 185-191. Link: https://goo.gl/8rlNE2
- Mitchell MD, Mannino DM, Steinke DT, Kryscio RJ, Bush HM, et al. (2011) Association of smoking and chronic pain syndromes in Kentucky women. J Pain 12: 892-899. Link: https://goo.gl/g545kf
- Yunus MB, Arslan S, Aldag JC (2002) Relationship between fibromyalgia features and smoking. Scand J Rheumatol 31: 301-305. Link: https://goo.gl/WG89Ip
- Costallat BL, Cintra da Silva P, Martinez JE (2009) Does smoking influence fibromyalgia syndrome patients’ health status? J Musculoskelet Pain 17: 131–138. Link: https://goo.gl/6p7jUh
- John U, Hanke M, Meyer C, Volzke H, Baumeister SE, et al. (2006) Tobacco smoking in relation to pain in a national general population survey. Prev Med 43: 477-481. Link: https://goo.gl/Ztd8mE
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, et al. (2008) An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician 11: 643-653. Link: https://goo.gl/cmmpBS
- Edwards CL, Fillingim RB, Keefe F (2001) Race, ethnicity and pain. Pain 94: 133-137. Link: https://goo.gl/UvW9oW
- Todd KH, Deaton C, D'Adamo AP, Goe L (2000) Ethnicity and analgesic practice. Ann Emerg Med 35: 11-16. Link: https://goo.gl/hSlFRg
- Gansky SA, Plesh O (2007) Widespread pain and fibromyalgia in a biracial cohort of young women. J Rheumatol 34: 810-817. Link: https://goo.gl/gIj1VZ
- Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS (2005) African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med 67: 948-956. Link: https://goo.gl/b2k4TA
- Campbell CM, Edwards RR, Fillingim RB (2005) Ethnic differences in responses to multiple experimental pain stimuli. Pain 113: 20-26. Link: https://goo.gl/hM86fL

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
