Archives of Hematology Case Reports and Reviews
Simultaneous diagnosis of Multiple Myeloma and Myelodysplasia: A case report
K Benabed1-3*, J Ferey1, M Riviere1, MY Benabed2 and S Cheze3
2Reims University Hospital, France
3Caen University Hospital, France
Cite this as
Benabed K, Ferey J, Riviere M, Benabed MY, Cheze S (2023) Simultaneous diagnosis of Multiple Myeloma and Myelodysplasia: A case report. Arch Hematol Case Rep Rev 8(1): 001-003. DOI: 10.17352/ahcrr.000041Copyright License
© 2023 Benabed K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Multiple Myeloma (MM) and Myelodysplastic Syndrome (MDS) are two hematological malignancies with different clinico-biological features to their specific pathogenesis and evolution. The simultaneous occurrence of these two blood diseases within the bone marrow, unrelated to prior chemotherapy, is a rare event: we report subsequently the case of a concomitant presentation of MM and MDS. This case will eventually raise several questions on the pathophysiological, and therapeutic level and on their course, in particular infections, thrombosis, extramedullary localizations, and circulating plasma cells.
Abbreviations
ASXL1: Additional Sex Combs like 1 (transcriptional regulator); CGH: Comparative Genomic Hybridization; ECOG-PS: Eastern Cooperative Oncology Group - Performance Status; FISH: Fluorescence In Situ Hybridization; RSV: Respiratory Syncytial Virus
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of myeloid disorders that cause morphologic bone marrow dysplasia associated with peripheral blood cytopenias and increased risk of transformation to Acute Myelogenous Leukemia (AML). Myelodysplastic syndromes occur more frequently in older males and in individuals with prior exposure to cytotoxic therapy [1]. Multiple Myeloma (MM) is a clonal plasma cell proliferative disorder characterized by a bone marrow infiltration of clonal plasma cells and monoclonal protein in the serum and/or urine. The excess production of these plasma cells and immunoglobulins can lead to lytic bone lesions, kidney injury, anemia, and hypercalcemia [2,3]. However, the co-occurrence of these two entities, unrelated to prior chemotherapy, is rarely reported in the literature. We report subsequently the case of a simultaneous presentation of MM and MDS.
Case presentation
We report here the case of an 82-year-old patient, Caucasian, with no notable history, especially no known history of cancer or hemopathy, or exposure to cytotoxic medications, except for benign prostatic hyperplasia. and dyslipidemia; He is a former farmer.
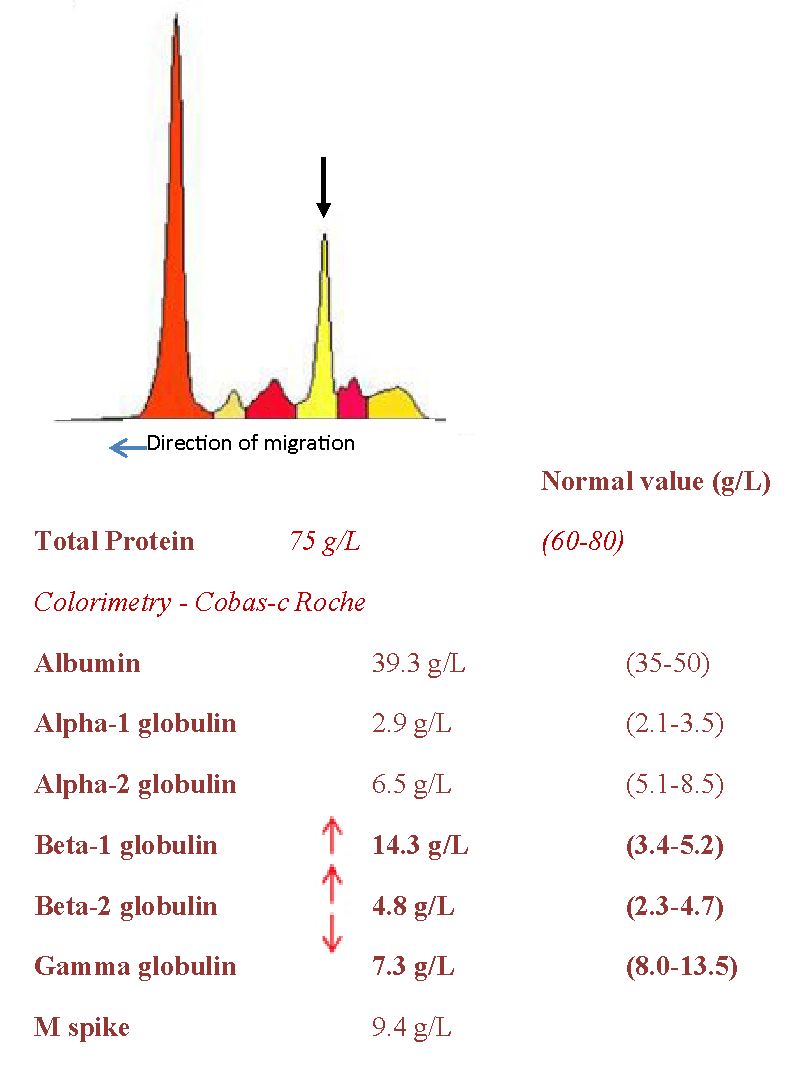
The patient was seen in medical consultation for the first time in May 2021, for a long-standing thrombocytopenia (107 G/L) and a low-level IgA kappa monoclonal gammopathy (9.4 g/l), leading to a first bone marrow aspiration showing some signs of DYSGRANULOPOIESIS, DYSERYTHROPOIESIS and 8% of DYSTROPHIC PLASMA CELLS (3.5% on Flow Cytometry). As for CGH array analysis and FISH on sorted plasma cells, they objectified recurrent abnormalities in the MM (a 1q21 gain; a gain of chromosomes 9 and 19 and a loss of chromosome 13; deletion of the long arms of a chromosome 14); no deletion of TP53 was demonstrated.
CLINICALLY, the patient only complained of fatigue (ECOG-PS 1); no bone pain or superficial tumor syndrome. We note the existence of trophic lesions of the distal extremities with chilblains (fingers and toes), old according to the patient, leading to a consultation in Internal Medicine of the Hospital which does not find any underlying autoimmune disease. The search for CRYOGLOBULIN came back negative. IMAGING (whole-body MRI) does not show suspicious bone lesions, except for a degenerative spine.
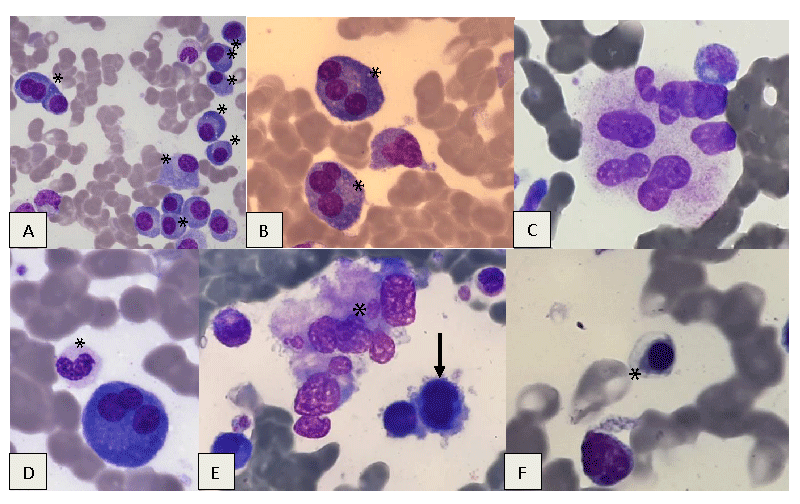
In March 2022, because of the progression of thrombocytopenia (55 G/l) and the occurrence of normochromic macrocytic (99.1fl) aregenerative anemia (hemoglobin 112 g/l) and neutropenia (0.972 G/l) with the presence of dysplastic features on the blood smear without circulating blasts, a new bone marrow aspiration is performed, this time finding a 20% dystrophic plasma cell infiltration associated with MDS with dysplasia of 3 lineages, without excess blasts (Figures 1,2). CONVENTIONAL KARYOTYPING, out of 21 mitoses, returned to normal.
Serum free light chains kappa 61.8 mg/l, lambda 28 mg/l and kappa/lambda ratio at 2.207.
Given the continuous aggravation of the pancytopenia, and of the lymphopenia (350/mm3), we decide to perform an Osteo-Medullary Biopsy (BOM), with double reporting, objectifying a marrow of increased cellularity in relation to age, evaluated at 60%; plasmacytosis estimated at 10%. Grade I myelofibrosis.
The Next Generation Sequencing (NGS) Myeloid Panel study detects two ASXL1 mutations, providing a clonality argument for a myeloid hemopathy, in this case, MDS (1).
The bone marrow aspiration performed at the iliac spine, at the same time as the BOM, finds 6% of plasma cells within a hypocellular marrow.
Lack of response to treatment with recombinant Erythropoietin (EPO) combined with short-time corticosteroid therapy for one week.
THE EVOLUTION was marked by the occurrence in October 2022 of febrile delirium.
Biologically, the complete blood count now shows very dystrophic circulating plasma cells (approximately 165/mm3) (2).
Analysis of Cerebrospinal Fluid (CSF) in microbiology shows the presence of Listeria monocytogenes, and cytologically a hypercellular fluid with a contingent of atypical plasma cells is demonstrated.
The search for Sars-CoV-2, influenza, and RSV viruses came back negative.
Recently, the patient was hospitalized again for an ischemic stroke (3), in January 2023.
In total: Coexistence of MM and MDS, with infectious (Listeria meningoencephalitis), tumor (neuromeningeal localization of MM), and vascular (stroke) complications.
Discussion
The simultaneous diagnosis of MM and MDS without a history of chemotherapy and/or radiotherapy is rarely reported in the literature. This case, unique in its clinical presentation and outcome, helps to delineate certain characteristics that impact the results observed.
The diagnostic step is the keystone of the initial management of the patient, who did not present CRAB/SLIM criteria, except for anemia which could be linked to MM or MDS. At this stage of diagnosis, MM could be wrongly classified as indolent. Exploration by NGS, FISH, and CGH-array at the same time confirmed the diagnosis of MM and MDS and has allowed us to establish the prognostic factors of these two associated hematological malignancies. In fact, the analysis of the data, for MM, objectified a 1q21 gain among other recurrent anomalies; for MDS an ASXL1 mutation. All these abnormalities are reported in the literature as having an unfavorable prognosis [4].
The occurrence of myeloid malignancies after the treatment for MM is well described in the literature [5-7]. On the other hand, there are only a limited number of reported cases of co-occurrence of MDS and MM where no previous chemotherapy was received. J.Várkonyi and al. described 8 cases of a concomitant development of MDS with MM or monoclonal gammopathy (MGUS) [8]. The median age of patients was 71.5 years [48 years - 88 years]. The degree of bone marrow infiltration by plasma cells ranges from 1% - 2% to 80% (median 15%, mean 30%), the immunoglobulin isotype secreted is variable (IgG, IgA, kappa light chain, IgM), and all patients presented with at least one peripheral cytopenia. These cases correspond more generally to lymphoproliferative disorder (MGUS cases) which may eventually progress to lymphoplasmacytic lymphoma, CLL, and other B-cell lymphomas. Coppelstone, et al. also reported a series of 20 patients with MDS and a concomitant lymphoid or plasmacytic neoplasm [9]: this raises the suspicion of an association between these two diseases. Coppelstone, et al. suggested a possible hypothesis that the presence of one of these neoplasms predisposes the development of the other, because of growth factors released influencing the other cell line [9]. Likewise, the numerous potential gene expression and molecular pathways involved in both MDS and MM may initiate or stimulate the progression of one or the other [10]. A possible genetic instability of a common precursor stem cell might also be an explanation [8,11]. An altered survival and function of natural killer cells (NK-cells) is described in MDS [12] and may possibly play a role in the development of B-cell lymphoproliferative disorder due to impaired anti-tumor immunity [9].
Conclusion
Further research is needed to elucidate the underlying mechanisms predisposing to coexistence of MM and MDS. The contribution of new generation diagnostic tools based not only on conventional cytogenetics and FISH, but also NGS and CGH-array which have now become essential for difficult and complex cases as illustrated in that case. Other similar cases and additional studies are necessary to establish therapeutic recommendations which still remain uncodified in this type of case.
Footnote
The expressed consent was obtained from the wife of the patient for the publication of the case.
- Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020 Nov;95(11):1399-1420. doi: 10.1002/ajh.25950. PMID: 32744763.
- Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, Gay F, Anderson KC. Multiple myeloma. Nat Rev Dis Primers. 2017 Jul 20;3:17046. doi: 10.1038/nrdp.2017.46. PMID: 28726797.
- Albagoush SA, Shumway C, Azevedo AM. Multiple Myeloma. In: StatPearls. Treasure Island (FL): StatPearls Publishing; Jan 2023; PMID: 30521185.
- Asada S, Fujino T, Goyama S, Kitamura T. The role of ASXL1 in hematopoiesis and myeloid malignancies. Cell Mol Life Sci. 2019 Jul;76(13):2511-2523. doi: 10.1007/s00018-019-03084-7. Epub 2019 Mar 30. PMID: 30927018.
- Gowin K, Skerget S, Keats JJ, Mikhael J, Cowan AJ. Plasma cell leukemia: A review of the molecular classification, diagnosis, and evidenced-based treatment. Leuk Res. 2021 Dec;111:106687. doi: 10.1016/j.leukres.2021.106687. Epub 2021 Aug 17. PMID: 34425325.
- Leleu X, Manier S, Dulery R. Myeloma and thrombosis Correspondences in Onco-haematology. July August September. 2010; 3
- Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008 Aug;35(4):418-29. doi: 10.1053/j.seminoncol.2008.04.012. PMID: 18692692; PMCID: PMC2600445.
- Judit V, Judit J, László G, Tamas M, Júlia T, Judit C, András M, Gábor T, Julianna S, Ferenc K. Myelodysplasia and Multiple Myeloma or Monoclonal Gammopathy. A Non-Fortuitous Coexistence. Hungarian Medical Journal. 2007; 1:235-240. 10.1556/HMJ.1.2007.2.10.
- Copplestone JA, Mufti GJ, Hamblin TJ, Oscier DG. Immunological abnormalities in myelodysplastic syndromes. II. Coexistent lymphoid or plasma cell neoplasms: a report of 20 cases unrelated to chemotherapy. Br J Haematol. 1986 May;63(1):149-59. doi: 10.1111/j.1365-2141.1986.tb07505.x. PMID: 3707860.
- Islam S, Sahu S. Dual diagnosis of De novo Myelodysplastic Syndrome and Multiple Myeloma: Youngest Case to be Reported and Literature Review. Clin Oncol. 2019; 4: 1623.
- Baumann MA, Libnoch JA, Hansen RM, Heckman MG, Hanson GA. Concurrent myelodysplasia and lymphoproliferation: a disorder of the true pluripotential stem cell? Q J Med. 1985 Jun;55(218):199-211. PMID: 4023169.
- Kiladjian JJ, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis JH, Fenaux P, Chouaib S, Caignard A. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006 Mar;20(3):463-70. doi: 10.1038/sj.leu.2404080. PMID: 16408099.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
